Machine Learning-Based Classification of Dependence in Ambulation in Stroke Patients Using Smartphone Video Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Video Data Collection
2.2. Assessment of Dependence in Ambulation
2.3. Pose Estimation and Tracking for Region-of-Interest Extraction
2.4. Video Pre-Processing for Deep Learning
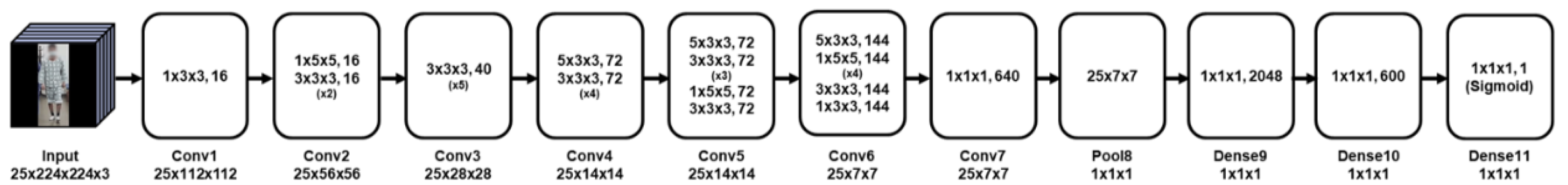
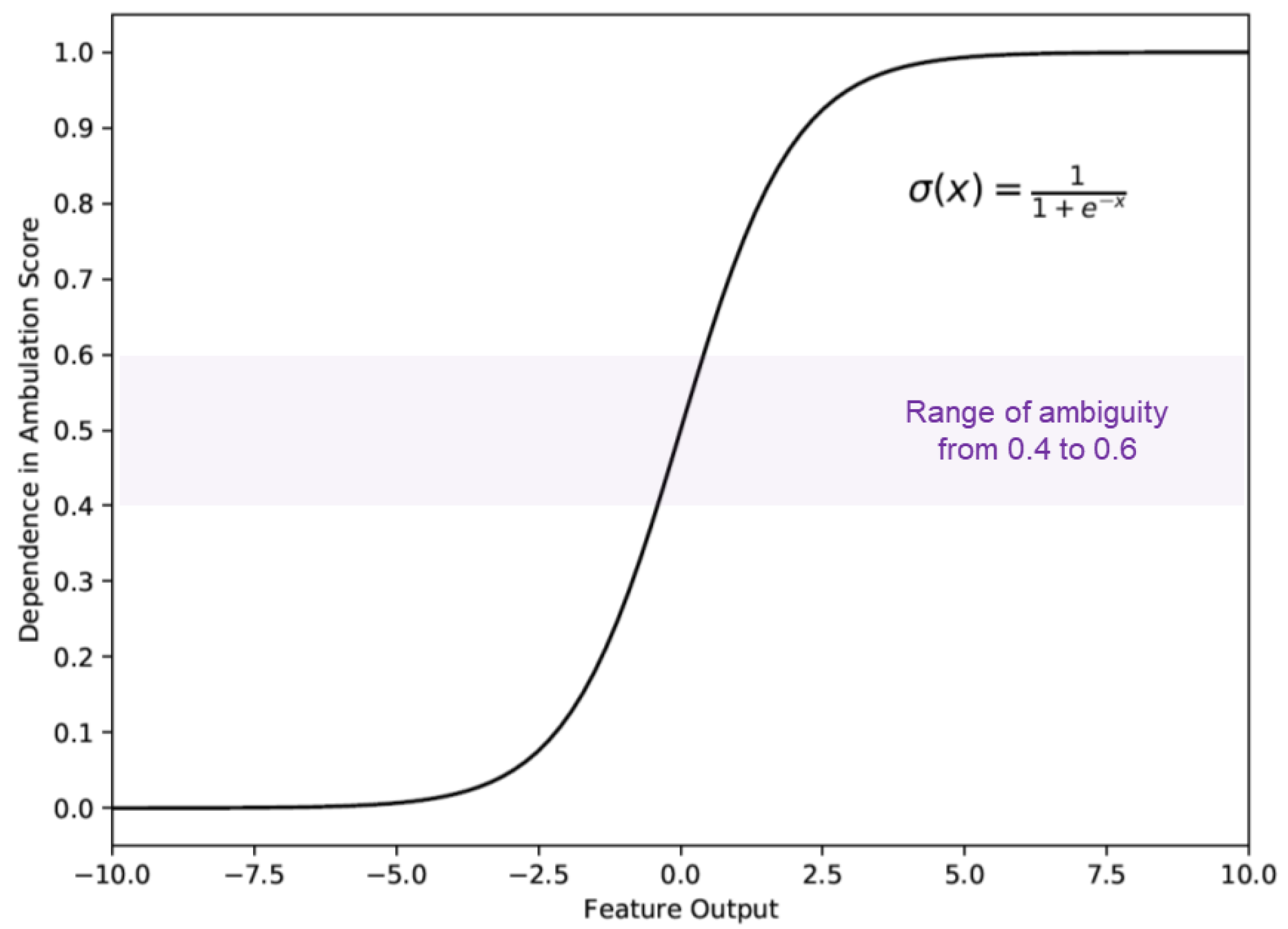
2.5. The 3D Convolutional Neural Network
2.6. Swing Time Asymmetry Measurement
2.7. Training and Testing
2.8. Evaluation Metrics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Ismail Ibrahim Ismail Alali, S.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 885. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Xu, T.; Clemson, L.; O'Loughlin, K.; Lannin, N.A.; Dean, C.; Koh, G. Risk factors for falls in community stroke survivors: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2018, 99, 563–573. e565. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Young, J. Incidence and consequences offalls due to stroke: A systematic inquiry. Bmj 1995, 311, 83–86. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.-G.; Shin, Y.-I.; Oh, G.-J.; Lee, Y.-S.; Joo, M.C.; Han, E.Y. Predictors of functional level and quality of life at 6 months after a first-ever stroke: The KOSCO study. J. Neurol. 2016, 263, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired: Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, M.; van de Water, A.T.; van de Port, I.G. Walking tests for stroke survivors: A systematic review of their measurement properties. Disabil. Rehabil. 2012, 34, 2207–2221. [Google Scholar] [CrossRef] [PubMed]
- Goh, H.-T.; Nadarajah, M.; Hamzah, N.B.; Varadan, P.; Tan, M.P. Falls and fear of falling after stroke: A case-control study. PMR 2016, 8, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar] [PubMed]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Louie, D.R.; Eng, J.J. Berg Balance Scale score at admission can predict walking suitable for community ambulation at discharge from inpatient stroke rehabilitation. J. Rehabil. Med. 2018, 50, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Popoola, O.P.; Wang, K. Video-based abnormal human behavior recognition—A review. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2012, 42, 865–878. [Google Scholar] [CrossRef]
- Ann, O.C.; Theng, L.B. Human activity recognition: A review. In Proceedings of the 2014 IEEE International Conference on Control System, Computing and Engineering (ICCSCE 2014), Penang, Malaysia, 28–30 November 2014; pp. 389–393. [Google Scholar]
- Arifoglu, D.; Bouchachia, A. Activity recognition and abnormal behaviour detection with recurrent neural networks. Procedia Comput. Sci. 2017, 110, 86–93. [Google Scholar] [CrossRef]
- Chintalapati, S.; Raghunadh, M. Automated attendance management system based on face recognition algorithms. In Proceedings of the 2013 IEEE International Conference on Computational Intelligence and Computing Research, Enathi, India, 26–28 December 2013; pp. 1–5. [Google Scholar]
- Sharma, R.P.; Verma, G.K. Human computer interaction using hand gesture. Procedia Comput. Sci. 2015, 54, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qiao, Y.; Tang, X. Action recognition with trajectory-pooled deep-convolutional descriptors. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 4305–4314. [Google Scholar]
- Pareek, P.; Thakkar, A. A survey on video-based human action recognition: Recent updates, datasets, challenges, and applications. Artif. Intell. Rev. 2021, 54, 2259–2322. [Google Scholar] [CrossRef]
- Thomas, G.; Gade, R.; Moeslund, T.B.; Carr, P.; Hilton, A. Computer vision for sports: Current applications and research topics. Comput. Vis. Image Underst. 2017, 159, 3–18. [Google Scholar] [CrossRef]
- Wu, D.; Sharma, N.; Blumenstein, M. Recent advances in video-based human action recognition using deep learning: A review. In Proceedings of the 2017 International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May 2017; pp. 2865–2872. [Google Scholar]
- Ji, S.; Xu, W.; Yang, M.; Yu, K. 3D convolutional neural networks for human action recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 35, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.; Bourdev, L.; Fergus, R.; Torresani, L.; Paluri, M. Learning spatiotemporal features with 3d convolutional networks. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015; pp. 4489–4497. [Google Scholar]
- Carreira, J.; Zisserman, A. Quo vadis, action recognition? a new model and the kinetics dataset. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 6299–6308. [Google Scholar]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.-E.; Sheikh, Y. OpenPose: Realtime multi-person 2D pose estimation using Part Affinity Fields. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 43, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Alharthi, A.S.; Yunas, S.U.; Ozanyan, K.B. Deep learning for monitoring of human gait: A review. IEEE Sens. J. 2019, 19, 9575–9591. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.-R.; Thuc, H.L.U.; Lee, Y.-J.; Hwang, J.-N.; Yoo, J.-H.; Choi, K.-H. A review on video-based human activity recognition. Computers 2013, 2, 88–131. [Google Scholar] [CrossRef]
- Bewley, A.; Ge, Z.; Ott, L.; Ramos, F.; Upcroft, B. Simple online and realtime tracking. In Proceedings of the 2016 IEEE International Conference on Image Processing (ICIP), Phoenix, AZ, USA, 25–28 September 2016; pp. 3464–3468. [Google Scholar]
- Kondratyuk, D.; Yuan, L.; Li, Y.; Zhang, L.; Tan, M.; Brown, M.; Gong, B. Movinets: Mobile video networks for efficient video recognition. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Virtual, 19–21 June 2021; pp. 16020–16030. [Google Scholar]
- Smith, L.N. Cyclical learning rates for training neural networks. In Proceedings of the 2017 IEEE Winter Conference on Applications of Computer Vision (WACV), Santa Rosa, CA, USA, 27–29 March 2017; pp. 464–472. [Google Scholar]
- Khera, P.; Kumar, N. Role of machine learning in gait analysis: A review. J. Med Eng. Technol. 2020, 44, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, L.; De Laet, T.; Di Lello, E.; Bruyninckx, H.; Molenaers, G.; Van Campenhout, A.; Aertbeliën, E.; Schwartz, M.; Wambacq, H.; De Cock, P. Probabilistic gait classification in children with cerebral palsy: A Bayesian approach. Res. Dev. Disabil. 2011, 32, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Kim, S.K.; Choi, S.B.; Kim, D.Y.; Kim, D.W. Interpretation of movement during stair ascent for predicting severity and prognosis of knee osteoarthritis in elderly women using support vector machine. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 192–196. [Google Scholar]
- Paulo, J.; Peixoto, P.; Amorim, P. Trajectory-based gait pattern shift detection for assistive robotics applications. Intell. Serv. Robot. 2019, 12, 255–264. [Google Scholar] [CrossRef]
- Cho, J.-s.; Cho, Y.-S.; Moon, S.-B.; Kim, M.-J.; Lee, H.D.; Lee, S.Y.; Ji, Y.-H.; Park, Y.-S.; Han, C.-S.; Jang, S.-H. Scoliosis screening through a machine learning based gait analysis test. Int. J. Precis. Eng. Manuf. 2018, 19, 1861–1872. [Google Scholar] [CrossRef]
- Guo, G.; Guffey, K.; Chen, W.; Pergami, P. Classification of normal and pathological gait in young children based on foot pressure data. Neuroinformatics 2017, 15, 13–24. [Google Scholar] [CrossRef]
- Kashi, S.; Polak, R.F.; Lerner, B.; Rokach, L.; Levy-Tzedek, S. A machine-learning model for automatic detection of movement compensations in stroke patients. IEEE Trans. Emerg. Top. Comput. 2020, 9, 1234–1247. [Google Scholar] [CrossRef]
- Ouahabi, A. A review of wavelet denoising in medical imaging. In Proceedings of the 2013 8th International Workshop on Systems, Signal Processing and Their Applications (WoSSPA), Algiers, Algeria, 12–15 May 2013; pp. 19–26. [Google Scholar]
- Ouahabi, A.; Taleb-Ahmed, A. Deep learning for real-time semantic segmentation: Application in ultrasound imaging. Pattern Recognit. Lett. 2021, 144, 27–34. [Google Scholar] [CrossRef]
- Arbaoui, A.; Ouahabi, A.; Jacques, S.; Hamiane, M. Concrete Cracks Detection and Monitoring Using Deep Learning-Based Multiresolution Analysis. Electronics 2021, 10, 1772. [Google Scholar] [CrossRef]


| Parameters | Patients (n = 206) |
|---|---|
| Age (mean ± SD, years) | 63.24 ± 14.36 |
| Sex (male: female, n) | 108:98 |
| Stroke (ischemic: hemorrhagic, n) | 113:93 |
| Involved stroke lesion | |
| Right: left:both hemisphere (n) | 82:105:19 |
| Supratentorial: infratentorial lesion (n) | 156:50 |
| Vascular territory in ischemic stroke (n = 113) (ACA:MCA:PCA:BA/SCA/PICA/AICA/VA, n) | 0:81:3:29 |
| Classification of hemorrhagic stroke (n = 93) (ICH:IVH:SAH:SDH, n) | 69:1:18:5 |
| Time from stroke onset to recorded video (mean ± SD, days) | 120.17 ± 281.52 |
| Level of dependence in ambulation when recording video | |
| FAC score (mean ± SD) | 1.73 ± 1.82 |
| FAC < 4 (dependent):FAC ≥ 4 (independent) (n, %) | 158 (76.7):48 (23.3) |
| BBS score (mean ± SD) | 23.75 ± 20.56 |
| BBS < 45 (dependent): BBS ≥ 45 (independent) (n, %) | 152 (73.7):54 (26.3) |
| 3D-CNN When Training Based on Assessment Scores | Accuracy | Precision | Recall | F-1 Score |
|---|---|---|---|---|
| FAC | 0.845 ± 0.065 | 0.853 ± 0.057 | 0.928 ± 0.055 | 0.888 ± 0.050 |
| BBS | 0.851 ± 0.037 | 0.863 ± 0.065 | 0.916 ± 0.046 | 0.886 ± 0.032 |
| FAC and BBS | 0.863 ± 0.032 | 0.874 ± 0.024 | 0.940 ± 0.035 | 0.905 ± 0.022 |
| 3D-CNN with Swing Time Asymmetry | Accuracy | Precision | Recall | F-1 Score |
| 0.887 ± 0.044 | 0.891 ± 0.041 | 0.957 ± 0.028 | 0.922 ± 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.T.; Park, E.; Jung, T.-D. Machine Learning-Based Classification of Dependence in Ambulation in Stroke Patients Using Smartphone Video Data. J. Pers. Med. 2021, 11, 1080. https://doi.org/10.3390/jpm11111080
Lee JT, Park E, Jung T-D. Machine Learning-Based Classification of Dependence in Ambulation in Stroke Patients Using Smartphone Video Data. Journal of Personalized Medicine. 2021; 11(11):1080. https://doi.org/10.3390/jpm11111080
Chicago/Turabian StyleLee, Jong Taek, Eunhee Park, and Tae-Du Jung. 2021. "Machine Learning-Based Classification of Dependence in Ambulation in Stroke Patients Using Smartphone Video Data" Journal of Personalized Medicine 11, no. 11: 1080. https://doi.org/10.3390/jpm11111080
APA StyleLee, J. T., Park, E., & Jung, T.-D. (2021). Machine Learning-Based Classification of Dependence in Ambulation in Stroke Patients Using Smartphone Video Data. Journal of Personalized Medicine, 11(11), 1080. https://doi.org/10.3390/jpm11111080






