A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial
Abstract
1. Introduction
2. Methods
3. Ethics Statement
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IE | inflammatory endotype |
| AE | adaptive endotype |
| CE | coagulopathic endotype |
| CAD | coronary artery disease |
| HAT | hydrocortisone ascorbic acid thiamine |
| SOFA | Sepsis-Related Organ Failure Assessment |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| APACHE IV | Acute Physiology and Chronic Health Evaluation IV |
| INR | international normalized ratio |
References
- Singer, M. Biomarkers in sepsis. Curr. Opin. Pulm. Med. 2013, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Wong, H.R. Risk Stratification and Prognosis in Sepsis: What Have We Learned from Microarrays? Clin. Chest Med. 2016, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R. Sepsis genomics and precision medicine. In Genomic and Precision Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 83–93. [Google Scholar]
- Opal, S.M.; Dellinger, R.P.; Vincent, J.-L.; Masur, H.; Angus, D.C. The next generation of sepsis clinical trial designs: What is next after the demise of recombinant human activated protein C?*. Crit. Care Med. 2014, 42, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol. Ther. 2018, 189, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care Lond. Engl. 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-B.; Wang, Z.-H.; Liao, X.-L.; Guo, W.-X.; Wen, J.-Y.; Qin, T.-H.; Wang, S.H. Efficacy of vitamin C in patients with sepsis: An updated meta-analysis. Eur. J. Pharmacol. 2020, 868, 172889. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; Sculthorpe, R.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients with Septic Shock: The Vitamins Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Vassallo, A.V.; Patel, V.V.; Sullivan, J.B.; Cavanaugh, J.; Elbaga, Y. Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis: The Oranges Trial. Chest 2020, 158, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Calfee, C.S.; Thompson, B.T.; Angus, D.C.; Liu, V.X. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am. J. Respir. Crit. Care Med. 2016, 194, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Perumal, T.M.; Henao, R.; Nichols, M.; Howrylak, J.A.; Choi, A.M.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Davenport, E.E.; et al. A community approach to mortality prediction in sepsis via gene expression analysis. Nat. Commun. 2018, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, M.B.; Buturovic, L.; Luethy, R.; Midic, U.; Moore, A.R.; Roque, J.A.; Shaller, B.D.; Asuni, T.; Rawling, D.; Remmel, M.; et al. A generalizable 29-mRNA neural-network classifier for acute bacterial and viral infections. Nat. Commun. 2020, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Gunsolus, I.L.; Sweeney, T.E.; Liesenfeld, O.; Ledeboer, N.A. Diagnosing and Managing Sepsis by Probing the Host Response to Infection: Advances, Opportunities, and Challenges. J. Clin. Microbiol. 2019, 57, e00425-19. [Google Scholar] [CrossRef] [PubMed]
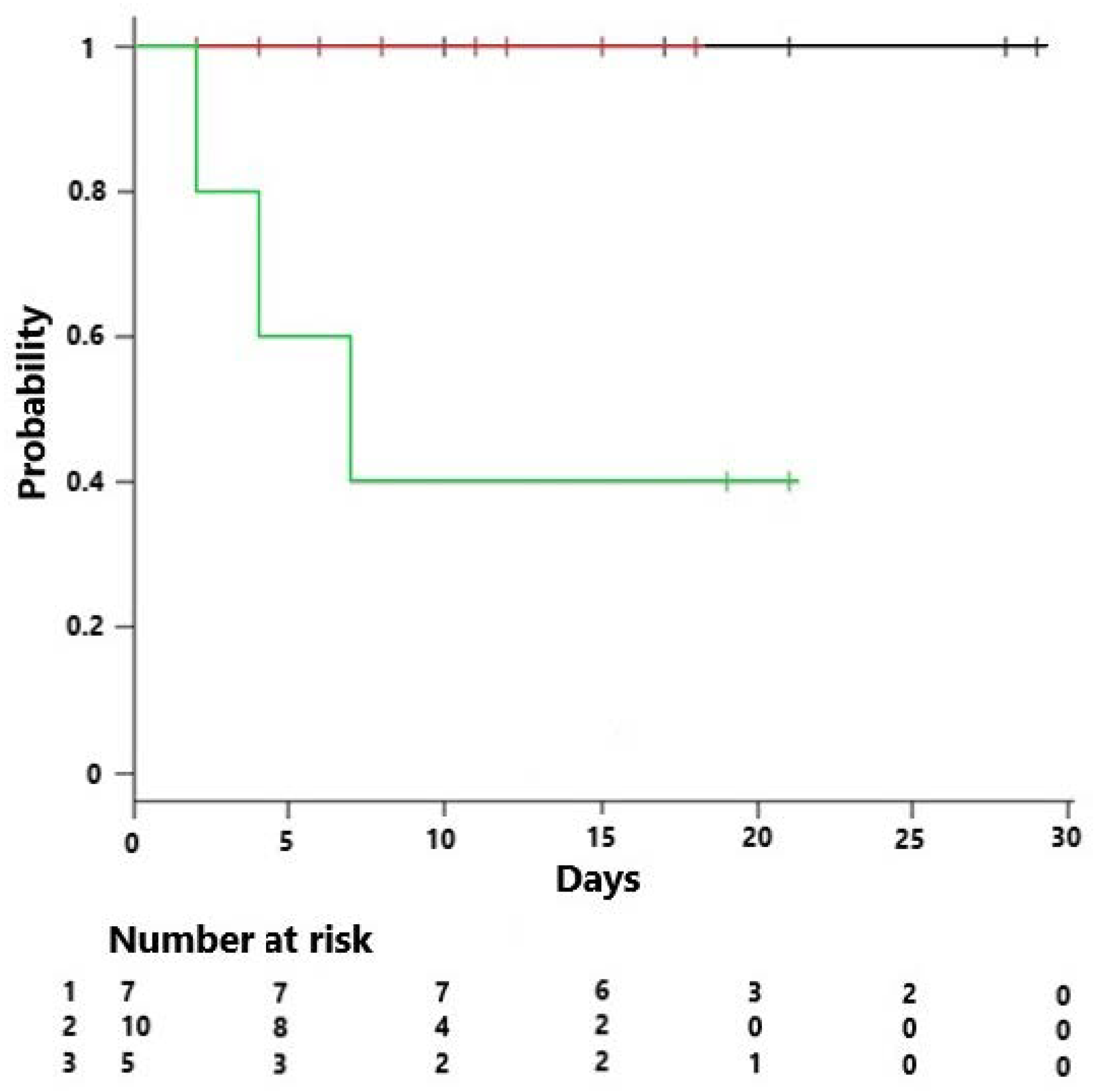
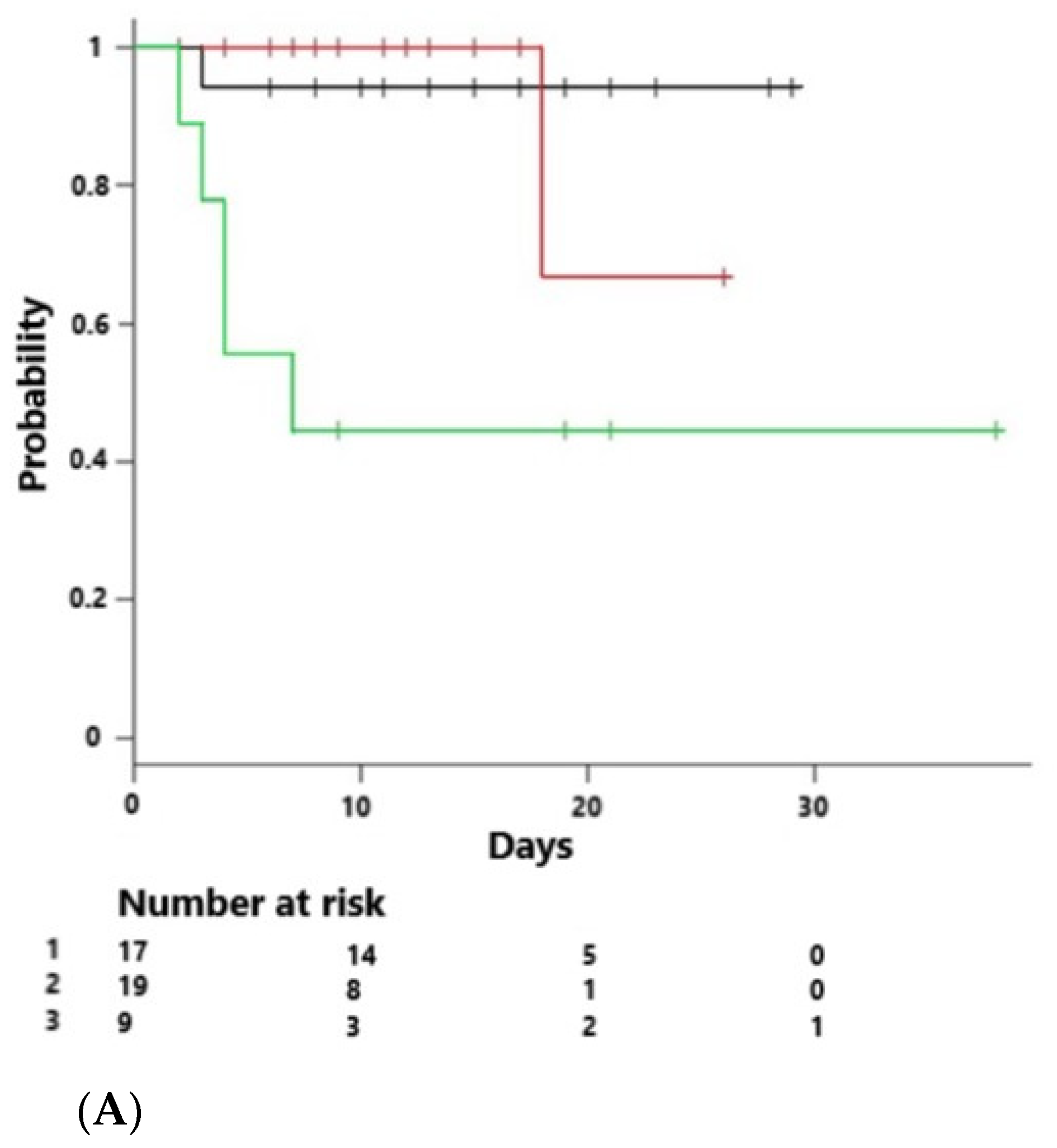

|
Survivors (n = 43) |
Non-Survivors (n = 8) | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Age | 65 (59, 70) | 79 (71, 86) | 0.011 | ||
| Race (Caucasian) | 41 (95%) | 7 (88%) | 0.41 | 0.34 | 0.027–4.29 |
| Wt. (kg) | 75 (66, 91) | 76 (71, 96) | 0.24 | ||
| Sex (male) | 20 (47%) | 3 (38%) | 0.71 | 0.69 | 0.14–3.25 |
| Diabetes | 17 (40%) | 4 (50%) | 0.7 | 1.52 | 0.33–6.9 |
| CHF | 9 (21%) | 1 (13%) | 1 | 0.54 | 0.059–5.0 |
| CAD | 8(19%) | 7(87%) | <0.001 | 30 | 3.2–265 |
| COPD | 14 (33%) | 4 (50%) | 0.43 | 2.07 | 0.45–9.52 |
| CKD | 7 (16.3%) | 2 (25%) | 0.62 | 1.71 | 0.28–10.3 |
| Pneumonia | 17 (40%) | 3 (38%) | 1 | 0.91 | 0.15–4.35 |
| Primary bacteremia | 7 (16%) | 1 (13%) | 1 | 0.73 | 0.073–6.94 |
| Mechanical ventilation | 16 (37%) | 7 (88%) | 0.016 | 11.2 | 1.33–105 |
| Vasopressors | 32 (74.4%) | 5 (62%) | 0.66 | 0.57 | 0.51–2.8 |
| AKI | 35 (81%) | 7 (88%) | 1 | 1.6 | 0.17–14.9 |
| HD | 6 (14%) | 1 (13%) | 1 | 0.88 | 0.091–8.4 |
| Inflammatory | 17 (39.5%) | 2 (25%) | 0.42 | 0.51 | 0.092–2.82 |
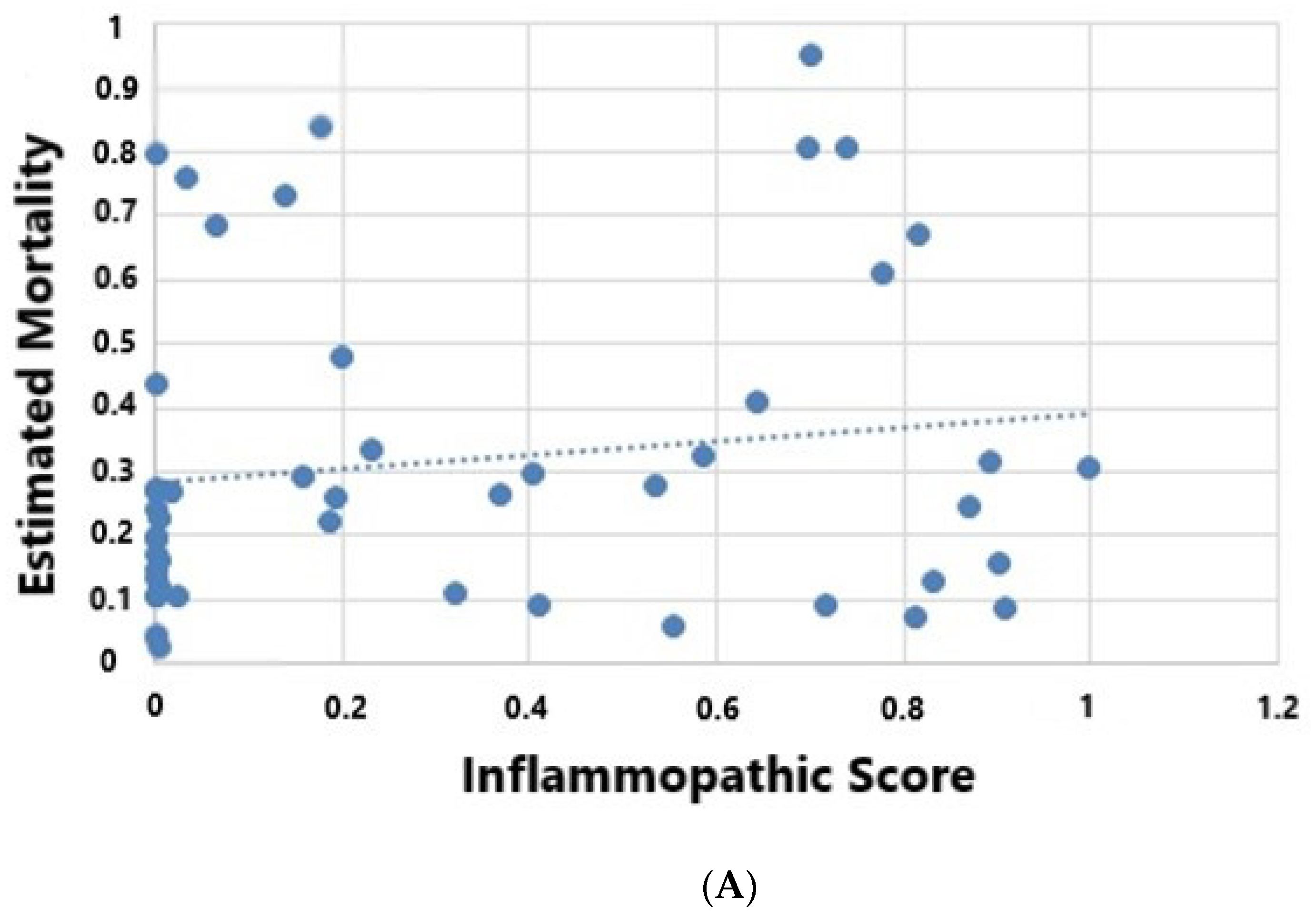
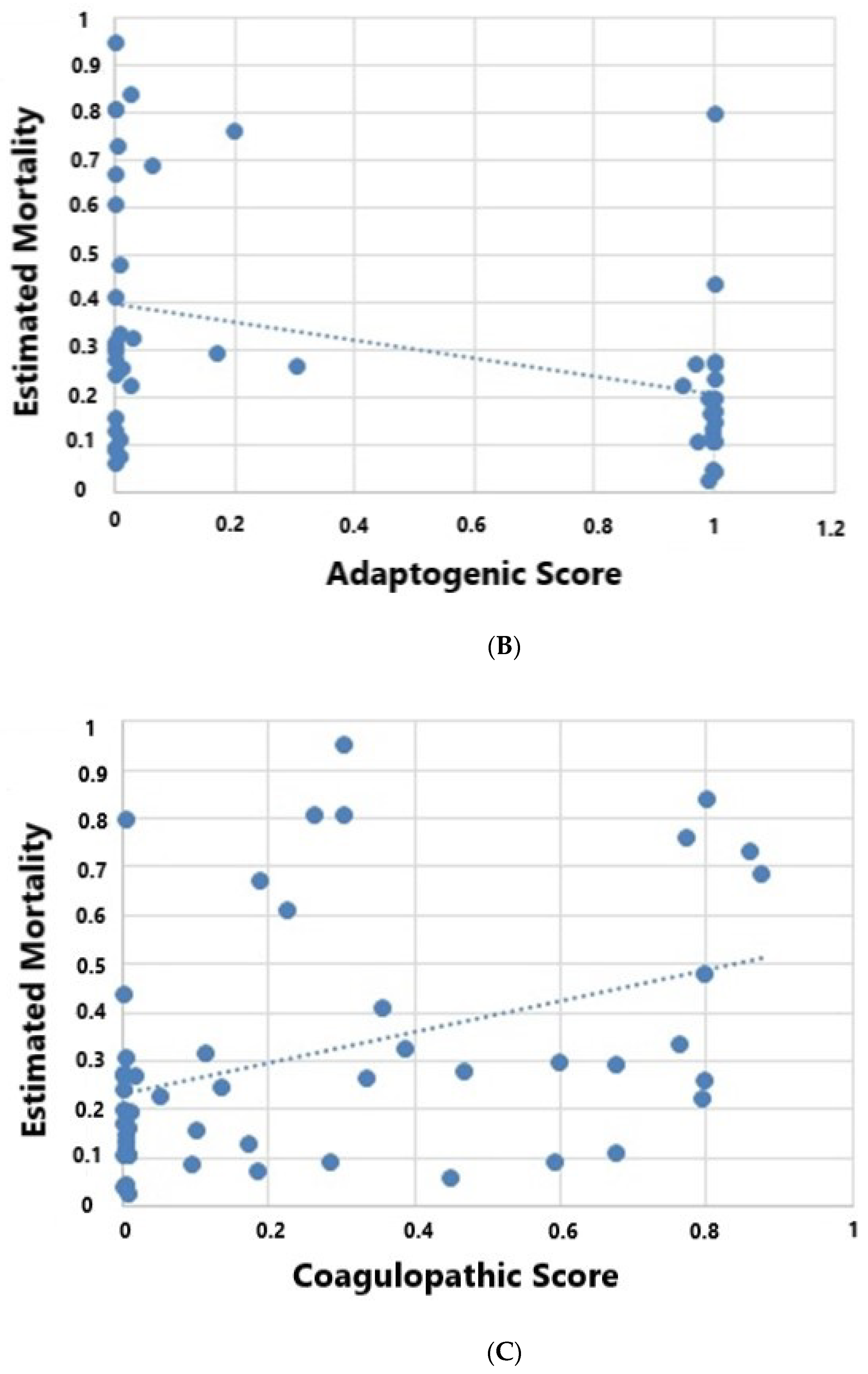
| Inflammatory Score | 0.18 (0.001, 0.69) | 0.18 (0.08, 0.63) | 0.42 | ||
| Coagulopathic | 7 (16%) | 5 (62%) | 0.012 | 8.57 | 1.65–44.0 |
| Coagulopathic Score | 0.09 (0.003, 0.35) | 0.77 (0.19, 0.84) | 0.031 | ||
| Adaptive | 19 (44%) | 1 (13%) | 0.12 | 0.2 | 0.020–1.65 |
| Adaptive Score | 0.17 (0.0001, 0.1) | 0.01 (0.0007, 0.05) | 0.16 | ||
| WBC × 109/L | 15 (11, 22) | 15 (6, 22) | 0.99 | ||
| Lactate (mM/L) | 3.1 (2, 4) | 4.9 (2.4, 9) | 0.2 | ||
| SCr (mg/dL) | 1.7 (1, 3.4) | 1.5 (1.1, 2.1) | 0.74 | ||
| Platelets (× 109/L) | 228 (168, 333) | 192 (90, 334) | 0.68 | ||
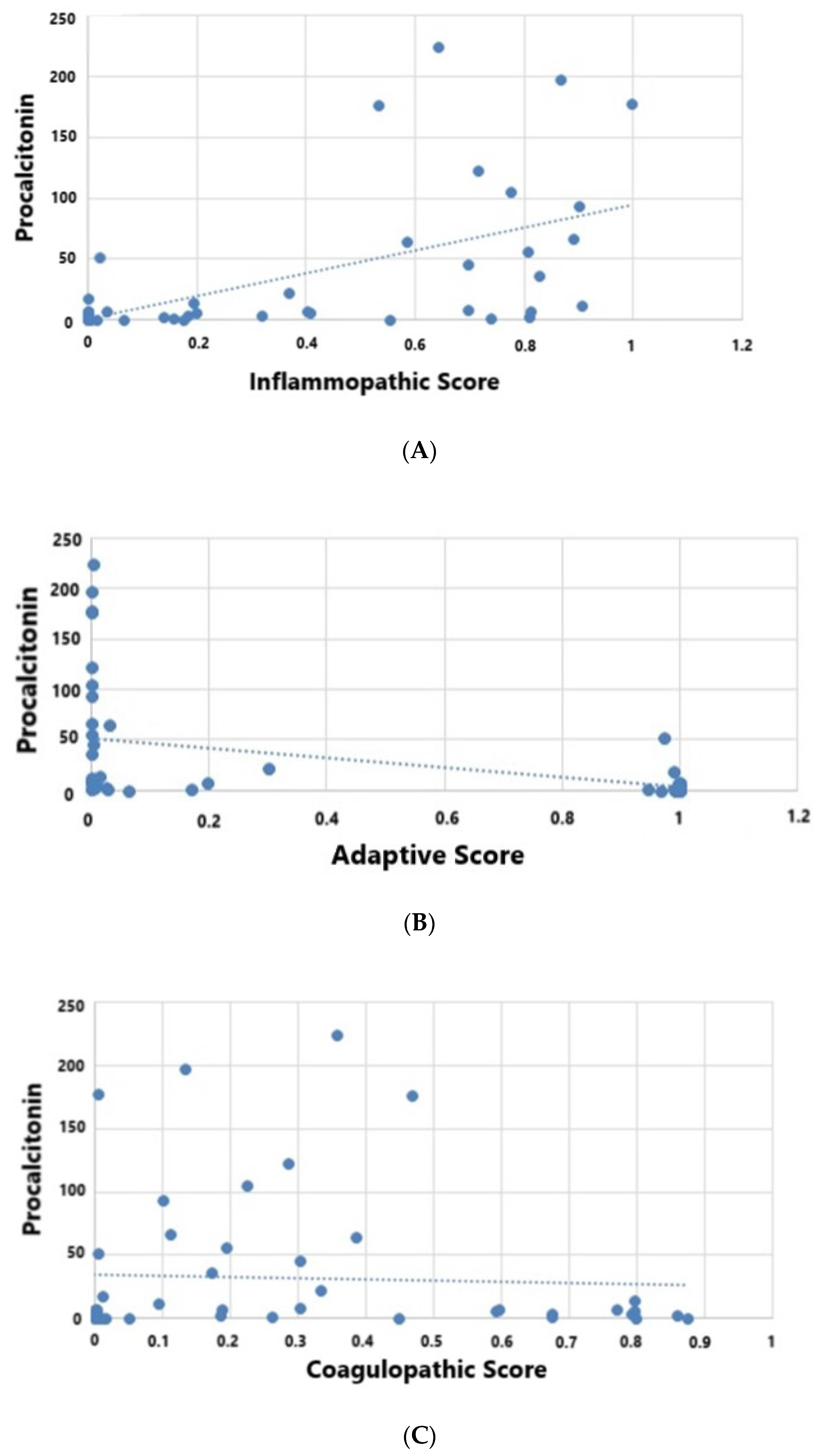
| Procalcitonin (ng/mL) | 3.7 (0.8, 15) | 1.65 (0.16, 8.5) | 0.8 | ||
| Tbili (mg/dL) | 0.7 (0.4, 1.6) | 1.4 (0.6, 2.2) | 0.34 | ||
| Ascorbic acid(mg/dL) | 0.3 (0.1, 0.4) | 0.52 (0.24, 0.67) | 0.13 | ||
| Thiamine(nmol/L) | 139 (113, 208) | 118 (105, 138) | 0.19 | ||
| SOFA | 8 (5, 8) | 9 (7.5, 10) | 0.45 | ||
| APACHE II | 21 (17, 28) | 26 (22, 33) | 0.12 | ||
| Apache IV | 76 (66, 91) | 107 (90, 119) | 0.002 | ||
| Apache IV predicted mortality (%) | 21 (11, 31) | 64 (11, 31) | 0.017 | ||
| PaO2/FiO2 | 256 (151, 341) | 186 (95, 321) | 0.58 | ||
| Total Lymphocyte | 0.5 (0.3, 1) | 0.35 (0.12, 0.7) | 0.09 | ||
| INR | 1.2 (1, 1.5) | 2 (1.5, 2.3) | 0.002 |
| Inflammatory (n = 19) | Adaptive (n = 20) | Coagulopathic (n = 12) | p | |
|---|---|---|---|---|
| Age | 64 (59, 73) | 67 (54, 81) | 76 (64, 82) | 0.16 |
| Race (Caucasian) | 19 (100%) | 18 (90%) | 11 (84%) | 0.53 |
| Weight (kg) | 70 (60, 84) | 86 (63, 100) | 76 (70, 100) | 0.54 |
| Sex (male) | 10 (53%) | 5 (25%) | 8 (61%) | 0.061 |
| CAD | 5 (26%) | 4 (20%) | 6 (46%) | 0.18 |
| Diabetes | 4 (20%) | 10 (50%) | 7 (54%) | 0.071 |
| CHF | 2 (10%) | 5 (25%) | 3 (23%) | 0.28 |
| COPD | 8 (42%) | 5 (25%) | 5 (38%) | 0.46 |
| CKD | 5 (26%) | 4 (20%) | 0 (0%) | 0.16 |
| Morbid obesity (BMI > 40) | 2 (10%) | 5 (25%) | 1 (8%) | 0.33 |
| Pneumonia | 8 (42%) | 6 (30%) | 6 (46%) | 0.66 |
| Primary bacteremia | 6 (31%) | 1 (5%) | 1 (8%) | 0.05 |
| Mechanical ventilation | 10 (53%) | 5 (25%) | 8 (61%) | 0.051 |
| Vasopressors | 14 (74%) | 13 (65%) | 10 (77%) | 0.52 |
| AKI | 15 (79%) | 16 (80%) | 11 (85%) | 0.62 |
| Positive blood cultures | 11 (58%) | 3 (15%) | 2 (15%) | 0.007 |
| WBC × 109/L | 14 (5, 27) | 14 (10, 20) | 18 (15, 22) | 0.12 |
| Lactate (mM/L) | 3.8 (2.8, 3.6) | 2.7 (1.6, 3.6) | 4.5 (2, 6.5) | 0.19 |
| SCr (mg/dL) | 2 (1.3, 3.6) | 1.36 (0.96, 2.1) | 1.6 (1.2, 2.1) | 0.21 |
| Platelets (× 109/L) | 189 (93, 235) | 263 (205, 370) | 213 (167, 350) | 0.024 |
| Procalcitonin (ng/mL) | 22 (4.6, 60) | 0.8 (0.2, 3.6) | 3.6 (1.6, 7) | <0.001 |
| Tbili (mg/dL) | 0.9 (0.6, 2) | 0.5 (0.4, 0.7) | 1.2 (0.62, 2) | 0.006 |
| PaO2/FiO2 | 211 (133, 293) | 277 (166, 366) | 224 (86, 347) | 0.43 |
| SOFA | 9 (7, 13) | 6 (4, 9) | 8 (6, 10) | 0.036 |
| INR | 1.28 (1.1, 1.5) | 1.25 (0.96, 1.6) | 1.4 (1, 2) | 0.87 |
| Total lymphocytes | 0.4 (0.2, 0.6) | 0.9 (0.4, 1.4) | 0.4 (0.25, 0.8) | 0.038 |
| APACHE II | 25 (18, 32) | 20 (15, 30) | 23 (20, 32) | 0.079 |
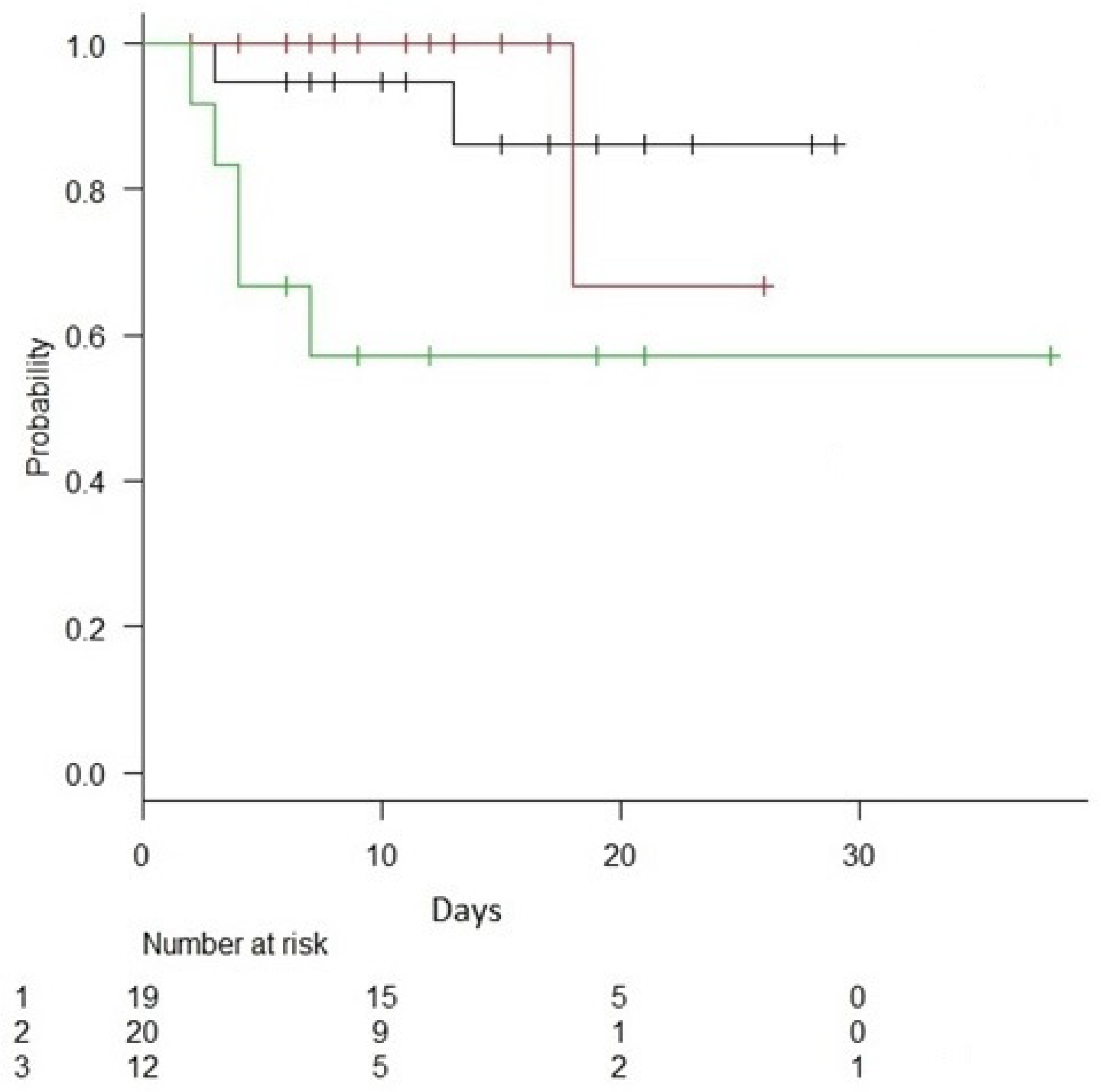
| Mortality across endotypes (%) | 10% | 5% | 40% | 0.032 |
| APACHE IV | 89 (65, 113) | 74 (65, 79) | 93 (73, 115) | 0.053 |
| Apache IV predicted mortality (%) | 0.29 (0.12, 0.62) | 0.17 (0.1, 0.26) | 0.3 (0.23, 0.7) | 0.023 |
| Ascorbic acid (mg/dL) | 0.3 (0.1, 0.4) | 0.3 (0.12, 0.4) | 0.4 (0.28, 0.65) | 0.19 |
| Thiamine(nmol/L) | 187 (117, 253) | 126 (104, 153) | 139 (107, 175) | 0.20 |
| HAT Therapy (n = 23) | Control (+ Steroids) (n = 6) | Control (no Steroids) (n = 22) | p | |
|---|---|---|---|---|
| Race (Caucasian) | 22 (96%) | 6 (100%) | 20 (91%) | 0.64 |
| Age | 64 (58, 80) | 73 (64, 79) | 67 (60, 77) | 0.77 |
| Sex (M) | 13 (57%) | 13 (50%) | 7 (32%) | 0.24 |
| Weight | 74 (60, 89) | 105 (78, 121) | 74 (63, 96) | 0.076 |
| Pneumonia | 9 (39%) | 5 (83%) | 6 (27%) | 0.045 |
| Primary Bacteremia | 3 (13%) | 2 (33%) | 3 (14%) | 0.44 |
| CAD | 5 (21%) | 1 (17%) | 9 (41%) | 0.28 |
| CHF | 4 (17%) | 2 (33%) | 4 (18%) | 0.66 |
| DM | 6 (26%) | 3 (50%) | 12 (54%) | 0.14 |
| Mechanical ventilation | 11 (48%) | 4 (67%) | 8 (30%) | 0.38 |
| Vasopressors | 20 (87%) | 4 (67%) | 13 (59%) | 0.1 |
| ARF | 12 (52%) | 3 (50%) | 7 (32%) | 0.1 |
| HD | 3 (13%) | 0 (0%) | 4 (18%) | 0.51 |
| Inflammopathic | 9 (37%) | 1 (5%) | 10 (50%) | 0.46 |
| Inflammatory Score | 0.31 (0.0001, 0.73) | 0.36 (0.002, 0.81) | 0.15 (0.007, 0.6) | 0.58 |
| Coagulopathic | 10 (43%) | 2 (33%) | 7 (32%) | 0.73 |
| Coagulopathic Score | 0.13 (0.002, 0.35) | 0.39 (0.13, 0.77) | 0.074 (0.003, 0.50) | 0.33 |
| Adaptive | 4 (17%) | 3 (50%) | 5 (13%) | 0.57 |
| Adaptive Score | 0.009 (0.0001, 0.9) | 0.035 (0.0001, 0.39) | 0.09 (0.007, 0.9) | 0.48 |
| WBC (K/mL) | 14 (7, 20) | 18 (12, 23) | 16 (12, 25) | 0.29 |
| Lactate (mM/L) | 2.6 (1.9, 4.1) | 4 (1.72, 4.5) | 3.5 (2.9, 6.5) | 0.29 |
| Creatinine (mg/dL) | 1.63 (1.26, 3.62) | 1.7 (1.25, 2.01) | 2.5 (0.74, 4.63) | 0.46 |
| Total Lympocyte | 0.4 (0.2, 0.8) | 0.8 (0.45, 1.6) | 1.4 (1.06, 1.55) | 0.096 |
| Platelets (K/mL) | 200 (85, 277) | 185 (161, 273) | 269 (203, 311) | 0.2 |
| INR | 1.28 (1.1, 2.1) | 1.04 (1.03, 1.05) | 1.4 (1.06, 1.55) | 0.23 |
| Procalcitonin (ng/mL) | 3 (0.19, 8) | 7 (6.4, 22) | 2.5 (0.74, 4.7) | 0.1 |
| Tbili (mg/dL) | 0.8 (0.5, 1.7) | 0.85 (0.47, 1.2) | 0.7 (0.4, 1.85) | 0.98 |
| PaO2/FiO2 | 200 (85, 277) | 185 (161, 273) | 96 (69, 206) | 0.06 |
| SOFA | 9 (7, 11) | 8.5 (5.5, 10.7) | 6 (4, 9) | 0.16 |
| APACHE II | 23 (19, 30) | 21 (15, 39) | 21 (17, 30) | 0.76 |
| APACHE IV | 82 (73, 91) | 84 (57, 121) | 70 (65, 97) | 0.49 |
| APACHE IV predicted mortality (%) | 27 (17, 41) | 34 (12, 69) | 16 (10, 31) | 0.26 |
| Ascorbic acid (mg/dL) | 0.3 (0.1, 0.4) | 0.3 (0.12, 0.4) | 0.45 (0.12, 0.67) | 0.14 |
| Thiamine (nmol/L) | 187 (151, 153) | 126 (104, 153) | 140 (115, 178) | 0.19 |
| HAT Therapy | n = 10 | n = 9 | n = 4 |
| IE | AE | CE | |
| survival | 9 | 8 | 2 |
| mortality | 1 | 1 | 2 |
| Controls given steroids off protocol | n = 2 | n = 1 | n = 3 |
| IE | AE | CE | |
| survival | 1 | 1 | 3 |
| mortality | 1 | 0 | 0 |
| Controls no steroids | n = 7 | n = 10 | n = 5 |
| IE | AE | CE | |
| survival | 7 | 10 | 2 |
| mortality | 0 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, J.; Vassallo, A.V.; Liesenfeld, O.; Levine, J.S.; Patel, V.V.; Sullivan, J.B.; Cavanaugh, J.B.; Elbaga, Y.; Sweeney, T.E. A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial. J. Pers. Med. 2021, 11, 9. https://doi.org/10.3390/jpm11010009
Iglesias J, Vassallo AV, Liesenfeld O, Levine JS, Patel VV, Sullivan JB, Cavanaugh JB, Elbaga Y, Sweeney TE. A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial. Journal of Personalized Medicine. 2021; 11(1):9. https://doi.org/10.3390/jpm11010009
Chicago/Turabian StyleIglesias, Jose, Andrew V. Vassallo, Oliver Liesenfeld, Jerrold S. Levine, Vishal V. Patel, Jesse B. Sullivan, Joseph B. Cavanaugh, Yasmine Elbaga, and Timothy E. Sweeney. 2021. "A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial" Journal of Personalized Medicine 11, no. 1: 9. https://doi.org/10.3390/jpm11010009
APA StyleIglesias, J., Vassallo, A. V., Liesenfeld, O., Levine, J. S., Patel, V. V., Sullivan, J. B., Cavanaugh, J. B., Elbaga, Y., & Sweeney, T. E. (2021). A 33-mRNA Classifier Is Able to Produce Inflammopathic, Adaptive, and Coagulopathic Endotypes with Prognostic Significance: The Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis (ORANGES) Trial. Journal of Personalized Medicine, 11(1), 9. https://doi.org/10.3390/jpm11010009






