Preoperative Predicting the WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma by Computed Tomography-Based Radiomics Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition
2.3. Histopathological Assessment of Nuclear Grade
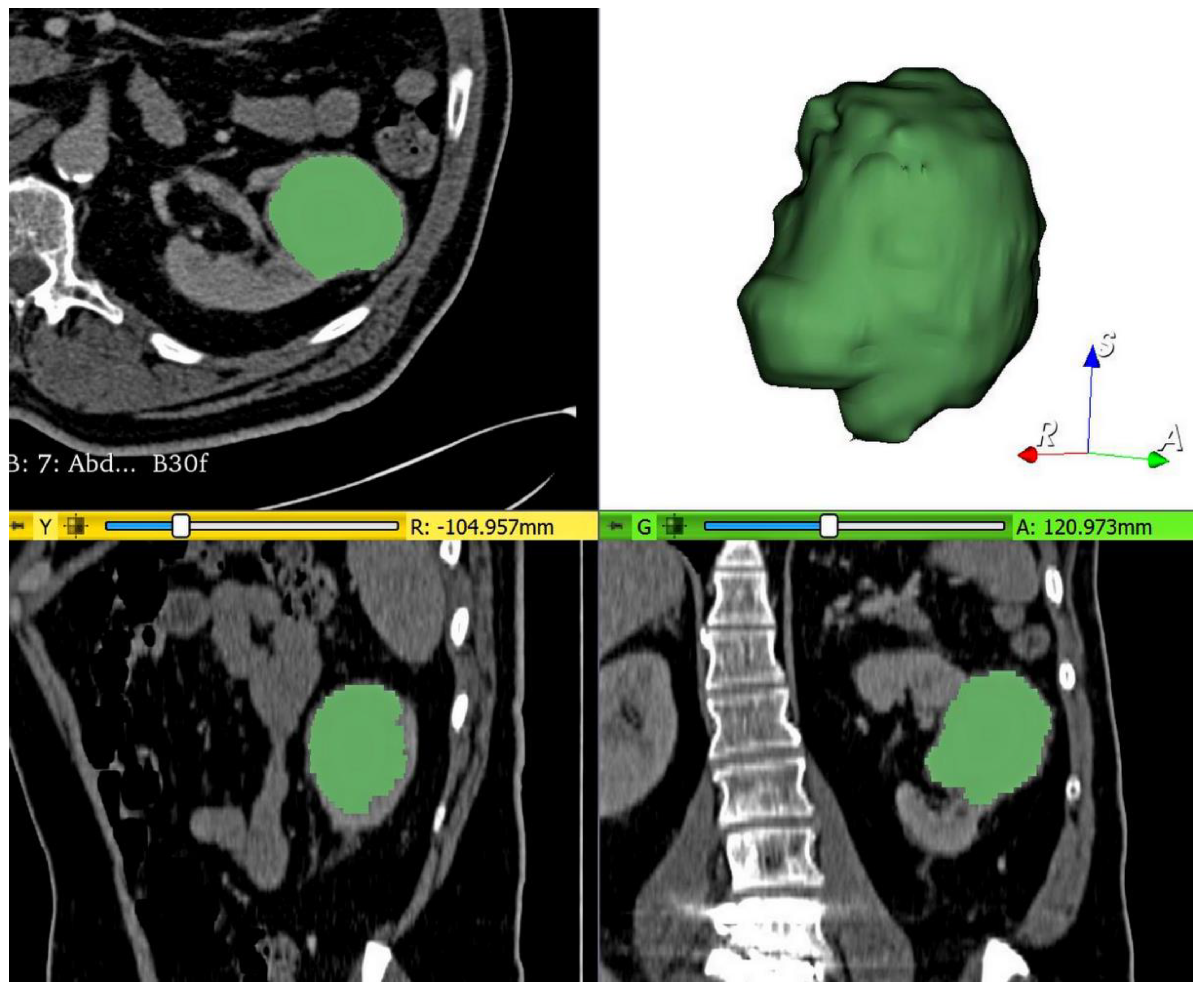
2.4. Tumor Segmentation, Preprocessing, and Radiomics Feature Extraction
2.5. Reliability Validation of Texture Features
2.6. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Feature Selection and Radiomics Score Building: Training Set
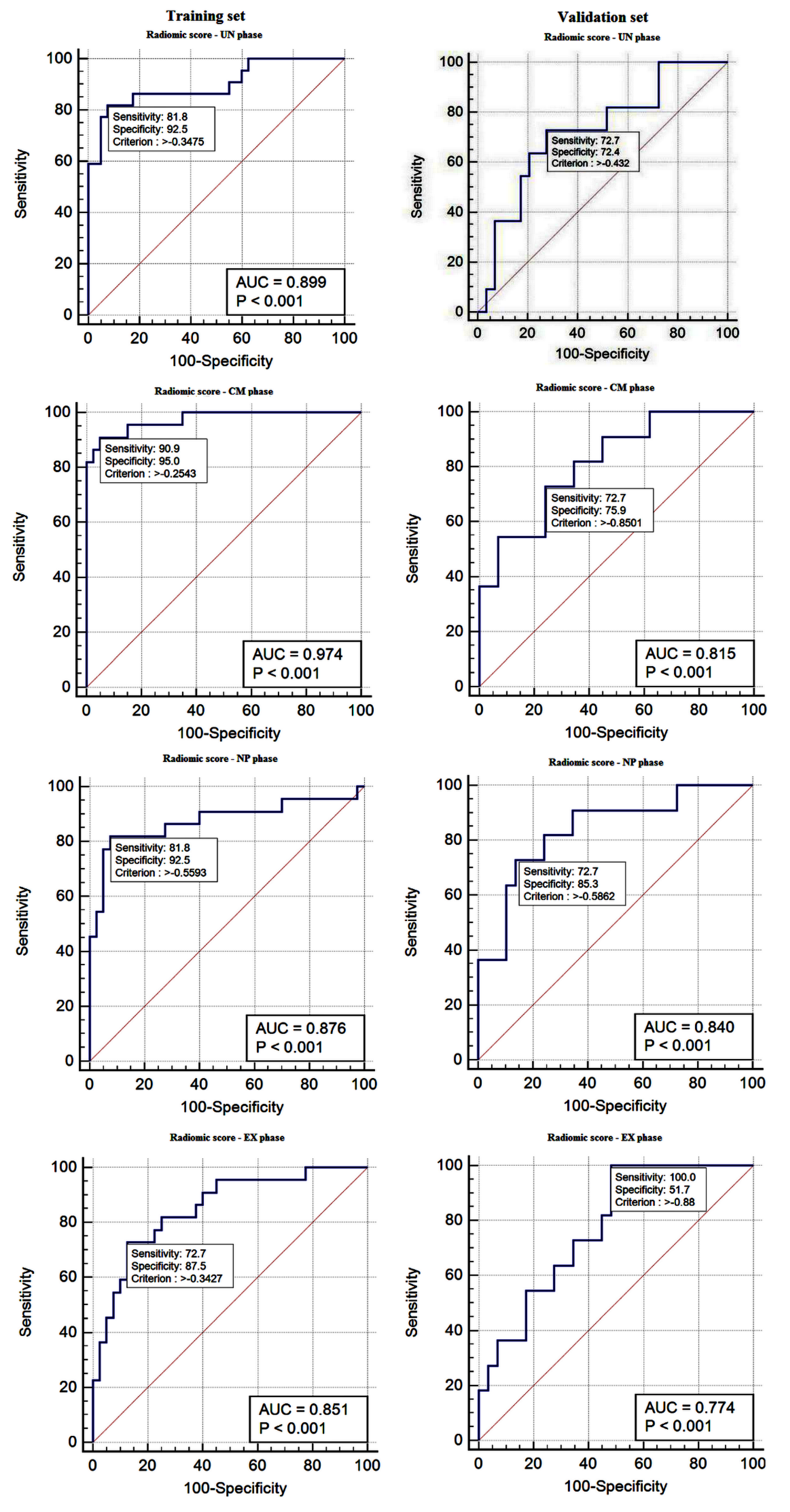
3.3. Performance of the Radiomics Scores: Training Set
3.4. Validation of the Radiomics Score
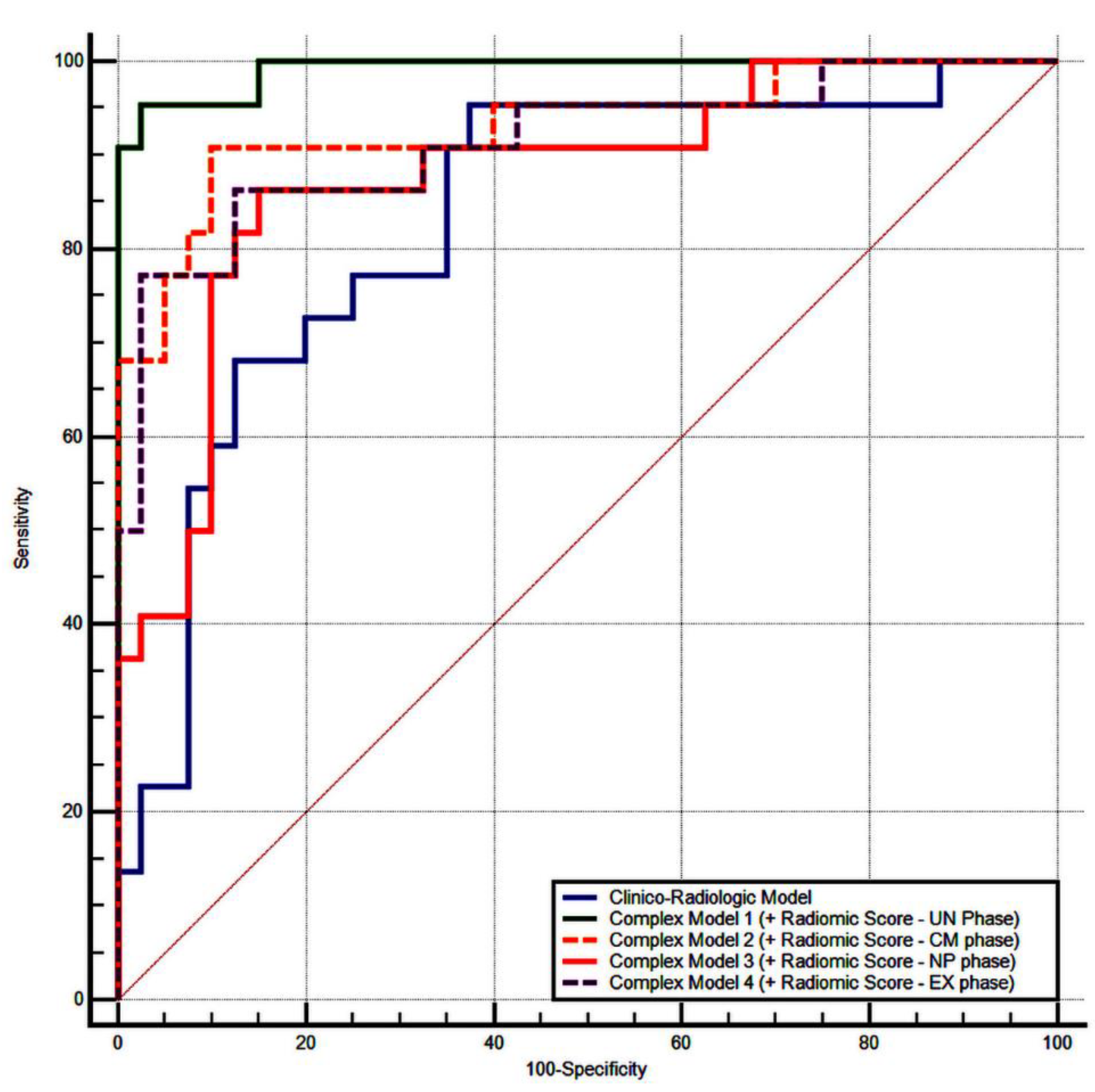
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medina-Rico, M.; López-Ramos, H.; Lobo, M.; Romo, J.; Prada, J.G. Epidemiology of renal cancer in developing countries: Review of the literature. Can. Urol. Assoc. J. 2018, 12, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, Y.; Zhang, J.; Fang, Z.; Liu, Z.; Xu, Z.; Fan, Y. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. Onco Targets Ther. 2018, 11, 5535–5544. [Google Scholar] [CrossRef]
- Perrino, C.M.; Cramer, H.M.; Chen, S.; Idrees, M.T.; Wu, H.H. World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grading in fine-needle aspiration biopsies of renal masses. Diagn. Cytopathol. 2018, 46, 895–900. [Google Scholar] [CrossRef]
- Bhatt, J.R.; Finelli, A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat. Rev. Urol. 2014, 11, 517–525. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Lechevallier, E.; Andre, M.; Daniel, L.; Coulange, C. Accuracy and Clinical Role of Fine Needle Percutaneous Biopsy with Computerized Tomography Guidance of Small (Less Than 4.0 Cm) Renal Masses. J. Urol. 2004, 171, 1802–1805. [Google Scholar] [CrossRef]
- Lebret, T.; Poulain, J.E.; Molinié, V.; Herve, J.M.; Denoux, Y.; Guth, A.; Scherrer, A.; Botto, H. Percutaneous Core Biopsy for Renal Masses: Indications, Accuracy and Results. J. Urol. 2007, 178, 1184–1188. [Google Scholar] [CrossRef]
- Blumenfeld, A.J.; Guru, K.; Fuchs, G.J.; Kim, H.L. Percutaneous Biopsy of Renal Cell Carcinoma Underestimates Nuclear Grade. Urology 2010, 76, 610–613. [Google Scholar] [CrossRef]
- Ficarra, V.; Brunelli, M.; Novara, G.; D’Elia, C.; Segala, D.; Gardiman, M.; Artibani, W.; Martignoni, G. Accuracy of on-bench biopsies in the evaluation of the histological subtype, grade, and necrosis of renal tumours. Pathology 2011, 43, 149–155. [Google Scholar] [CrossRef]
- Jeldres, C.; Sun, M.; Liberman, D.; Leghezzani, G.; de la Taille, A.; Tostain, J.; Valeri, A.; Cindolo, L.; Ficarra, V.; Artibani, W.; et al. Can renal mass biopsy assessment of tumor grade be safely substituted for by a predictive model? J. Urol. 2009, 182, 2585–2589. [Google Scholar] [CrossRef] [PubMed]
- Millet, I.; Curros, F.; Serre, I.; Taourel, P.; Thuret, R. Can Renal Biopsy Accurately Predict Histological Subtype and Fuhrman Grade of Renal Cell Carcinoma? J. Urol. 2012, 188, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Smaldone, M.C.; Uzzo, R.G.; Haifler, M.; Bratslavsky, G.; Leibovich, B.C. Renal Mass Biopsy: Always, Sometimes, or Never? Eur. Urol. 2016, 70, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keek, S.A.; Leijenaar, R.T.; Jochems, A.; Woodruff, H.C. A review on radiomics and the future of theranostics for patient selection in precision medicine. Brit. J. Radiol. 2018, 91, 20170926. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Capobianco, E.; Dominietto, M. From Medical Imaging to Radiomics: Role of Data Science for Advancing Precision Health. J. Pers. Med. 2020, 10, 15. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Imaging 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Velcheti, V.; Madabhushi, A. Novel Quantitative Imaging for Predicting Response to Therapy: Techniques and Clinical Applications. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; Savadjiev, P.; Chatterjee, A.; Muthukrishnan, N.; Reinhold, C.; Forghani, B. Radiomics and Artificial Intelligence for Biomarker and Prediction Model Development in Oncology. Comput. Struct. Biotechnol. J. 2019, 17, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Cui, E.-M.; Lei, Y.; Luo, L. CT-based machine learning model to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Abdom. Radiol. 2019, 44, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xing, Z.; Jiang, Z.; Chen, J.; Pan, L.; Qiu, J.; Xing, W. CT-based radiomic model predicts high grade of clear cell renal cell carcinoma. Eur. J. Radiol. 2018, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, Y.; Cui, J.; Yang, R.; Meng, X.; Cai, Z.; Zhang, J.; Xu, W.; Wen, D.; Yin, H. Clear cell renal cell carcinoma: CT-based radiomics features for the prediction of Fuhrman grade. Eur. J. Radiol. 2018, 109, 8–12. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Dong, D.; Bai, X.; Huang, Q.; Guo, A.; Ye, H.; Tian, J.; Wang, H.-Y. Multiparametric MRI Radiomic Model for Preoperative Predicting WHO / ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma. J. Magn. Reson. Imaging 2020, 52, 1557–1566. [Google Scholar] [CrossRef]
- Zhou, H.; Mao, H.; Dong, D.; Fang, M.; Gu, D.; Liu, X.; Xu, M.; Yang, S.; Zou, J.; Yin, R.; et al. Development and External Validation of Radiomics Approach for Nuclear Grading in Clear Cell Renal Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 4057–4065. [Google Scholar] [CrossRef]
- Feng, Z.; Shen, Q.; Li, Y.; Hu, Z. CT texture analysis: A potential tool for predicting the Fuhrman grade of clear-cell renal carcinoma. Cancer Imaging 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Han, D.; Yu, Y.; Yu, N.; Dang, S.; Wu, H.; Jialiang, R.; He, T. Prediction models for clear cell renal cell carcinoma ISUP/WHO grade: Comparison between CT radiomics and conventional contrast-enhanced CT. Br. J. Radiol. 2020, 93, 20200131. [Google Scholar] [CrossRef] [PubMed]
- Huhdanpaa, H.; Hwang, D.; Cen, S.Y.; Quinn, B.; Nayyar, M.; Zhang, X.; Chen, F.; Desai, B.; Liang, G.; Gill, I.S.; et al. CT prediction of the Fuhrman grade of clear cell renal cell carcinoma (RCC): Towards the development of computer-assisted diagnostic method. Abdom. Imaging 2015, 40, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat. Med. 2012, 31, 3972–3981. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.; Lo, L.-H. Examining Test-Retest Reliability. Nurs. Res. 2002, 51, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Soufi, M.; Ninomiya, K.; Kamezawa, H.; Yamada, M. Potentials of radiomics for cancer diagnosis and treatment in comparison with computer-aided diagnosis. Radiol. Phys. Technol. 2018, 11, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.; Deist, T.M.; Peerlings, J.; De Jong, E.E.; Van Timmeren, J.; Sanduleanu, S.; LaRue, R.T.H.M.; Even, A.J.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Krajewski, K.M.; Braschi-Amirfarzan, M.; Ramaiya, N.H. Advanced Renal Cell Carcinoma: Role of the Radiologist in the Era of Precision Medicine. Radiology 2017, 284, 333–351. [Google Scholar] [CrossRef]
- Dong, X.; Xing, L.; Wu, P.; Fu, Z.; Wan, H.; Li, D.; Yin, Y.; Sun, X.; Yu, J. Three-dimensional positron emission tomography image texture analysis of esophageal squamous cell carcinoma. Nucl. Med. Commun. 2013, 34, 40–46. [Google Scholar] [CrossRef]
- Mu, W.; Chen, Z.; Liang, Y.; Shen, W.; Yang, F.; Dai, R.; Wu, N.; Tian, J. Staging of cervical cancer based on tumor heterogeneity characterized by texture features on18F-FDG PET images. Phys. Med. Biol. 2015, 60, 5123–5139. [Google Scholar] [CrossRef] [PubMed]
- Petresc, B.; Lebovici, A.; Caraiani, C.; Feier, D.S.; Graur, F.; Buruian, M.M. Pre-Treatment T2-WI Based Radiomics Features for Prediction of Locally Advanced Rectal Cancer Non-Response to Neoadjuvant Chemoradiotherapy: A Preliminary Study. Cancers 2020, 12, 1894. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Abaleke, S.; Young, R.C.; Chatwin, C.R.; Miles, K.A. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010, 10, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Lindner, V.; De Fromont, M.; Molinié, V.; Letourneux, H.; Meyer, N.; Martin, M.; Jacqmin, D. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma. Cancer 2005, 103, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Letourneux, H.; Lindner, V.; Lang, H.; Massfelder, T.; Meyer, N.; Saussine, C.; Jacqmin, D. Reproductibilité du grade nucléaire de Fuhrman. Avantages d’un regroupement en deux grades (Reproducibility of Fuhrman nuclear grade: Advantages of a two-grade system). Prog. Urol. 2006, 16, 281–285. [Google Scholar] [PubMed]
- Al-Aynati, M.; Chen, V.; Salama, S.; Shuhaibar, H.; Treleaven, D.; Vincic, L. Interobserver and intraobserver variability using the Fuhrman grading system for renal cell carcinoma. Arch. Pathol. Lab. Med. 2003, 127, 593–596. [Google Scholar] [PubMed]
- Bektaş, S.; Bahadir, B.; Kandemir, N.O.; Barut, F.; Gul, A.E.; Ozdamar, S.O. Intraobserver and Interobserver Variability of Fuhrman and Modified Fuhrman Grading Systems for Conventional Renal Cell Carcinoma. Kaohsiung J. Med Sci. 2009, 25, 596–600. [Google Scholar] [CrossRef]
- Cui, E.; Li, Z.; Ma, C.; Li, Q.; Lei, Y.; Lan, Y.; Yu, J.; Zhou, Z.; Li, R.; Long, W.; et al. Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. Eur. Radiol. 2020, 30, 2912–2921. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Zhang, T.; Han, F.; Song, B. Predictive models composed by radiomic features extracted from multi-detector computed tomography images for predicting low-and high-grade clear cell renal cell carcinoma: A STARD-compliant article. Medicine 2019. [Google Scholar] [CrossRef]
- Shu, J.; Wen, D.; Xi, Y.; Xia, Y.; Cai, Z.; Xu, W.; Meng, X.; Liu, B.; Yin, H. Clear cell renal cell carcinoma: Machine learning-based computed tomography radiomics analysis for the prediction of WHO/ISUP grade. Eur. J. Radiol. 2019, 121, 108738. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Xu, K.; Li, W.; Huo, Z.; Liu, H.; Shen, T.; Pan, F.; Jiang, Y.; Zhang, M. Prediction of ISUP grading of clear cell renal cell carcinoma using support vector machine model based on CT images. Medicine 2019, 98, e15022. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G. Radiomics and Artificial Intelligence for Renal Mass Characterization. Radiol. Clin. North Am. 2020, 58, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Yoshizako, T.; Hisatoshi, A.; Mori, H.; Tamaki, Y.; Ishikawa, N.; Kitagaki, H. The additional utility of apparent diffusion coefficient values of clear-cell renal cell carcinoma for predicting metastasis during clinical staging. Acta Radiol. Open 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Niver, B.E.; Fitzgerald, E.F.; Babb, J.S.; Chandarana, H.; Melamed, J. Utility of the Apparent Diffusion Coefficient for Distinguishing Clear Cell Renal Cell Carcinoma of Low and High Nuclear Grade. Am. J. Roentgenol. 2010, 195, W344–W351. [Google Scholar] [CrossRef]
- Maruyama, M.; Yoshizako, T.; Uchida, K.; Araki, H.; Tamaki, Y.; Ishikawa, N.; Shiina, H.; Kitagaki, H. Comparison of utility of tumor size and apparent diffusion coefficient for differentiation of low- and high-grade clear-cell renal cell carcinoma. Acta Radiol. 2015, 56, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Petersen, J.H.; Budtz-Jørgensen, E.; Endahl, L. Interpreting Parameters in the Logistic Regression Model with Random Effects. Biometrics 2000, 56, 909–914. [Google Scholar] [CrossRef]
- Bektas, C.T.; Kocak, B.; Yardimci, A.H.; Turkcanoglu, M.H.; Yucetas, U.; Koca, S.B.; Erdim, C.; Kilickesmez, O. Clear Cell Renal Cell Carcinoma: Machine Learning-Based Quantitative Computed Tomography Texture Analysis for Prediction of Fuhrman Nuclear Grade. Eur. Radiol. 2019, 29, 1153–1163. [Google Scholar] [CrossRef]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radiol. Med. 2020, 125, 754–762. [Google Scholar] [CrossRef]
- Xu, K.; Liu, L.; Li, W.; Sun, X.; Shen, T.; Pan, F.; Jiang, Y.; Guo, Y.; Ding, L.; Zhang, M. CT-Based Radiomics Signature for Preoperative Prediction of Coagulative Necrosis in Clear Cell Renal Cell Carcinoma. Korean J. Radiol. 2020, 21, 670–683. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J. Urol. 2002, 168, 2395–2400. [Google Scholar] [CrossRef]
- Klatte, T.; Patard, J.-J.; De Martino, M.; Bensalah, K.; Verhoest, G.; De La Taille, A.; Abbou, C.-C.; Allhoff, E.P.; Carrieri, G.; Riggs, S.B.; et al. Tumor Size Does Not Predict Risk of Metastatic Disease or Prognosis of Small Renal Cell Carcinomas. J. Urol. 2008, 179, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef] [PubMed]
| Training Set | Validation Set | |||||
|---|---|---|---|---|---|---|
| Characteristic | Low Grade | High Grade | p-Value | Low Grade | High Grade | p-Value |
| Number | 40 | 22 | 29 | 11 | ||
| Age (years) | 58.2 ± 12.92 | 66.36 ± 10.45 | 0.009 * | 61.89 ± 13.30 | 66.63 ± 14.66 | 0.36 |
| Gender | 0.91 | 1.00 | ||||
| Male | 26 (65%) | 14 (35%) | 20 (74.1%) | 7 (25.9%) | ||
| Female | 14 (63.6%) | 8 (36.4%) | 9 (69.2%) | 4 (30.8%) | ||
| Tumor size (mm) | 46.65 ± 28.53 | 73.22 ± 26.25 | 0.001 * | 53.17 ± 22.68 | 79.45 ± 25.15 | 0.008 * |
| Tumor stage (n) | 0.001 * | 0.01 * | ||||
| 1 | 30 (85.7%) | 5 (14.3%) | 18 (94.7%) | 1 (5.3%) | ||
| 2 | 3 (50%) | 3 (50%) | 6 (100%) | - | ||
| 3 | 7 (35%) | 13 (65%) | 5 (35.7) | 9 (64.3%) | ||
| 4 | - | 1 (100%) | - | 1 (100%) | ||
| Vein thrombosis | 0.02 * | 0.009 * | ||||
| No | 33 (73.3%) | 12 (26.6%) | 22 (88%) | 3 (12%) | ||
| Yes | 7 (41.1%) | 10 (58.8%) | 7 (46.6%) | 8 (53.3%) | ||
| Tumor necrosis | 0.47 | 1.00 | ||||
| No | 7 (77.7%) | 2 (22.2%) | 2 (66.6%) | 1 (33.3%) | ||
| Yes | 33 (60%) | 22 (40%) | 27 (72.9%) | 10 (27.0%) | ||
| Perinephritic invasion | 0.009 * | 0.49 | ||||
| No | 34 (73.9%) | 12 (26.0%) | 12 (80%) | 3 (20%) | ||
| Yes | 6 (37.5%) | 10 (62.5%) | 17 (68%) | 8 (32%) | ||
| Intratumoral neovascularity | 0.003 * | 0.29 | ||||
| No | 30 (78.9%) | 8 (21.0%) | 11 (85.6%) | 2 (15.3%) | ||
| Yes | 10 (41.6%) | 14 (58.3%) | 18 (66.6%) | 9 (33.3%) | ||
| Hemorrhage | 0.01 * | 0.48 | ||||
| No | 32 (74.4%) | 11 (25.5%) | 16 (80%) | 4 (20%) | ||
| Yes | 8 (42.1%) | 11 (57.8%) | 13 (65%) | 7 (35%) | ||
| Lymphadenopathy | 0.05 | 0.12 | ||||
| No | 35 (71.4%) | 14 (28.5%) | 27 (77.1%) | 8 (22.8%) | ||
| Yes | 5 (38.4%) | 8 (61.5%) | 2 (40%) | 3 (60%) | ||
| Radiomic Group | Radiomic Feature | Associated Filter | Coefficient |
|---|---|---|---|
| UN phase | |||
| Intercept | −0.872 | ||
| Texture feature | JointAverage | LoG filter (2 mm) | 0.409 |
| Texture feature | SizeZoneNonUniformity | LoG filter (2 mm) | 0.010 |
| Texture feature | DependenceVariance | LoG filter (4 mm) | 0.362 |
| First-order | Minimum | LoG filter (4 mm) | −0.296 |
| Texture feature | LongRunEmphasis | LoG filter (4 mm) | 0.477 |
| Texture feature | SmallAreaHighGrayLevelEmphasis | LoG filter (4 mm) | 0.091 |
| Texture feature | LargeAreaLowGrayLevelEmphasis | Wavelet-LHL | 0.039 |
| Texture feature | LongRunLowGrayLevelEmphasis | Wavelet-LLH | −0.431 |
| Texture feature | SmallAreaLowGrayLevelEmphasis | Wavelet-LLH | −0.349 |
| Texture feature | LongRunLowGrayLevelEmphasis | Wavelet-HHL | 0.343 |
| CM phase | |||
| Intercept | −1.184 | ||
| Texture feature | SmallAreaLowGrayLevelEmphasis | Original | −0.387 |
| First-order | Skewness | LoG filter (2 mm) | −0.311 |
| First-order | Minimum | LoG filter (2 mm) | −0.052 |
| First-order | 10Percentile | LoG filter (2 mm) | 0.303 |
| Texture feature | LowGrayLevelEmphasis | LoG filter (4 mm) | −0.373 |
| Texture feature | LongRunHighGrayLevelEmphasis | Wavelet-HLL | 0.306 |
| Texture feature | LowGrayLevelZoneEmphasis | Wavelet-HLL | −0.076 |
| Texture feature | Imc2 | Wavelet-LHL | 0.797 |
| First-order | Mean | Wavelet-LHL | 0.516 |
| Texture feature | GrayLevelNonUniformity | Wavelet-LHL | −0.153 |
| Texture feature | SmallAreaEmphasis | Wavelet-LHL | 0.823 |
| Texture feature | LongRunLowGrayLevelEmphasis | Wavelet-LLH | −0.429 |
| First-order | Maximum | Wavelet-HLH | 0.583 |
| Texture feature | GrayLevelVariance | Wavelet-HHL | 0.084 |
| First-order | Entropy | Wavelet-HHL | 0.049 |
| Texture feature | RunVariance | Wavelet-HHL | 0.064 |
| Texture feature | ShortRunLowGrayLevelEmphasis | Wavelet-LLL | −0.379 |
| NP phase | |||
| Intercept | −0.765 | ||
| Texture feature | HighGrayLevelRunEmphasis | Original | 0.325 |
| Texture feature | ShortRunHighGrayLevelEmphasis | LoG filter (6 mm) | −0.087 |
| Texture feature | Imc2 | Wavelet-HLL | 0.191 |
| Texture feature | ShortRunHighGrayLevelEmphasis | Wavelet-HLL | 0.225 |
| Texture feature | Contrast | Wavelet-LHL | 0.192 |
| Texture feature | SmallAreaHighGrayLevelEmphasis | Wavelet-LHL | 0.353 |
| Texture feature | ZoneEntropy | Wavelet-LHL | 0.049 |
| First-order | Entropy | Wavelet-LHH | 0.070 |
| Texture feature | DependenceNonUniformityNormalized | Wavelet-LLH | 0.013 |
| Texture feature | SumEntropy | Wavelet-HLH | −0.190 |
| Texture feature | Imc2 | Wavelet-HLH | 0.331 |
| Texture feature | GrayLevelVariance | Wavelet-HHL | 0.019 |
| Texture feature | Idn | Wavelet-LLL | 0.223 |
| Texture feature | SmallAreaLowGrayLevelEmphasis | Wavelet-LLL | −0.189 |
| EX phase | |||
| Intercept | −0.653 | ||
| Texture feature | DependenceVariance | LoG filter (4 mm) | −0.234 |
| First-order | Kurtosis | LoG filter (4 mm) | 0.139 |
| Texture feature | RunVariance | LoG filter (4 mm) | 0.163 |
| Texture feature | SizeZoneNonUniformity | LoG filter (4 mm) | 0.032 |
| Texture feature | DependenceNonUniformityNormalized | Wavelet-HLL | 0.165 |
| Texture feature | SmallDependenceLowGrayLevelEmphasis | Wavelet-HLL | −0.046 |
| Texture feature | SmallAreaHighGrayLevelEmphasis | Wavelet-LHL | 0.028 |
| WHO/ISUP Nuclear Grades | Radiomic Score Mean ± SD | p-Value | MDCT Phase |
|---|---|---|---|
| Training set | |||
| Low grade (n = 40) | −1.68 ± 1.16 | p < 0.001 | UN |
| −2.50 ± 1.95 | p < 0.001 | CM | |
| −1.26 ± 0.68 | p < 0.001 | NP | |
| −0.92 ± 0.53 | p < 0.001 | EX | |
| High grade (n = 22) | 0.60 ± 1.34 | p < 0.001 | UN |
| 1.21 ± 1.29 | p < 0.001 | CM | |
| 0.15 ± 1.18 | p < 0.001 | NP | |
| −0.16 ± 0.51 | p < 0.001 | EX | |
| Validation set | |||
| Low grade (n = 29) | −1.18 ± 1.70 | p = 0.051 | UN |
| −2.21 ± 2.42 | p < 0.001 | CM | |
| −1.12 ± 0.72 | p = 0.001 | NP | |
| −0.80 ± 0.62 | p = 0.009 | EX | |
| High grade (n = 11) | −0.03 ± 1.32 | p = 0.051 | UN |
| 1.53 ± 3.43 | p < 0.001 | CM | |
| 0.19 ± 1.56 | p = 0.001 | NP | |
| −0.24 ± 0.45 | p = 0.009 | EX |
| Variable | AUC (95% CI) | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | Cut-Off Value | p-Value |
|---|---|---|---|---|---|---|---|
| Training set | |||||||
| Radiomic score: UN phase | 0.89 (0.796–0.961) | 81.82 (59.7–94.8) | 92.50 (79.6–98.4) | 85.7 (63.7–97.0) | 90.2 (76.9–97.3) | −0.34 | <0.001 |
| Radiomic score: CM phase | 0.97 (0.89–0.99) | 90.91 (70.8–98.9) | 95.00 (83.1–99.4) | 90.9 (70.8–98.9) | 95.0 (83.1–99.4) | −0.25 | <0.001 |
| Radiomic score: NP phase | 0.87 (0.76–0.94) | 81.82 (59.7–94.8) | 92.50 (79.6–98.4) | 85.7 (63.7–97.0) | 90.2 (76.9–97.3) | −0.55 | <0.001 |
| Radiomic score: EX phase | 0.85 (0.73–0.92) | 72.73 (49.8–89.3) | 87.50 (73.2–95.8) | 76.2 (52.8–91.8) | 85.4 (70.8–94.4) | −0.34 | <0.001 |
| Validation set | |||||||
| Radiomic score: UN phase | 0.72 (0.56–0.85) | 72.73 (39.0–94.0) | 72.41 (52.8–87.3) | 50.0 (24.7–75.3) | 87.5 (67.6–97.3) | −0.43 | 0.0157 |
| Radiomic score: CM phase | 0.81 (0.66–0.92) | 72.73 (39.0–94.0) | 75.90 (56.5–89.7) | 53.3 (26.6–78.7) | 88.0 (68.8–97.5) | −0.85 | <0.001 |
| Radiomic score: NP phase | 0.84 (0.69–0.93) | 72.73 (39.0–94.0) | 85.30 (68.3–96.1) | 66.7 (34.9–90.1) | 89.3 (71.8–97.7) | −0.58 | <0.001 |
| Radiomic score: EX phase | 0.77 (0.61–0.89) | 100.0 (71.5–100.0) | 51.72 (32.5–70.6) | 44.0 (24.4–65.1) | 100.0 (78.2–100.0) | −0.88 | <0.001 |
| Variable | Coefficient | Std. Error | p-Value | Odds Ratio (OR) |
|---|---|---|---|---|
| Age (years) | 0.05 | 0.03 | 0.10 | 1.05 |
| Tumor size (mm) | 0.006 | 0.01 | 0.74 | 1.00 |
| Vein thrombosis: positive | −1.32 | 1.22 | 0.27 | 0.26 |
| Perinephric invasion: positive | 1.60 | 1.39 | 0.25 | 4.98 |
| Tumor stage (2, 3, or 4) | 2.09 | 1.07 | 0.05 | 8.13 |
| Intratumoral neovascularity: positive | 1.04 | 0.99 | 0.29 | 2.85 |
| Hemorrhage: positive | −1.81 | 1.55 | 0.24 | 0.16 |
| Constant | −5.55 |
| Variable | Coefficient | Std. Error | p-Value | Odds Ratio (OR) |
|---|---|---|---|---|
| UN phase | ||||
| Age (years) | 0.03 | 0.05 | 0.4997 | 1.03 |
| Tumor size (mm) | −0.02 | 0.02 | 0.4156 | 0.97 |
| Vein thrombosis: positive | 1.64 | 1.81 | 0.3653 | 5.16 |
| Perinephric invasion: positive | −0.17 | 1.73 | 0.9176 | 0.83 |
| Tumor stage (2, 3, or 4) | 0.99 | 1.42 | 0.4860 | 2.70 |
| Intratumoral neovascularity: positive | 0.07 | 1.17 | 0.9485 | 1.07 |
| Hemorrhage: positive | −1.75 | 1.87 | 0.34 | 0.17 |
| Radiomic score: UN phase | 1.83 | 0.59 | 0.0021 | 6.27 |
| Constant | −0.90 | |||
| CM phase | ||||
| Age (years) | 0.12 | 0.09 | 0.1772 | 1.13 |
| Tumor size (mm) | −0.08 | 0.07 | 0.2576 | 0.91 |
| Vein thrombosis: positive | 1.13 | 16.78 | 0.9460 | 3.11 |
| Perinephric invasion: positive | 2.76 | 17.36 | 0.8735 | 15.86 |
| Tumor stage (2, 3, or 4) | 3.62 | 3.38 | 0.2837 | 37.59 |
| Intratumoral neovascularity: positive | −2.36 | 2.85 | 0.4075 | 3.11 |
| Hemorrhage: positive | −4.07 | 17.52 | 0.8161 | 0.01 |
| Radiomic score: CM phase | 4.92 | 2.37 | 0.0384 | 137.75 |
| Constant | −1.50 | |||
| NP phase | ||||
| Age (years) | 0.11 | 0.05 | 0.03 | 1.12 |
| Tumor size (mm) | −0.03 | 0.02 | 0.2121 | 0.96 |
| Vein thrombosis: positive | −3.15 | 1.68 | 0.0610 | 0.04 |
| Perinephric invasion: positive | 3.88 | 2.23 | 0.0827 | 48.76 |
| Tumor stage (2, 3, or 4) | 2.19 | 1.50 | 0.1449 | 8.98 |
| Intratumoral neovascularity: positive | 0.34 | 1.24 | 0.7830 | 1.41 |
| Hemorrhage: positive | ||||
| Radiomic score: NP phase | 2.78 | 0.91 | 0.0023 | 16.17 |
| Constant | −4.64 | |||
| EX phase | ||||
| Age (years) | 0.05 | 0.04 | 0.2408 | 1.05 |
| Tumor size (mm) | −0.05 | 0.03 | 0.0879 | 0.94 |
| Vein thrombosis: positive | −0.24 | 1.42 | 0.8639 | 0.78 |
| Perinephric invasion: positive | 1.01 | 1.57 | 0.5184 | 2.77 |
| Tumor stage (2, 3, or 4) | 1.87 | 1.19 | 0.1170 | 6.49 |
| Intratumoral neovascularity: positive | 0.09 | 1.07 | 0.9263 | 1.10 |
| Hemorrhage: positive | −1.20 | 1.71 | 0.4832 | 0.29 |
| Radiomic score: EX phase | 4.64 | 1.75 | 0.0081 | 103.88 |
| Constant | 1.40 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovanu, C.-G.; Boca, B.; Lebovici, A.; Tamas-Szora, A.; Feier, D.S.; Crisan, N.; Andras, I.; Buruian, M.M. Preoperative Predicting the WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma by Computed Tomography-Based Radiomics Features. J. Pers. Med. 2021, 11, 8. https://doi.org/10.3390/jpm11010008
Moldovanu C-G, Boca B, Lebovici A, Tamas-Szora A, Feier DS, Crisan N, Andras I, Buruian MM. Preoperative Predicting the WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma by Computed Tomography-Based Radiomics Features. Journal of Personalized Medicine. 2021; 11(1):8. https://doi.org/10.3390/jpm11010008
Chicago/Turabian StyleMoldovanu, Claudia-Gabriela, Bianca Boca, Andrei Lebovici, Attila Tamas-Szora, Diana Sorina Feier, Nicolae Crisan, Iulia Andras, and Mircea Marian Buruian. 2021. "Preoperative Predicting the WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma by Computed Tomography-Based Radiomics Features" Journal of Personalized Medicine 11, no. 1: 8. https://doi.org/10.3390/jpm11010008
APA StyleMoldovanu, C.-G., Boca, B., Lebovici, A., Tamas-Szora, A., Feier, D. S., Crisan, N., Andras, I., & Buruian, M. M. (2021). Preoperative Predicting the WHO/ISUP Nuclear Grade of Clear Cell Renal Cell Carcinoma by Computed Tomography-Based Radiomics Features. Journal of Personalized Medicine, 11(1), 8. https://doi.org/10.3390/jpm11010008





