Patients’ Characterization of Medication, Emotions, and Incongruent Perceptions around Adherence
Abstract
:1. Introduction
2. Methods
2.1. Recruitment
2.2. Measure
2.3. Procedure
2.4. Data Analysis
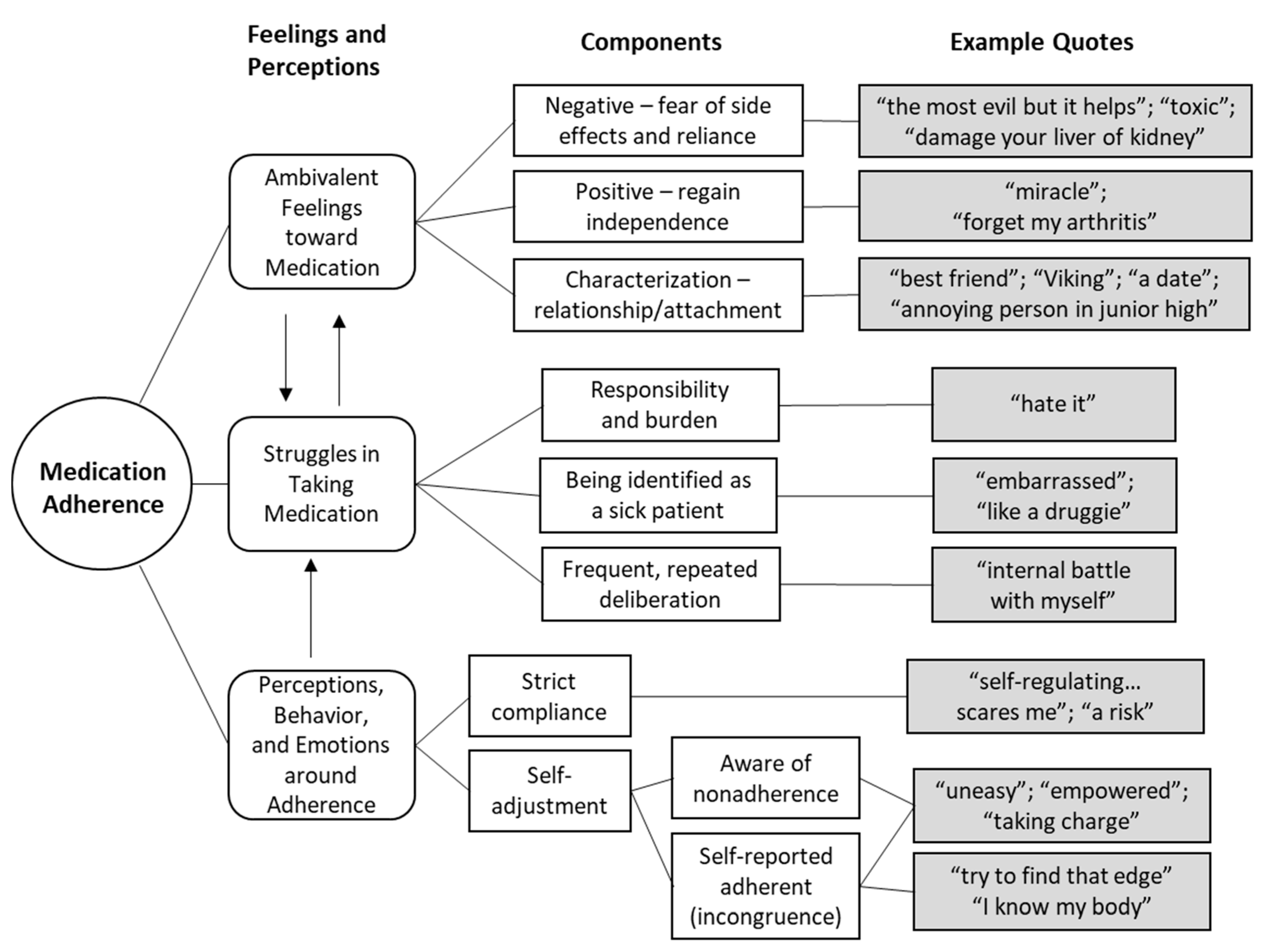
3. Results
3.1. Ambivalent Feelings toward Medication
“I drink gallons of water a day because I’ve got to flush that out of my system, that stuff is toxic. … my organs are probably just screaming on the inside.”(FG 1, SRA 5)
“I’m a little concerned because the medication which seems to be working well, you know, can kind of damage your liver or kidneys.”(FG 1, SRA 5)
“I hate prednisone. It’s like the most evil but it helps.”(FG 4, SRA 4)
“[My medication] feels like the annoying person in junior high that’s always there … [I’m] like ‘can you leave me alone?’ … I know my medication is helping me; it’s good to have that friend.”(FG 4, SRA 5)
“I guess you’d say [the medicine is my] best friend … I can’t say it makes you feel perfect, but I think without it I would feel much worse.”(FG 1, SRA 5)
“It’s a Viking. So, it’s strong and fights for me and defends me. But if I’m on the bad side of my Viking, it might hurt me.”(FG 2, SRA 5)
“I have a ‘date’ with (drug name) once a week. Nobody likes needles but I do love the (drug name) needle because … I promise you by, like the second day, you can feel it; I can breathe and move. It’s $3500 [for a three-month supply]—it’s like the most expensive thing in my home. But I will sell things so I can get it … [However], every time I take this needle out I’m like, oh my God, don’t let five years from now I grow an extra ear or anything.”(FG 1, SRA 5)
3.2. Struggles in Taking Medication
“I really hated it because it meant that I was sick, and I was the only one that had to take medicine in my family. Even my grandmother is healthier than me.”(IDI 2, SRA 4)
“I’m embarrassed when I go places, or even when our kids would have friends over … I feel like a druggie taking all this medicine when I’m 40 years old.”(FG 4, SRA 5)
“I was able to write and function and forget about my arthritis.”(IDI 7, SRA 5)
“It’s like an internal battle with myself, and I go back and forth for a couple of days before I just break down and say it’s easier just to take it.”(IDI 3, SRA 5)
“I know it’s like that evil thing … You know you would be better if you could get off of it, but I cannot get off of it.”(FG 4, SRA 5)
3.3. Actions and Attitudes around Non/Adherence
3.3.1. In/Congruence between Perceptions and Behavior
“I take them exactly as prescribed. It’s strong medication, and if you start self-regulating, I just can’t imagine the side effects … that really scares me.”(FG 2, SRA 4)
“It upset my stomach, so I started taking less and less, and trying to find that edge where it was healthy, but I wasn’t getting sick.”(FG 3, SRA 5)
“Sometimes on a bad day, I will get frustrated because I feel like my medication is not working anyways, so then I don’t want the side effects as well if I take it. So, perhaps on bad days, I’m less compliant.”(IDI 6, SRA 3)
“[My doctors] would prescribe that, but I would take half of that when I feel like I need it or more if I felt like I needed it.”(FG 1, SRA 5)
“I just took one extra one, and it worked, so I guess I need to talk to the doctor about me having to take a little bit more than just that one pill when I am having an episode.”(FG 2, SRA 4)
3.3.2. Emotions and Empowerment from Self-Adjusting Medication
“I would say uneasy … because if I skip a dosage, or if I’m kind of playing around with it, I’m not sure what the outcome is going to be. So, it’s like a risk I’m taking.”(FG 3, SRA 4)
“[I feel] like I’m a disappointment sometimes to [my care team].”(FG 4, SRA 4)
“I’ll get paranoid that my joints are secretly, you know quietly getting damaged and that I’m not helping them.”(FG 4, SRA 5)
“I would say [I feel] empowered to be able to decide if I was able to take it or not whereas before I felt reliant.”(IDI 2, SRA 4)
“Empowered. I mean, it’s in my hands … I feel like I’m taking charge of my own health.”(FG 1, SRA 5)
“In control. It makes you feel like you’re doing a little bit of something on your own and not being told exactly, ‘You got to do this’.”(FG 4, SRA 5)
“I make decisions based on my side-effects. If they are intolerable, I figure [my doctor] is not in my body, he doesn’t know.”(FG 4, SRA 4)
“The doctor put us on a dosage, but because we know our own bodies, we know that we may feel better. So maybe we don’t need as much medication as they actually prescribed.”(FG 1, SRA 5)
“I know my body, and I know by experience … At the beginning I wouldn’t do it [self-adjust my medication) and I followed the instructions of the doctor and [it] got me really in a bad spot … But then [I felt] in control because now I learned how to manage my own body, right.”(FG 2, SRA 5)
“I think empowered and in control definitely applied later when [my doctor] started communicating with me and asking me what I wanted to do.”(IDI 1, SRA 4)
“I feel in control because if [there is] something that’s not right [when I take my medication differently] I know I can always pick up the phone and call the nurse and they’ll send a message to my PA nurse, and they’ll call me back. So, I can kind of govern my own self.”(FG 2, SRA 5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid Arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Birch, J.T.; Bhattacharya, S. Emerging Trends in Diagnosis and Treatment of Rheumatoid Arthritis. Prim. Care Clin. Off. Pract. 2010, 37, 779–792. [Google Scholar] [CrossRef]
- Ahlstrand, I.; Björk, M.; Thyberg, I.; Börsbo, B.; Falkmer, T. Pain and Daily Activities in Rheumatoid Arthritis. Disabil. Rehabil. 2012, 34, 1245–1253. [Google Scholar] [CrossRef]
- Miagoux, Q.; Singh, V.; de Mézquita, D.; Chaudru, V.; Elati, M.; Petit-Teixeira, E.; Niarakis, A. Inference of an Integrative, Executable Network for Rheumatoid Arthritis Combining Data-Driven Machine Learning Approaches and a State-of-the-Art Mechanistic Disease Map. J. Pers. Med. 2021, 11, 785. [Google Scholar] [CrossRef]
- Donahue, K.E.; Gartlehner, G.; Jonas, D.E.; Lux, L.J.; Thieda, P.; Jonas, B.L.; Hansen, R.A.; Morgan, L.C.; Lohr, K.N. Systematic Review: Comparative Effectiveness and Harms of Disease-Modifying Medications for Rheumatoid Arthritis. Ann. Intern. Med. 2008, 148, 124. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Smith, C.; Doré, C.J.; Scott, D.L. A Meta-Analysis of the Efficacy and Toxicity of Combining Disease-Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis Based on Patient Withdrawal. Rheumatology 2005, 44, 1414–1421. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Van den Bemt, B.J.; van Lankveld, W.G. How Can We Improve Adherence to Therapy by Patients with Rheumatoid Arthritis? Nat. Clin. Pract. Rheumatol. 2007, 3, 681. [Google Scholar] [CrossRef] [PubMed]
- Van den Bemt, B.J.F.; van den Hoogen, F.H.J.; Benraad, B.; Hekster, Y.A.; van Riel, P.L.C.M.; van Lankveld, W. Adherence Rates and Associations with Nonadherence in Patients with Rheumatoid Arthritis Using Disease Modifying Antirheumatic Drugs. J. Rheumatol. 2009, 36, 2164–2170. [Google Scholar] [CrossRef]
- Scheiman-Elazary, A.; Duan, L.; Shourt, C.; Agrawal, H.; Ellashof, D.; Cameron-Hay, M.; Furst, D.E. The Rate of Adherence to Antiarthritis Medications and Associated Factors among Patients with Rheumatoid Arthritis: A Systematic Literature Review and Metaanalysis. J. Rheumatol. 2016, 43, 512–523. [Google Scholar] [CrossRef]
- Hovstadius, B.; Petersson, G. Non-Adherence to Drug Therapy and Drug Acquisition Costs in a National Population—A Patient-Based Register Study. BMC Health Serv. Res. 2011, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Sabaté, E.; World Health Organization (Eds.) Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-154599-0. [Google Scholar]
- Costa, E.; Giardini, A.; Savin, M.; Menditto, E.; Lehane, E.; Laosa, O.; Pecorelli, S.; Monaco, A.; Marengoni, A. Interventional Tools to Improve Medication Adherence: Review of Literature. Patient Prefer. Adherence 2015, 9, 1303–1314. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.J.; Nuckols, T.K.; Coles, C.; Le, M.M.; Schnipper, J.L.; Shane, R.; Jackevicius, C.; Lee, J.; Pevnick, J.M.; Members of the PHARM-DC Group. A Systematic Overview of Systematic Reviews Evaluating Medication Adherence Interventions. Am. J. Health Syst. Pharm. 2020, 77, 138–147. [Google Scholar] [CrossRef]
- Pinho, S.; Cruz, M.; Ferreira, F.; Ramalho, A.; Sampaio, R. Improving Medication Adherence in Hypertensive Patients: A Scoping Review. Prev. Med. 2021, 146, 106467. [Google Scholar] [CrossRef]
- Kvarnström, K.; Westerholm, A.; Airaksinen, M.; Liira, H. Factors Contributing to Medication Adherence in Patients with a Chronic Condition: A Scoping Review of Qualitative Research. Pharmaceutics 2021, 13, 1100. [Google Scholar] [CrossRef]
- Kardas, P.; Lewek, P.; Matyjaszczyk, M. Determinants of Patient Adherence: A Review of Systematic Reviews. Front. Pharmacol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugtenburg, J.G.; Timmers, L.; Elders, P.J.; Vervloet, M.; van Dijk, L. Definitions, Variants, and Causes of Nonadherence with Medication: A Challenge for Tailored Interventions. Patient Prefer. Adherence 2013, 7, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A New Taxonomy for Describing and Defining Adherence to Medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Salt, E.; Frazier, S.K. Adherence to Disease-Modifying Antirheumatic Drugs in Patients with Rheumatoid Arthritis: A Narrative Review of the Literature. Orthop. Nurs. 2010, 29, 260–275. [Google Scholar] [CrossRef] [Green Version]
- Burmester, G.R.; Pope, J.E. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Villa-Hermosilla, M.-C.; Fernández-Carballido, A.; Hurtado, C.; Barcia, E.; Montejo, C.; Alonso, M.; Negro, S. Sulfasalazine Microparticles Targeting Macrophages for the Treatment of Inflammatory Diseases Affecting the Synovial Cavity. Pharmaceutics 2021, 13, 951. [Google Scholar] [CrossRef]
- Pasma, A.; van ’t Spijker, A.; Luime, J.J.; Walter, M.J.M.; Busschbach, J.J.V.; Hazes, J.M.W. Facilitators and Barriers to Adherence in the Initiation Phase of Disease-Modifying Antirheumatic Drug (DMARD) Use in Patients with Arthritis Who Recently Started Their First DMARD Treatment. J. Rheumatol. 2015, 42, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Harrold, L.R.; Briesacher, B.A.; Peterson, D.; Beard, A.; Madden, J.; Zhang, F.; Gurwitz, J.H.; Soumerai, S.B. Cost-Related Medication Nonadherence in Older Rheumatoid Arthritis Patients. J. Rheumatol. 2013, 40, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Clifford, S.; Barber, N.; Horne, R. Understanding Different Beliefs Held by Adherers, Unintentional Nonadherers, and Intentional Nonadherers: Application of the Necessity–Concerns Framework. J. Psychosom. Res. 2008, 64, 41–46. [Google Scholar] [CrossRef]
- Zwikker, H.E.; van Dulmen, S.; den Broeder, A.A.; van den Bemt, B.J.; van den Ende, C.H. Perceived Need to Take Medication Is Associated with Medication Non-Adherence in Patients with Rheumatoid Arthritis. Patient Prefer. Adherence 2014, 8, 1635–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Raza, K.; Nightingale, P.; Horne, R.; Chapman, S.; Greenfield, S.; Gill, P. Determinants of Adherence to Disease Modifying Anti-Rheumatic Drugs in White British and South Asian Patients with Rheumatoid Arthritis: A Cross Sectional Study. BMC Musculoskelet. Disord. 2015, 16, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quandt, S.A.; Arcury, T.A. Qualitative Methods in Arthritis Research: Overview and Data Collection. Arthritis Rheum. 1997, 10, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.; Tymms, K.; Fallon, K.; Sumpton, D.; Tugwell, P.; Tunnicliffe, D.; Tong, A. Qualitative Research in Rheumatology: An Overview of Methods and Contributions to Practice and Policy. J. Rheumatol. 2021, 48, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.-V.; Samuelson, K.; Hagell, P.; Fridlund, B.; Forslind, K.; Svensson, B.; Thomé, B. Living with Persistent Rheumatoid Arthritis: A BARFOT Study. J. Clin. Nurs. 2017, 26, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Shaw, Y.; Metes, I.D.; Michaud, K.; Donohue, J.M.; Roberts, M.S.; Levesque, M.C.; Chang, J.C. Rheumatoid Arthritis Patients’ Motivations for Accepting or Resisting Disease-Modifying Antirheumatic Drug Treatment Regimens. Arthritis Care Res. 2018, 70, 533–541. [Google Scholar] [CrossRef]
- Shariff, Z.; Kirby, D.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I. Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms That Improve Adherence and Acceptance in Older People. Pharmaceutics 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Drame, K.; Geutjens, S.; Airaksinen, M. Does the Polypill Improve Patient Adherence Compared to Its Individual Formulations? A Systematic Review. Pharmaceutics 2020, 12, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Pensé-Lhéritier, A.; Aoussat, A. Standardized Method to Assess Medicines’ Acceptability: Focus on Paediatric Population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive Validity of A Medication Adherence Measure in an Outpatient Setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Carl, T. The Hill-Bone Scales. Available online: https://nursing.jhu.edu/faculty_research/research/projects/hill-bone/hill-bone-scales.html (accessed on 25 September 2021).
- Hughes, L.D.; Done, J.; Young, A. A 5 Item Version of the Compliance Questionnaire for Rheumatology (CQR5) Successfully Identifies Low Adherence to DMARDs. BMC Musculoskelet. Disord. 2013, 14, 286. [Google Scholar] [CrossRef] [Green Version]
- Lam, W.Y.; Fresco, P. Medication Adherence Measures: An Overview. BioMed Res. Int. 2015, 2015, e217047. [Google Scholar] [CrossRef] [Green Version]
- Blatt, S.J. The Validity of Projective Techniques and Their Research and Clinical Contribution. J. Pers. Assess. 1975, 39, 327–343. [Google Scholar] [CrossRef]
- Bell, J.E. Projective Techniques: A Dynamic Approach to the Study of the Personality; Longmans, Green & Co.: Oxford, UK, 1948. [Google Scholar]
- Haire, M. Projective Techniques in Marketing Research. J. Mark. 1950, 14, 649–656. [Google Scholar] [CrossRef]
- Mesías, F.J.; Escribano, M. Projective Techniques–Chapter 4. In Methods in Consumer Research; Ares, G., Varela, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 79–102. ISBN 978-0-08-102089-0. [Google Scholar]
- Fereday, J.; Muir-Cochrane, E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. Int. J. Qual. Methods 2006, 5, 80–92. [Google Scholar] [CrossRef]
- Arcury, T.A.; Quandt, S.A. Qualitative Methods in Arthritis Research: Sampling and Data Analysis. Arthritis Rheum. 1998, 11, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.; Kallikorm, R.; Põlluste, K.; Lember, M. Compliance with Treatment of Rheumatoid Arthritis. Rheumatol. Int. 2012, 32, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Carder, P.C.; Vuckovic, N.; Green, C.A. Negotiating Medications: Patient Perceptions of Long-Term Medication Use. J. Clin. Pharm. Ther. 2003, 28, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Popa-Lisseanu, M.G.G.; Greisinger, A.; Richardson, M.; O’Malley, K.J.; Janssen, N.M.; Marcus, D.M.; Tagore, J.; Suarez-Almazor, M.E. Determinants of Treatment Adherence in Ethnically Diverse, Economically Disadvantaged Patients with Rheumatic Disease. J. Rheumatol. 2005, 32, 913–919. [Google Scholar]
- Jones, B.; Hunt, A.; Hewlett, S.; Harcourt, D.; Dures, E. Rheumatology Patients’ Perceptions of Patient Activation and the Patient Activation Measure: A Qualitative Interview Study. Musculoskeletal Care 2021. [Google Scholar] [CrossRef]
- National Collaborating Centre for Primary Care. Patients’ experience of medicine-taking. In Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence; Royal College of General Practitioners: London, UK, 2009. [Google Scholar]
- Li, L.; Cui, Y.; Yin, R.; Chen, S.; Zhao, Q.; Chen, H.; Shen, B. Medication Adherence Has an Impact on Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Patient Prefer. Adherence 2017, 11, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Salt, E.; Frazier, S.K. Predictors of Medication Adherence in Patients with Rheumatoid Arthritis. Drug Dev. Res. 2011, 72, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liu, J.; Koleva, Y.; Fonseca, V.; Kalsekar, A.; Pawaskar, M. Concordance of Adherence Measurement Using Self-Reported Adherence Questionnaires and Medication Monitoring Devices. Pharmacoeconomics 2010, 28, 1097–1107. [Google Scholar] [CrossRef]
- Foley, L.; Larkin, J.; Lombard-Vance, R.; Murphy, A.W.; Hynes, L.; Galvin, E.; Molloy, G.J. Prevalence and Predictors of Medication Non-Adherence among People Living with Multimorbidity: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e044987. [Google Scholar] [CrossRef]
- Varallo, G.; Scarpina, F.; Giusti, E.M.; Suso-Ribera, C.; Cattivelli, R.; Guerrini Usubini, A.; Capodaglio, P.; Castelnuovo, G. The Role of Pain Catastrophizing and Pain Acceptance in Performance-Based and Self-Reported Physical Functioning in Individuals with Fibromyalgia and Obesity. J. Pers. Med. 2021, 11, 810. [Google Scholar] [CrossRef]
- Van Munster, M.; Stümpel, J.; Thieken, F.; Pedrosa, D.J.; Antonini, A.; Côté, D.; Fabbri, M.; Ferreira, J.J.; Růžička, E.; Grimes, D.; et al. Moving towards Integrated and Personalized Care in Parkinson’s Disease: A Framework Proposal for Training Parkinson Nurses. J. Pers. Med. 2021, 11, 623. [Google Scholar] [CrossRef]
- Metta, V.; Batzu, L.; Leta, V.; Trivedi, D.; Powdleska, A.; Mridula, K.R.; Kukle, P.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; et al. Parkinson’s Disease: Personalized Pathway of Care for Device-Aided Therapies (DAT) and the Role of Continuous Objective Monitoring (COM) Using Wearable Sensors. J. Pers. Med. 2021, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Betteridge, N.; Brown, T.M.; Woolcott, J.; Kivitz, A.J.; Zerbini, C.; Whalley, D.; Olayinka-Amao, O.; Chen, C.; Dahl, P.; et al. Treatment Mode Preferences in Rheumatoid Arthritis: Moving Toward Shared Decision-Making. Patient Prefer. Adherence 2020, 14, 119–131. [Google Scholar] [CrossRef] [Green Version]
- De Belvis, A.G.; Pellegrino, R.; Castagna, C.; Morsella, A.; Pastorino, R.; Boccia, S. Success Factors and Barriers in Combining Personalized Medicine and Patient Centered Care in Breast Cancer. Results from a Systematic Review and Proposal of Conceptual Framework. J. Pers. Med. 2021, 11, 654. [Google Scholar] [CrossRef]
- Lin, C.; Tu, R.; Bier, B.; Tu, P. Uncovering the Imprints of Chronic Disease on Patients’ Lives and Self-Perceptions. J. Pers. Med. 2021, 11, 807. [Google Scholar] [CrossRef]
- Castro, E.M.; Van Regenmortel, T.; Vanhaecht, K.; Sermeus, W.; Van Hecke, A. Patient Empowerment, Patient Participation and Patient-Centeredness in Hospital Care: A Concept Analysis Based on a Literature Review. Patient Educ. Couns. 2016, 99, 1923–1939. [Google Scholar] [CrossRef]
- Bartkeviciute, B.; Lesauskaite, V.; Riklikiene, O. Individualized Health Care for Older Diabetes Patients from the Perspective of Health Professionals and Service Consumers. J. Pers. Med. 2021, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Sousa Lobo, J.M.; et al. Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.; Manger, B.; Alvaro-Gracia, J.; Johnstone, R.; Gomez-Reino, J.; Eberhardt, E.; Wolfe, F.; Schwartzman, S.; Furfaro, N.; Kavanaugh, A. Patient Perceptions Concerning Pain Management in the Treatment of Rheumatoid Arthritis. J. Int. Med. Res. 2010, 38, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared Decision Making: A Model for Clinical Practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, A.L.; Coleska, A.; Burns, P.B.; Chung, K.C. The Evolution of Patient Decision-Making Regarding Medical Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| n = 27 | n (%) | Mean (SD) |
|---|---|---|
| Gender | ||
| Woman | 21 (77.8%) | |
| Man | 6 (22.2%) | |
| Age (years) | 46.4 (14.56) | |
| 18–29 | 4 (14.8%) | |
| 30–49 | 13 (48.1%) | |
| 50–64 | 7 (25.9%) | |
| 65+ | 3 (11.1%) | |
| Ethnicity | ||
| Caucasian | 20 (74.0%) | |
| African American | 3 (11.1%) | |
| Hispanic | 4 (14.8%) | |
| First diagnosed (years ago) | 11.0 (8.1) | |
| 1–4 | 4 (14.8%) | |
| 5–9 | 8 (29.6%) | |
| 10–14 | 8 (29.6%) | |
| 15–19 | 5 (18.5%) | |
| 20–24 | 1 (3.7%) | |
| 25+ | 1 (3.7%) | |
| Self-reported adherence | 4.5 (0.7) | |
| 1 = never | 0 | |
| 2 = not often | 0 | |
| 3 = sometimes | 3 (11.1%) | |
| 4 = most of the time | 8 (29.6%) | |
| 5 = always | 16 (59.3%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, P.; Smith, D.; Clark, R.; Bayzle, L.; Tu, R.; Lin, C. Patients’ Characterization of Medication, Emotions, and Incongruent Perceptions around Adherence. J. Pers. Med. 2021, 11, 975. https://doi.org/10.3390/jpm11100975
Tu P, Smith D, Clark R, Bayzle L, Tu R, Lin C. Patients’ Characterization of Medication, Emotions, and Incongruent Perceptions around Adherence. Journal of Personalized Medicine. 2021; 11(10):975. https://doi.org/10.3390/jpm11100975
Chicago/Turabian StyleTu, Pikuei, Danielle Smith, Rachel Clark, Laura Bayzle, Rungting Tu, and Cheryl Lin. 2021. "Patients’ Characterization of Medication, Emotions, and Incongruent Perceptions around Adherence" Journal of Personalized Medicine 11, no. 10: 975. https://doi.org/10.3390/jpm11100975
APA StyleTu, P., Smith, D., Clark, R., Bayzle, L., Tu, R., & Lin, C. (2021). Patients’ Characterization of Medication, Emotions, and Incongruent Perceptions around Adherence. Journal of Personalized Medicine, 11(10), 975. https://doi.org/10.3390/jpm11100975






