Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
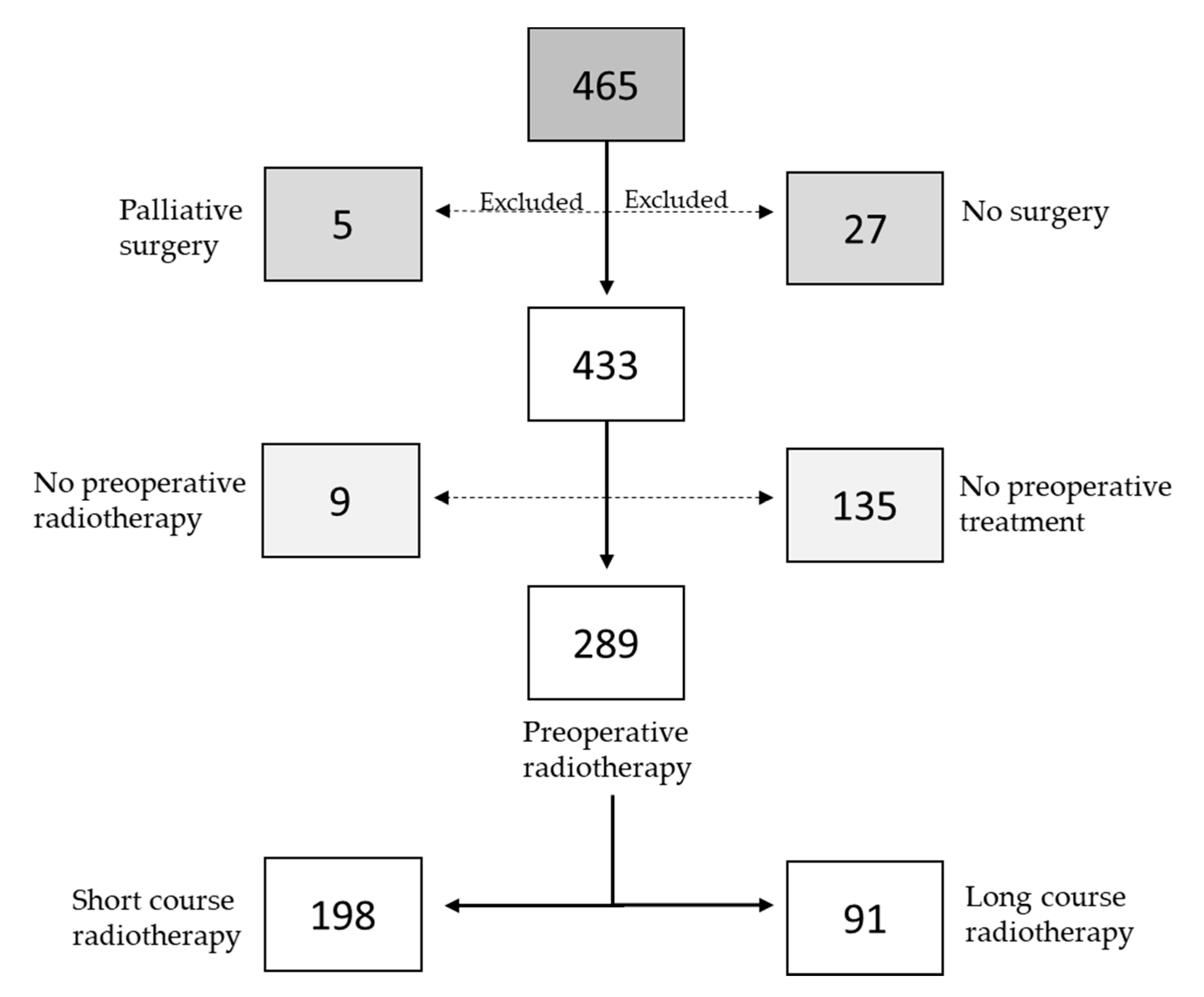
2.1. Patients
2.2. Data Collection
2.3. Staging and Evaluation of Treatment Effect
2.4. Treatment
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics in MAC and NMAC Patients
3.2. Survival in MAC and NMAC Patients
3.3. Survival in Relation to Preoperative RT in MAC and NMAC Patients
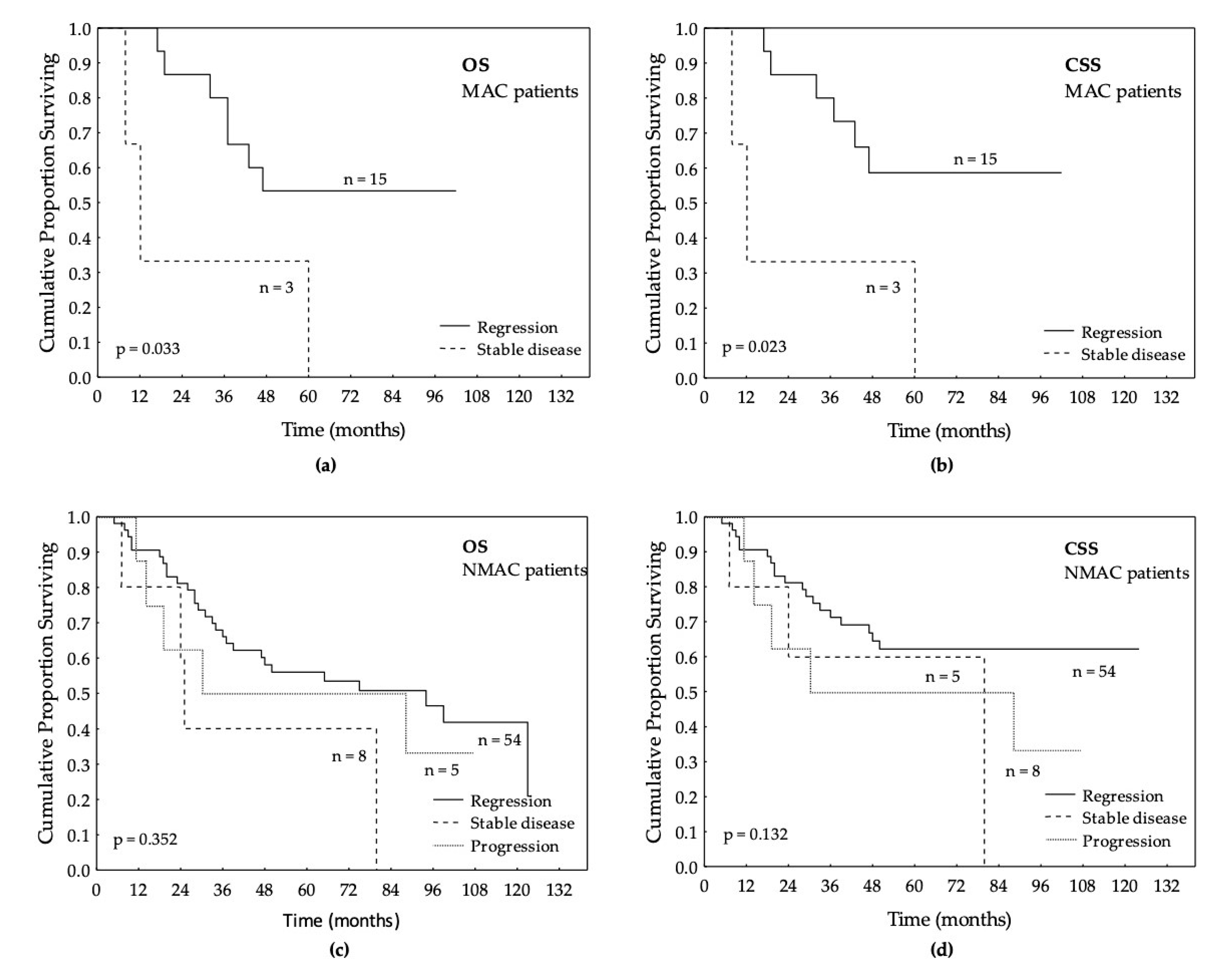
3.4. Local Response after LCRT in MAC and NMAC Patients’ Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| MAC | Mucinous adenocarcinoma |
| WHO | World Health organization |
| NMAC | Non-mucinous adenocarcinoma |
| CT | Chemotherapy |
| CRT | Chemoradiotherapy |
| RT | Radiotherapy |
| RC | Rectal cancer |
| SCRT | Short-course radiotherapy |
| LCRT | Long-course radiotherapy |
| TME | Total mesorectal excision |
| MRI | Magnetic resonance imaging |
| OS | Overall survival |
| CSS | Cancer specific survival |
| DFS | Disease-free survival |
| DRFS | Distant-recurrence-free survival |
| LRFS | Local-recurrence-free survival |
| APR | Abdominoperineal resection |
| TEM | Trans endoscopic microsurgery |
| HR | Hazard ratio |
| CI | Confidence interval |
| ESMO | European Society of Medical Oncology |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; O’Connell, J.B.; Maggard, M.A.; Sack, J.; Ko, C.Y. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis. Colon Rectum. 2005, 48, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Hyngstrom, J.R.; Hu, C.Y.; Xing, Y.; You, Y.N.; Feig, B.W.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Cormier, J.N.; Chang, G.J. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: Analysis from the National Cancer Data Base. Ann. Surg. Oncol. 2012, 19, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Kato, T.; Hirai, T.; Yasui, K.; Morimoto, T.; Shimizu, Y.; Kodera, Y.; Yamamura, Y. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis. Colon Rectum. 2003, 46, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Loupakis, F.; Graziano, F.; Bisonni, R.; Torresi, U.; Vincenzi, B.; Mari, D.; Giordani, P.; Alessandroni, P.; Salvatore, L.; et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann. Oncol. 2012, 23, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.M.; Yeh, C.Y.; Changchien, C.R.; Chen, J.S.; Tang, R.; Chen, J.R. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int. J. Colorectal Dis. 2010, 25, 941–947. [Google Scholar] [CrossRef]
- Melis, M.; Hernandez, J.; Siegel, E.M.; McLoughlin, J.M.; Ly, Q.P.; Nair, R.M.; Lewis, J.M.; Jensen, E.H.; Alvarado, M.D.; Coppola, D.; et al. Gene expression profiling of colorectal mucinous adenocarcinomas. Dis. Colon Rectum. 2010, 53, 936–943. [Google Scholar] [CrossRef]
- Umpleby, H.C.; Ranson, D.L.; Williamson, R.C. Peculiarities of mucinous colorectal carcinoma. Br. J. Surg. 1985, 72, 715–718. [Google Scholar] [CrossRef]
- Hugen, N.; van de Velde, C.J.; Bosch, S.L.; Fütterer, J.J.; Elferink, M.A.; Marijnen, C.A.; Rutten, H.J.; de Wilt, J.H.; Nagtegaal, I.D. Modern treatment of rectal cancer closes the gap between common adenocarcinoma and mucinous carcinoma. Ann. Surg. Oncol. 2015, 22, 2669–2676. [Google Scholar] [CrossRef]
- Nozoe, T.; Anai, H.; Nasu, S.; Sugimachi, K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J. Surg. Oncol. 2000, 75, 103–107. [Google Scholar] [CrossRef]
- Akino, F.; Mitomi, H.; Nakamura, T.; Ohtani, Y.; Ichinoe, M.; Okayasu, I. High apoptotic activity and low epithelial cell proliferation with underexpression of p21(WAF1/CIP1) and p27Kip1 of mucinous carcinomas of the colorectum: Comparison with well-differentiated type. Am. J. Clin. Pathol. 2002, 117, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.H.; Robey-Cafferty, S.S.; Cleary, K.R. Mucinous carcinomas of the colon and rectum. An analysis of 62 stage B and C lesions. Arch. Pathol. Lab. Med. 1991, 115, 1022–1025. [Google Scholar] [PubMed]
- Halvorsen, T.B.; Seim, E. Influence of mucinous components on survival in colorectal adenocarcinomas: A multivariate analysis. J. Clin. Pathol. 1988, 41, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Verhoeven, R.H.; Radema, S.A.; de Hingh, I.H.; Pruijt, J.F.; Nagtegaal, I.D.; Lemmens, V.E.; de Wilt, J.H. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann. Oncol. 2013, 24, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Song, Y.X.; Xu, Y.Y.; Sun, Z.; Sun, J.X.; Xu, H.M.; Wang, Z. Does the prognosis of colorectal mucinous carcinoma depend upon the primary tumour site? Results from two independent databases. Histopathology 2013, 63, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.C.; Yang, X.Y.; Wang, Z.Q. Prognostic significance of the mucin component in stage III rectal carcinoma patients. Asian Pac. J. Cancer Prev. 2014, 15, 8101–8105. [Google Scholar] [CrossRef][Green Version]
- Lupinacci, R.M.; Mello, E.S.; Coelho, F.F.; Kruger, J.A.; Perini, M.V.; Pinheiro, R.S.; Fonseca, G.M.; Cecconello, I.; Herman, P. Prognostic implication of mucinous histology in resected colorectal cancer liver metastases. Surgery 2014, 155, 1062–1068. [Google Scholar] [CrossRef]
- Shin, U.S.; Yu, C.S.; Kim, J.H.; Kim, T.W.; Lim, S.B.; Yoon, S.N.; Yoon, Y.S.; Kim, C.W.; Kim, J.C. Mucinous rectal cancer: Effectiveness of preoperative chemoradiotherapy and prognosis. Ann. Surg. Oncol. 2011, 18, 2232–2239. [Google Scholar] [CrossRef]
- Camma, C.; Giunta, M.; Fiorica, F.; Pagliaro, L.; Craxi, A.; Cottone, M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA 2000, 284, 1008–1015. [Google Scholar] [CrossRef]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8507 patients from 22 randomised trials. Meta-Analysis 2001, 358, 1291–1304. [Google Scholar]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Aristei, C.; Glimelius, B.; Minsky, B.D.; Beets-Tan, R.; Borras, J.M.; Haustermans, K.; Maingon, P.; Overgaard, J.; Pahlman, L.; et al. Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother. Oncol. 2009, 92, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, B.; Lu, X.; Bai, R.; Feng, L.; Wang, Q.; Zhao, Y.; He, S. Preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer: Meta-analysis with trial sequential analysis of long-term survival data. PloS ONE. 2018, 13, e0200142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.R.; Liu, S.X.; Zhang, T.S.; Chen, L.X.; Xia, J.; Hu, Z.D.; Li, B. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2014, 23, 211–221. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallbook, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Yoo, R.N.; Kim, H.J. Total neoadjuvant therapy in locally advanced rectal cancer: Role of systemic chemotherapy. Ann. Gastroenterol. Surg. 2019, 3, 356–367. [Google Scholar] [CrossRef]
- Cedermark, B.; Dahlberg, M.; Glimelius, B.; Pahlman, L.; Rutqvist, L.E.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar]
- Pahlman, L.; Glimelius, B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann. Surg. 1990, 211, 187–195. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- Grillo-Ruggieri, F.; Mantello, G.; Berardi, R.; Cardinali, M.; Fenu, F.; Iovini, G.; Montisci, M.; Fabbietti, L.; Marmorale, C.; Guerrieri, M.; et al. Mucinous rectal adenocarcinoma can be associated to tumor downstaging after preoperative chemoradiotherapy. Dis. Colon. Rectum. 2007, 50, 1594–1603. [Google Scholar] [CrossRef]
- Zhang, H.; Evertsson, S.; Sun, X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int. J. Oncol. 1999, 14, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Krynski, J.; Michalski, W.; Oledzki, J.; Kusnierz, J.; Zajac, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Brown, G.; Glynne-Jones, R.; de Wilt, J.H.; Nagtegaal, I.D. Advances in the care of patients with mucinous colorectal cancer. Nat. Rev. Clin. Oncol. 2016, 13, 361–369. [Google Scholar] [CrossRef] [PubMed]

| All Patients | MAC | NMAC | p-Values 1 | |
|---|---|---|---|---|
| n = 433 (100%) | n = 54 (12%) | n = 379 (88%) | ||
| n (%) | n (%) | n (%) | ||
| Preoperative RT | 0.361 | |||
| Yes | 289 | 39 (72) | 250 (66) | |
| No | 144 | 15 (28) | 129 (34) | |
| Type of RT | 0.012 | |||
| SCRT | 198 | 20 (51) | 178 (71) | |
| LCRT | 91 | 19 (49) | 72 (29) | |
| Response after LCRT | 85 | 18 | 67 | 0.449 |
| Regression (partial or complete) | 69 | 15 (83) | 54 (81) | |
| Stable disease | 11 | 3 (17) | 8 (12) | |
| Progression | 5 | 0 | 5 (7) | |
| Type of surgery | 0.718 | |||
| APR | 158 | 22 (41) | 136 (36) | |
| TEM | 28 | 2 (4) | 26 (7) | |
| TME | 242 | 29 (54) | 213 (56) | |
| Proctocolectomy | 5 | 1 (2) | 4 (1) | |
| Resection margin | 0.005 | |||
| No tumor cells (R0) | 396 | 44 (81) | 352 (93) | |
| Remaining tumor cells (R1) | 37 | 10 (19) | 27 (7) | |
| Distance to anal verge (cm) | 0.815 | |||
| Mean (cm) | 8.0 | 7.5 | 8.1 | |
| Perioperative CT | <0.001 | |||
| Yes | 129 | 27 (50) | 102 (27) | |
| No | 304 | 27 (50) | 277 (73) | |
| Local recurrence | 0.796 | |||
| Yes | 36 | 4 (7) | 32 (8) | |
| No | 397 | 50 (93) | 347 (92) | |
| Distant recurrence | 0.009 | |||
| Yes | 120 | 23 (43) | 97 (26) | |
| No | 313 | 31 (57) | 282 (74) |
| All Patients | MAC | NMAC | p-Values 1 | |
|---|---|---|---|---|
| n = 433 (%) | n = 54 (%) | n = 379 (%) | ||
| Sex | 0.785 | |||
| Male | 266 | 30 (56) | 248 (58) | |
| Female | 185 | 24 (44) | 161 (42) | |
| Age (years) | 0.267 | |||
| ≤69 | 202 | 29 (54) | 173 (46) | |
| >69 | 231 | 25 (46) | 206 (54) | |
| TNM stage | 0.015 | |||
| I | 129 | 7 (13) | 122 (32) | |
| IIA | 110 | 10 (19) | 100 (26) | |
| IIB | 10 | 2 (4) | 8 (2) | |
| IIIA | 17 | 2 (4) | 15 (4) | |
| IIIB | 57 | 11 (20) | 46 (12) | |
| IIIC | 56 | 11 (20) | 45 (12) | |
| IV | 54 | 11 (20) | 43 (11) | |
| T-stage | 0.008 | |||
| T1 | 49 | 0 | 49 (13) | |
| T2 | 113 | 11 (20) | 102 (27) | |
| T3 | 230 | 35 (65) | 195 (52) | |
| T4 | 38 | 8 (15) | 30 (8) | |
| Unknown | 3 | 0 | 3 (1) | |
| N-stage | 0.013 | |||
| N0 | 244 | 21 (41) | 223 (63) | |
| N1 | 86 | 15 (29) | 71 (20) | |
| N2 | 77 | 15 (29) | 62 (17) | |
| Unknown | 26 | 3 (6) | 23 (6) | |
| Vascular invasion | 0.676 | |||
| Yes | 87 | 12 (22) | 75 (20) | |
| No | 346 | 42 (78) | 304 (80) | |
| Perineural growth | 0.340 | |||
| Yes | 58 | 5 (9) | 53 (14) | |
| No | 375 | 49 (91) | 326 (86) | |
| Differentiation | <0.001 | |||
| Well | 42 | 1 (2) | 41 (11) | |
| Moderately | 287 | 19 (35) | 251 (66) | |
| Poorly | 132 | 34 (63) | 87 (23) |
| All Patients | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR 1 (95% CI) | p-value | HR 1 (95% CI) | p-value | |
| Overall survival | 0.55 (0.38–0.80) | 0.002 | 0.59 (0.41–0.87) | 0.007 |
| Cancer-specific survival | 0.47 (0.30–0.73) | <0.001 | 0.57 (0.37–0.90) | 0.016 |
| Disease-free survival | 0.54 (0.36–0.80) | 0.002 | 0.62 (0.41–0.93) | 0.021 |
| Distant-recurrence-free survival | 0.52 (0.34–0.79) | 0.002 | 0.58 (0.38–0.90) | 0.014 |
| Local-recurrence-free survival | 0.41 (0.18–0.91) | 0.028 | 0.48 (0.21–1.08) | 0.076 |
| NMAC | ||||
| Univariate | Multivariate | |||
| HR 1 (95% CI) | p-value | HR 1 (95% CI) | p-value | |
| Overall survival | 0.53 (0.35–0.80) | 0.003 | 0.57 (0.37–0.88) | 0.011 |
| Cancer-specific survival | 0.43 (0.26–0.72) | 0.001 | 0.52 (0.30–0.88) | 0.014 |
| Disease-free survival | 0.46 (0.30–0.72) | <0.001 | 0.46 (0.29–0.74) | 0.001 |
| Distant-recurrence-free survival | 0.45 (0.28–0.72) | <0.001 | 0.44 (0.27–0.72) | 0.001 |
| Local-recurrence-free survival | 0.39 (0.17–0.93) | 0.033 | 0.45 (0.19–1.07) | 0.070 |
| MAC | ||||
| Univariate | Multivariate | |||
| HR 1 (95% CI) | p-value | HR 1 (95% CI) | p-value | |
| Overall survival | 0.86 (0.37–2.03) | 0.736 | 0.50 (0.19–1.30) | 0.157 |
| Cancer-specific survival | 0.94 (0.39–2.27) | 0.896 | 0.54 (0.28–2.34) | 0.209 |
| Disease-free survival | 1.55 (0.62–3.86) | 0.347 | 1.22 (0.45–3.29) | 0.696 |
| Distant-recurrence-free survival | 1.49 (0.60–3.72) | 0.393 | 1.33 (0.50–3.52) | 0.572 |
| Local-recurrence-free survival | 0.49 (0.04–5.36) | 0.555 | - 2 | - 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernmark, K.; Sun, X.-F.; Holmqvist, A. Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy. J. Pers. Med. 2020, 10, 226. https://doi.org/10.3390/jpm10040226
Vernmark K, Sun X-F, Holmqvist A. Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy. Journal of Personalized Medicine. 2020; 10(4):226. https://doi.org/10.3390/jpm10040226
Chicago/Turabian StyleVernmark, Karolina, Xiao-Feng Sun, and Annica Holmqvist. 2020. "Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy" Journal of Personalized Medicine 10, no. 4: 226. https://doi.org/10.3390/jpm10040226
APA StyleVernmark, K., Sun, X.-F., & Holmqvist, A. (2020). Mucinous and Non-Mucinous Rectal Adenocarcinoma—Differences in Treatment Response to Preoperative Radiotherapy. Journal of Personalized Medicine, 10(4), 226. https://doi.org/10.3390/jpm10040226






