Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients
Abstract
Highlights
- The single-pulse transcranial magnetic stimulation (TMS) (spTMS) allows probing and visualization of the spatiotemporal characteristics of each young (YNG), older (OLD), and schizophrenia (SCZ) group.
- Different TMS-evoked potential (TEP) characteristics induced by spTMS were observed in the OLD and SCZ compared with YNG.
- Specifically, changes in P30 and N45 deflections of TEPs by spTMS were important in classifying these groups across the motor cortex (M1) and dorsolateral prefrontal cortex (DLPFC).
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. TMS Procedure
2.3. EEG Recording and Pre-Processing
2.4. EEG Signal Processing
2.5. Single-Pulses TMS-Evoked Potential (TEP) Analyses
2.6. Statistical Analyses
3. Results
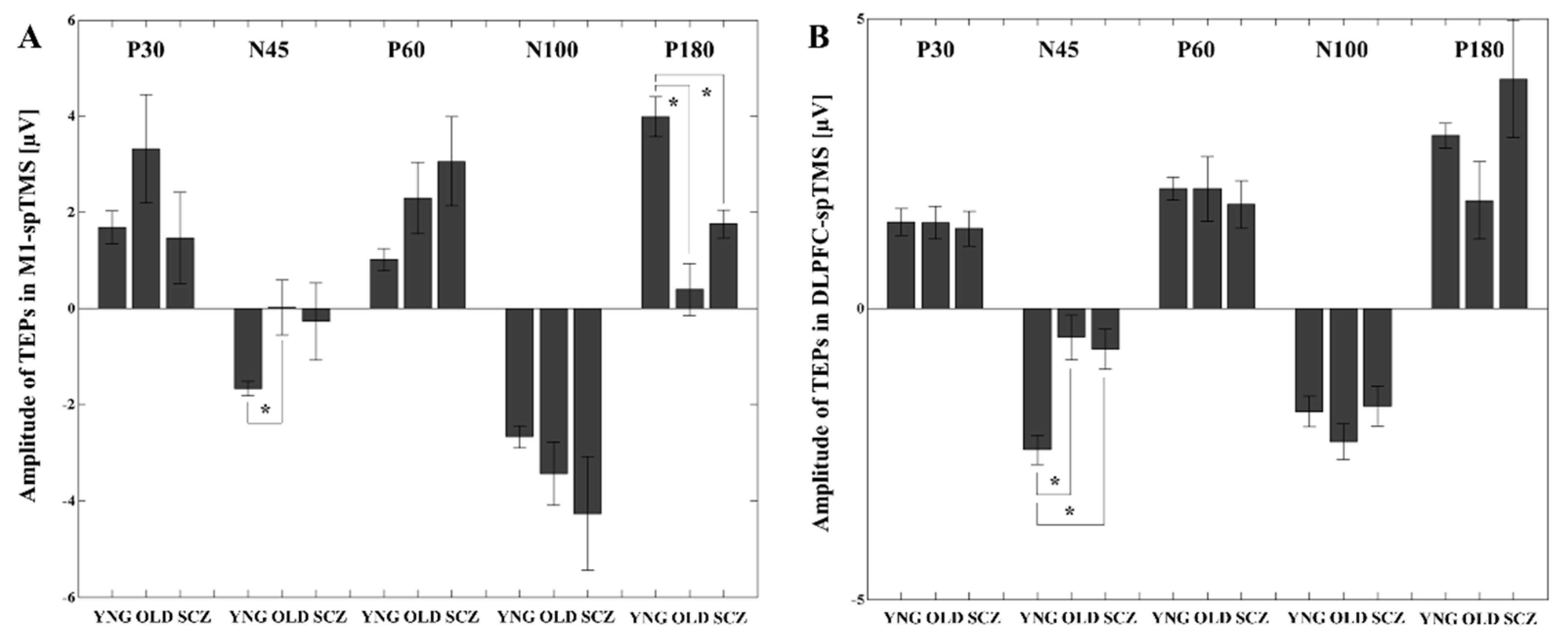
3.1. TEP Amplitude Differences between the YNG, OLD, and SCZ Groups
3.2. Predictive Factors to Classify the Groups Based on the Multiple Regression Analysis
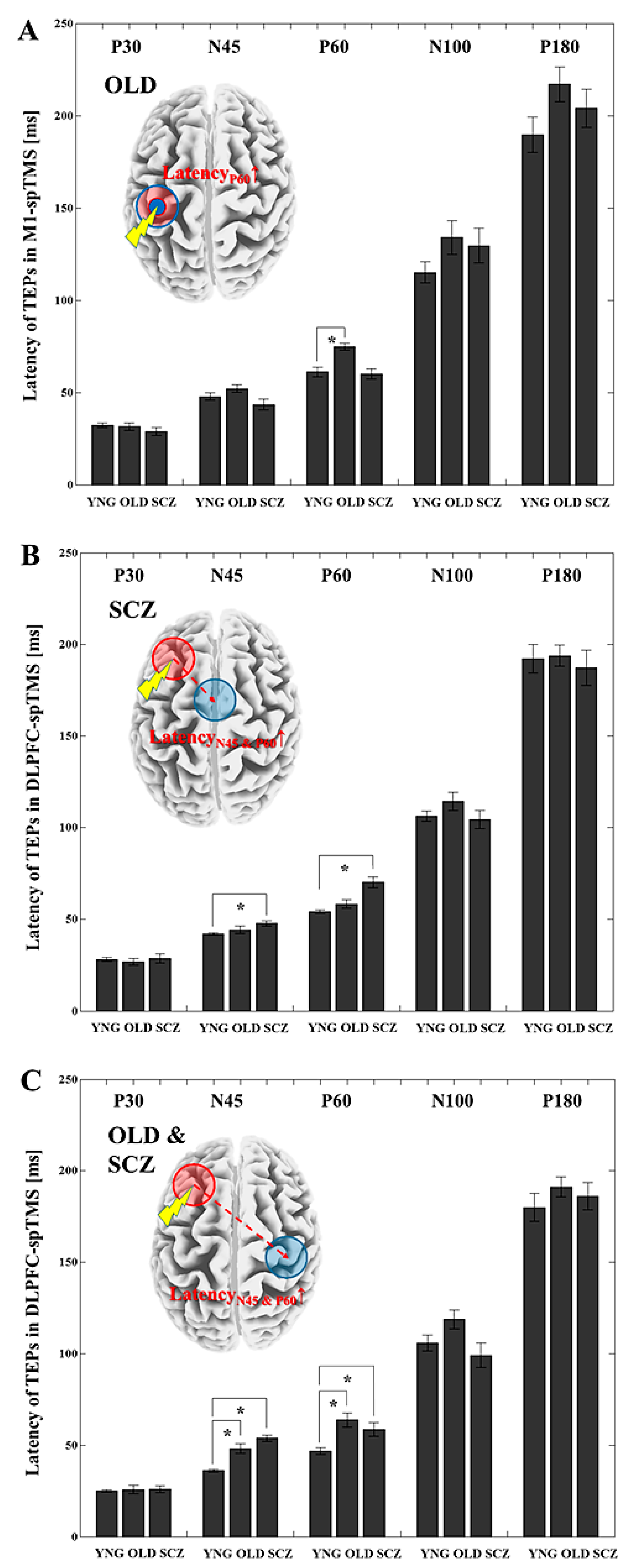
3.3. Latency Differences for Each TEP between the YNG, OLD, and SCZ Groups
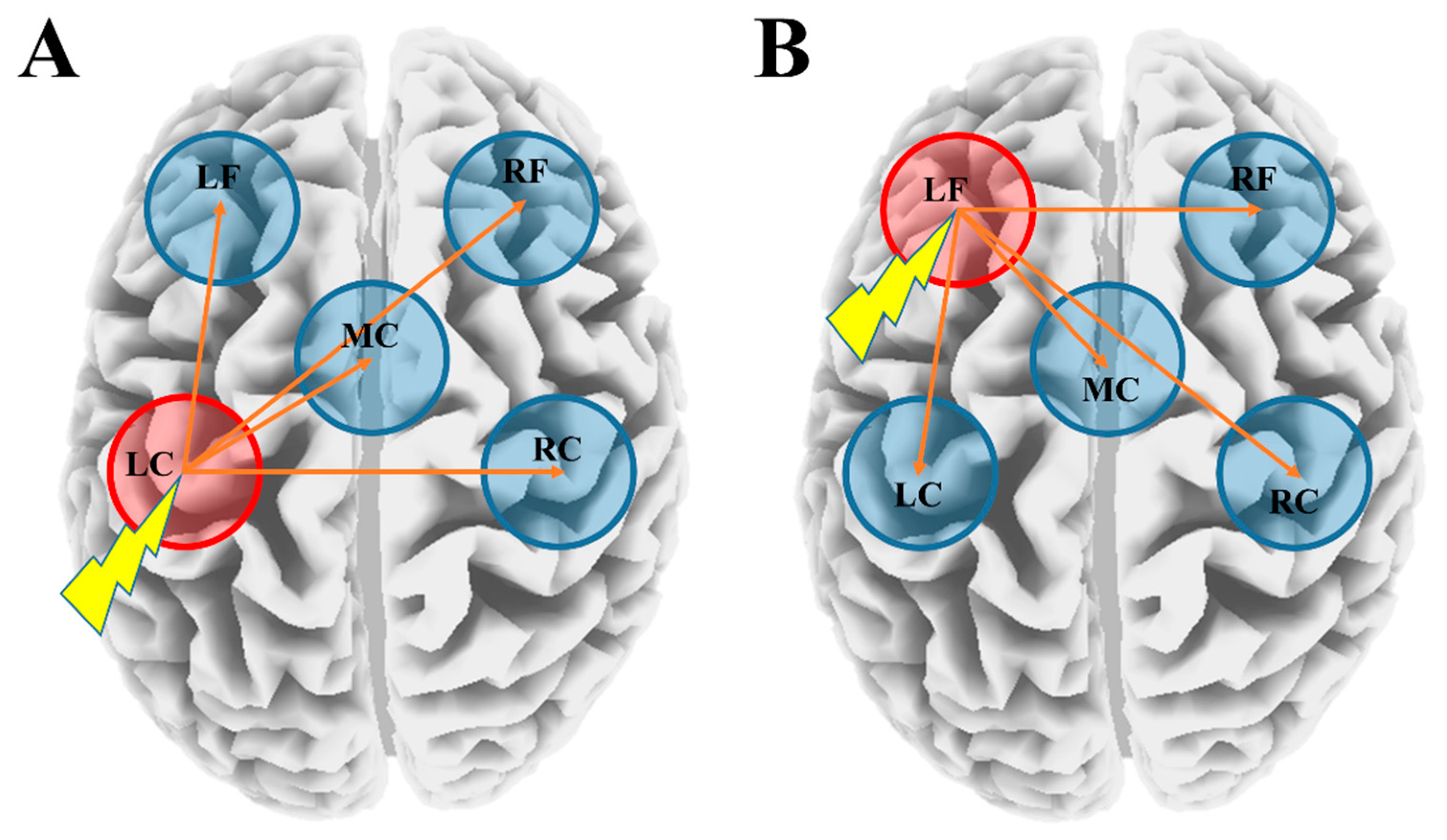
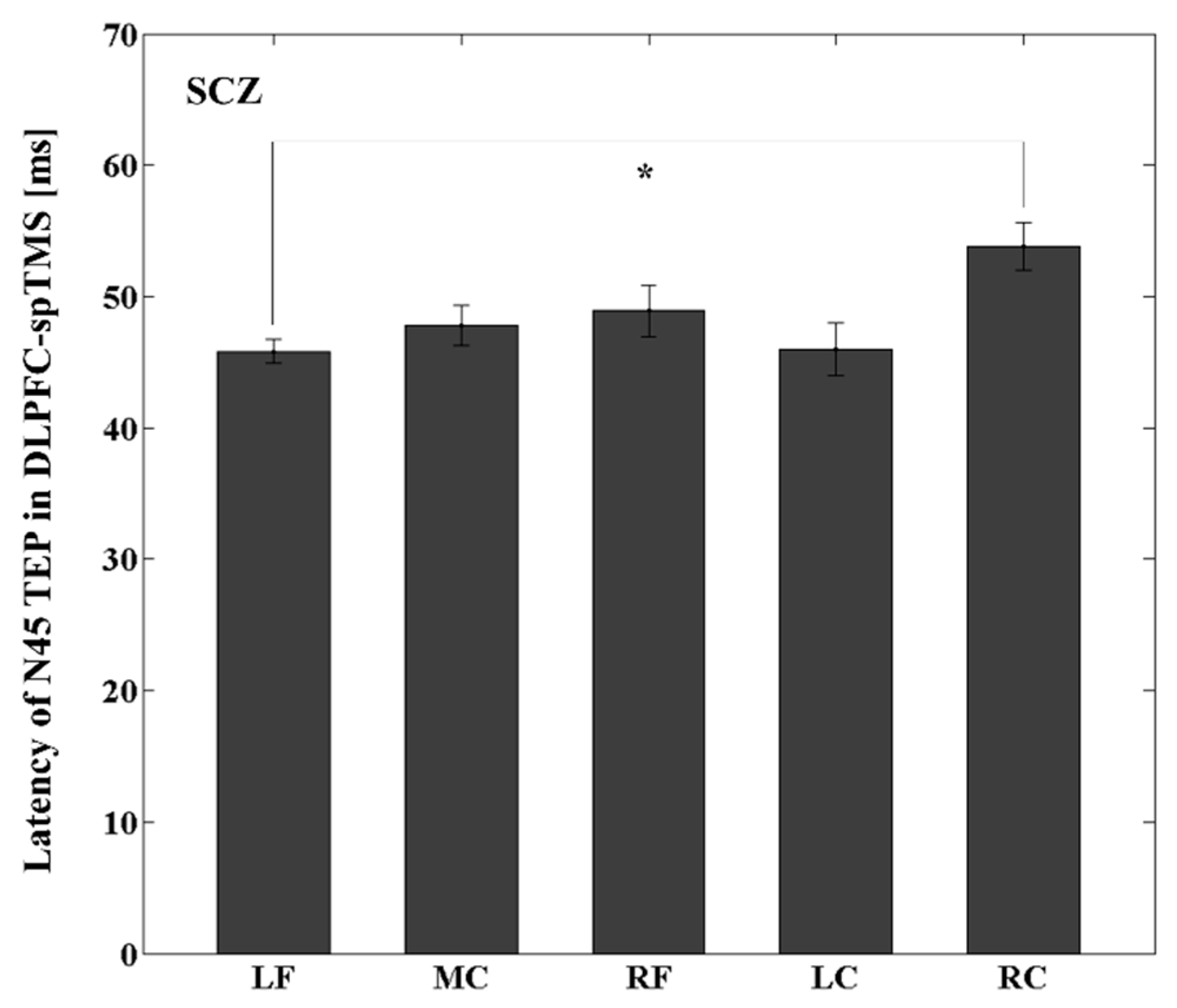
3.4. Latency Differences between the Stimulated ROI and Other ROIs within the YNG, OLD, and SCZ Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SpTMS | single-pulse transcranial magnetic stimulation |
| M1 | primary motor cortex |
| DLPFC | dorsolateral prefrontal cortex |
| TEP | TMS-evoked potential |
References
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, Z.J.; Farzan, F.; Barr, M.S.; Maller, J.J.; Chen, R.; Fitzgerald, P.B. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: A TMS-EEG study. Neuropsychopharmacology 2008, 33, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Farzan, F.; Barr, M.S.; Wong, W.; Chen, R.; Fitzgerald, P.B.; Daskalakis, Z.J. Suppression of gamma-oscillations in the dorsolateral prefrontal cortex following long interval cortical inhibition: A TMS-EEG study. Neuropsychopharmacology 2009, 34, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Maller, J.J.; Hoy, K.; Farzan, F.; Daskalakis, Z.J. GABA and cortical inhibition in motor and non-motor regions using combined TMS-EEG: A time analysis. Clin. Neurophysiol. 2009, 120, 1706–1710. [Google Scholar] [CrossRef]
- Fuggetta, G.; Fiaschi, A.; Manganotti, P. Modulation of cortical oscillatory activities induced by varying single-pulse transcranial magnetic stimulation intensity over the left primary motor area: A combined EEG and TMS study. Neuroimage 2005, 27, 896–908. [Google Scholar] [CrossRef]
- Paus, T.; Sipila, P.K.; Strafella, A.P. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: An EEG study. J. Neurophysiol. 2001, 86, 1983–1990. [Google Scholar] [CrossRef]
- Premoli, I.; Castellanos, N.; Rivolta, D.; Belardinelli, P.; Bajo, R.; Zipser, C.; Espenhahn, S.; Heidegger, T.; Müller-Dahlhaus, F.; Ziemann, U. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J. Neurosci. 2014, 34, 5603–5612. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Daskalakis, Y.J.; Fitzgerald, P.B. Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: A TMS-EEG study. Cortex 2015, 64, 68–77. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co-registration study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef]
- Lioumis, P.; Kičić, D.; Savolainen, P.; Mäkelä, J.P.; Kähkönen, S. Reproducibility of TMS-evoked EEG responses. Hum. Brain Mapp. 2009, 30, 1387–1396. [Google Scholar] [CrossRef]
- Ferreri, F.; Rossini, P.M. TMS and TMS-EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev. Neurosci. 2013, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Komssi, S.; Kahkonen, S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain. Res. Rev. 2006, 52, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Cash, R.F.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.B.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of glutamatergic and GABAA-Mediated neurotransmission in motor and dorsolateral prefrontal cortex using paired-pulse TMS-EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Komssi, S.; Kahkonen, S.; Ilmoniemi, R.J. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum. Brain Mapp. 2004, 21, 154–164. [Google Scholar] [CrossRef]
- Kahkonen, S.; Komssi, S.; Wilenius, J.; Ilmoniemi, R.J. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage 2005, 24, 955–960. [Google Scholar] [CrossRef]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural frequencies of human corticothalamic circuits. J. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef]
- Kahkonen, S.; Wilenius, J.; Komssi, S.; Ilmoniemi, R.J. Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin. Neurophysiol. 2004, 115, 583–588. [Google Scholar] [CrossRef]
- Kahkonen, S.; Komssi, S.; Wilenius, J.; Ilmoniemi, R.J. Prefrontal TMS produces smaller EEG responses than motor-cortex TMS: Implications for rTMS treatment in depression. Psychopharmacology 2005, 181, 16–20. [Google Scholar] [CrossRef]
- Casarotto, S.; Herukka, S.; Pigorini, A.; Napolitani, M.; Gosseries, O.; Niskanen, E.; Könönen, M.; Mervaala, E.; Rosanova, M.; Soininen, H.; et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport 2011, 22, 592–597. [Google Scholar] [CrossRef]
- Levit-Binnun, N.; Litvak, V.; Pratt, H.; Moses, E.; Zaroor, M.; Peled, A. Differences in TMS-evoked responses between schizophrenia patients and healthy controls can be observed without a dedicated EEG system. Clin. Neurophysiol. 2010, 121, 332–339. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Massimini, M.; Peterson, M.J.; Riedner, B.A.; Lazar, M.; Murphy, M.J.; Huber, R.; Rosanova, M.; Alexander, A.L.; Kalin, N.; et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: A TMS/EEG study. Am. J. Psychiatry 2008, 165, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Bagarinao, E.; Watanabe, H.; Maesawa, S.; Mori, D.; Hara, K.; Kawabata, K.; Yoneyama, N.; Ohdake, R.; Imai, K.; Masuda, M.; et al. An unbiased data-driven age-related structural brain parcellation for the identification of intrinsic brain volume changes over the adult lifespan. Neuroimage 2018, 169, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Konopaske, G.T.; Lange, N.; Coyle, J.T.; Benes, F.M. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 2014, 71, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Young, K.M. White matter plasticity in adulthood. Neuroscience 2014, 276, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Peters, A. The effects of normal aging on myelin and nerve fibers: A review. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef]
- Pajevic, S.; Basser, P.J.; Fields, R.D. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 2014, 276, 135–147. [Google Scholar] [CrossRef]
- Noda, Y. Toward the establishment of neurophysiological indicators for neuropsychiatric disorders using transcranial magnetic stimulation-evoked potentials: A systematic review. Psychiatry Clin. Neurosci. 2020, 74, 12–34. [Google Scholar] [CrossRef]
- Maatta, S.; Könönen, M.; Kallioniemi, E.; Lakka, T.; Lintu, N.; Lindi, V.; Ferreri, F.; Ponzo, D.; Säisänen, L. Development of cortical motor circuits between childhood and adulthood: A navigated TMS-HdEEG study. Hum. Brain Mapp. 2017, 38, 2599–2615. [Google Scholar] [CrossRef]
- Noda, Y.; Zomorrodi, R.; Cash, R.F.H.; Barr, M.S.; Farzan, F.; Rajji, T.K.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of the influence of age on GABAA and glutamatergic mediated functions in the dorsolateral prefrontal cortex using paired-pulse TMS-EEG. Aging 2017, 9, 556–572. [Google Scholar] [CrossRef]
- Noda, Y.; Barr, M.S.; Zomorrodi, R.; Cash, R.F.H.; Farzan, F.; Rajji, T.K.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Evaluation of short interval cortical inhibition and intracortical facilitation from the dorsolateral prefrontal cortex in patients with schizophrenia. Sci. Rep. 2017, 7, 17106. [Google Scholar] [CrossRef]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Measuring brain stimulation induced changes in cortical properties using TMS-EEG. Brain Stimul. 2015, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- McClintock, S.M.; Freitas, C.; Oberman, L.; Lisanby, S.H.; Pascual-Leone, A. Transcranial magnetic stimulation: A neuroscientific probe of cortical function in schizophrenia. Biol. Psychiatry 2011, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.H.; Davies, S.N.; Collingridge, G.L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J. Physiol. 1990, 424, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Deisz, R.A. GABA(B) receptor-mediated effects in human and rat neocortical neurones In Vitro. Neuropharmacology 1999, 38, 1755–1766. [Google Scholar] [CrossRef]
- Ferreri, F.; Pasqualetti, P.; Määttä, S.; Ponzo, D.; Ferrarelli, F.; Tononi, G.; Mervaala, E.; Miniussi, C.; Rossini, P.M. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 2011, 54, 90–102. [Google Scholar] [CrossRef]
- Peinemann, A.; Lehner, C.; Conrad, B.; Siebner, H.R. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci. Lett. 2001, 313, 33–36. [Google Scholar] [CrossRef]
- Marneweck, M.; Loftus, A.; Hammond, G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci. Res. 2011, 70, 408–414. [Google Scholar] [CrossRef]
- Heise, K.F.; Zimerman, M.; Hoppe, J.; Gerloff, C.; Wegscheider, K.; Hummel, F.C. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J. Neurosci. 2013, 33, 9039–9049. [Google Scholar] [CrossRef]
- Grachev, I.D.; Apkarian, A.V. Aging alters regional multichemical profile of the human brain: An. in vivo 1H-MRS study of young versus middle-aged subjects. J. Neurochem. 2001, 76, 582–593. [Google Scholar] [CrossRef]
- Grachev, I.D.; Swarnkar, A.; Szeverenyi, N.M.; Ramachandran, T.S.; Apkarian, A.V. Aging alters the multichemical networking profile of the human brain: An. In Vivo (1)H-MRS study of young versus middle-aged subjects. J. Neurochem. 2001, 77, 292–303. [Google Scholar] [CrossRef]
- Benes, F.M.; McSparren, J.; Bird, E.D.; SanGiovanni, J.P.; Vincent, S.L.B. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch. Gen. Psychiatry 1991, 48, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.M.; Kwok, E.W.; Vincent, S.L.; Todtenkopf, M.S. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol. Psychiatry 1998, 44, 88–97. [Google Scholar] [CrossRef]
- Woo, T.U.; Walsh, J.P.; Benes, F.M. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch. Gen. Psychiatry 2004, 61, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, S.; Huang, H.S. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res. Rev. 2006, 52, 293–304. [Google Scholar] [CrossRef]
- Benes, F.M.; Lim, B.; Matzilevich, D.; Walsh, J.P.; Subburaju, S.; Minns, M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. USA 2007, 104, 10164–10169. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ukai, S.; Kose, A.; Hashimoto, T.; Iwatani, J.; Okumura, M.; Tsuji, T.; Shinosaki, K. Reduction of cortical GABAergic inhibition correlates with working memory impairment in recent onset schizophrenia. Schizophr. Res. 2013, 146, 238–243. [Google Scholar] [CrossRef]
- Bridgman, A.C.; Barr, M.S.; Goodman, M.S.; Chen, R.; Rajji, T.K.; Daskalakis, Z.J.; George, T.P. Deficits in GABAA receptor function and working memory in non-smokers with schizophrenia. Schizophr. Res. 2016, 171, 125–130. [Google Scholar] [CrossRef]
- Umesawa, Y.; Matsushima, K.; Atsumi, T.; Kato, T.; Fukatsu, R.; Wada, M.; Ide, M. Altered GABA concentration in brain motor area is associated with the severity of motor disabilities in individuals with autism spectrum disorder. J. Autism Dev. Disord. 2020, 50, 2710–2722. [Google Scholar] [CrossRef]
- Gray, D.T.; Engle, J.R.; Rudolph, M.L.; Recanzone, G.H. Regional and age-related differences in GAD67 expression of parvalbumin- and calbindin-expressing neurons in the rhesus macaque auditory midbrain and brainstem. J. Comp. Neurol. 2014, 522, 4074–4084. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. TMS-EEG: TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci. Biobehav. Rev. 2016, 64, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Sullivan, C.M.; Rogasch, N.C.; Hoy, K.E.; Bailey, N.W.; Cash, R.F.H.; Fitzgerald, P.B. The effects of individualised intermittent theta burst stimulation in the prefrontal cortex: A TMS-EEG study. Hum. Brain Mapp. 2019, 40, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Deng, W.; He, Z.; Wang, Q.; Huang, C.; Jiang, L.; Gong, O.; Ziedonis, D.M.; King, J.A.; Ma, X.; et al. A splitting brain: Imbalanced neural networks in schizophrenia. Psychiatry Res. 2015, 232, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Di Santo, A.; Brown, K.; Ibáñez, J.; Casula, E.; Rawji, V.; Di Lazzaro, V.; Koch, G.; Rothwell, J. Disentangling EEG responses to TMS due to cortical and peripheral activations. Brain Stimul. 2020, 14, 4–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, Y.; Barr, M.S.; Zomorrodi, R.; Cash, R.F.H.; Lioumis, P.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients. J. Pers. Med. 2021, 11, 54. https://doi.org/10.3390/jpm11010054
Noda Y, Barr MS, Zomorrodi R, Cash RFH, Lioumis P, Chen R, Daskalakis ZJ, Blumberger DM. Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients. Journal of Personalized Medicine. 2021; 11(1):54. https://doi.org/10.3390/jpm11010054
Chicago/Turabian StyleNoda, Yoshihiro, Mera S. Barr, Reza Zomorrodi, Robin F. H. Cash, Pantelis Lioumis, Robert Chen, Zafiris J. Daskalakis, and Daniel M. Blumberger. 2021. "Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients" Journal of Personalized Medicine 11, no. 1: 54. https://doi.org/10.3390/jpm11010054
APA StyleNoda, Y., Barr, M. S., Zomorrodi, R., Cash, R. F. H., Lioumis, P., Chen, R., Daskalakis, Z. J., & Blumberger, D. M. (2021). Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients. Journal of Personalized Medicine, 11(1), 54. https://doi.org/10.3390/jpm11010054





