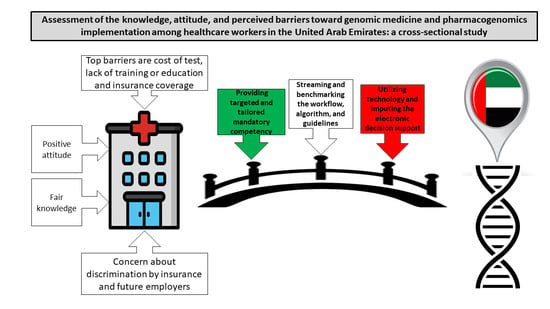
Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Assessment of General Knowledge on Genetics and PGX
3.2. Attitudes towards the Applications of PGX
3.2.1. Attitudes on Genetic Testing
3.2.2. Concerns and Ethics
3.2.3. Desire to Participate
3.2.4. Current and Future Outlook on PGX
3.2.5. Barriers to Implementation
3.2.6. Type of Preferred Education
3.3. Assessment of Personal Knowledge and Attitudes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chanfreau-Coffinier, C.; Hull, L.E.; Lynch, J.A.; DuVall, S.L.; Damrauer, S.M.; Cunningham, F.E.; Voight, B.F.; Matheny, M.E.; Oslin, D.W.; Icardi, M.S.; et al. Projected Prevalence of Actionable Pharmacogenetic Variants and Level A Drugs Prescribed among US Veterans Health Administration Pharmacy Users. JAMA Netw. Open 2019, 2, e195345. [Google Scholar] [CrossRef]
- Bush, W.S.; Crosslin, D.R.; Owusu-Obeng, A.; Wallace, J.; Almoguera, B.; Basford, M.A.; Bielinski, S.J.; Carrell, D.S.; Connolly, J.J.; Crawford, D.; et al. Genetic variation among 82 pharmacogenes: The PGRNseq data from the eMERGE network. Clin. Pharmacol. Ther. 2016, 100, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Burke, W.; Ginsburg, G.; Mills, R.; Agans, R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin. Genet. 2012, 82, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Muzoriana, N.; Gavi, S.; Nembaware, V.; Dhoro, M.; Matimba, A. Knowledge, Attitude, and Perceptions of Pharmacists and Pharmacy Students towards Pharmacogenomics in Zimbabwe. Pharmacy 2017, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, C.; Tsermpini, E.E.; Mavroidi, E.; Katsila, T.; Patrinos, G.P.; Squassina, A. Assessment of the pharmacogenomics educational environment in southeast Europe. Public Health Genom. 2014, 17, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Y.; Mosleh, R.; Hawash, M.; Jarrar, Q. Knowledge and attitudes of pharmacy students towards pharmacogenomics among universities in Jordan and west bank of Palestine. Pharmgenomics Pers. Med. 2019, 12, 247–255. [Google Scholar] [CrossRef]
- Christianson, A.; Howson, C.P.; Modell, B. March of Dimes Global Report on Birth Defects: The Hidden Toll of Dying and Disabled Children; March of Dimes: White Plains, NY, USA, 2006. [Google Scholar]
- Al-Gazali, L.; Ali, B.R. Mutations of a country: A mutation review of single gene disorders in the United Arab Emirates (UAE). Hum. Mutat. 2010, 31, 505–520. [Google Scholar] [CrossRef]
- Rogausch, A.; Prause, D.; Schallengerb, A.; Brockmöller, J.; Himmel, W. Patients’ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics 2006, 7, 49–59. [Google Scholar] [CrossRef]
- Mai, Y.; Mitropoulou, C.; Papadopoulou, X.E.; Vozikis, A.; Cooper, D.N.; Van Schaik, R.H.; Patrinos, G.P. Critical appraisal of the views of healthcare professionals with respect to pharmacogenomics and personalized medicine in Greece. Per. Med. 2014, 11, 15–26. [Google Scholar] [CrossRef]
- Formea, C.M.; Nicholson, W.T.; McCullough, K.B.; Berg, K.D.; Berg, M.L.; Cunningham, J.L.; Merten, J.A.; Ou, N.N.; Stollings, J.L. Development and evaluation of a pharmacogenomics educational program for pharmacists. Am. J. Pharm. Educ. 2013, 77. [Google Scholar] [CrossRef]
- Mccullough, K.B.; Formea, C.M.; Berg, K.D.; Burzynski, J.A.; Cunningham, J.L.; Ou, N.N.; Rudis, M.I.; Stollings, J.L.; Nicholson, W.T. Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 2011, 75. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.; Alshammari, S.; Ouda, G.; Koshy, S.; Awad, A. Knowledge, perceptions and confidence of physicians and pharmacists towards pharmacogenetics practice in Kuwait. PLoS ONE 2018, 13, e0203033. [Google Scholar] [CrossRef]
- Elewa, H.; Alkhiyami, D.; Alsahan, D.; Abdel-Aziz, A. A survey on the awareness and attitude of pharmacists and doctors towards the application of pharmacogenomics and its challenges in Qatar. J. Eval. Clin. Pract. 2015, 21, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Dodson, C. Knowledge and attitudes concerning pharmacogenomics among healthcare professionals. Per. Med. 2011, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Toomula, N.; Bindu, H. Pharmacogenomics-Personalized Treatment of Cancer, Diabetes and Cardiovascular Diseases. J. Pharm. Pharm. 2012, 3, 1000107. [Google Scholar] [CrossRef]
- WHO. World Health Organization-Noncommunicable Diseases United Arab Emirates Profile. Available online: https://www.who.int/nmh/countries/are_en.pdf (accessed on 23 October 2020).
- Karrar, S. CVD Preventive Health Measures and Public Health. Available online: https://www.haad.ae/CME/LinkClick.aspx?fileticket=u-GO5_LDri4= (accessed on 23 October 2020).
- Gurwitz, D. Pharmacogenetics education: 10 years of experience at Tel Aviv University. Pharmacogenomics 2010, 11, 647–649. [Google Scholar] [CrossRef]
- Abdela, O.A.; Bhagavathula, A.S.; Gebreyohannes, E.A.; Tegegn, H.G. Ethiopian health care professionals’ knowledge, attitude, and interests toward pharmacogenomics. Pharmgenomics Pers. Med. 2017, 10, 279–285. [Google Scholar] [CrossRef]
- Johansen Taber, K.A.; Dickinson, B.D. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers. Med. 2014, 7, 145–162. [Google Scholar] [CrossRef]
- Kudzi, W.; Addy, B.S.; Dzudzor, B. Knowledge of Pharmacogenetics among Healthcare Professionals and Faculty Members of Health Training Institutions in Ghana. Ghana Med. J. 2015, 49, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Chen, W.J.; Yeh, Y.L.; Foster, M.; Al-Shagrawi, S.; Chen, L.S. Characteristics and evaluation outcomes of genomics curricula for health professional students: A systematic literature review. Genet. Med. 2019, 21, 1675–1682. [Google Scholar] [CrossRef]
- Squiers, L.; Peinado, S.; Berkman, N.; Boudewyns, V.; McCormack, L. The health literacy skills framework. J. Health Commun. 2012, 17, 30–54. [Google Scholar] [CrossRef]
- Carver, R.B.; Castéra, J.; Gericke, N.; Evangelista, N.A.M.; El-Hani, C.N. Young adults’ belief in genetic determinism, and knowledge and attitudes towards modern genetics and genomics: The PUGGS questionnaire. PLoS ONE 2017, 12, e0169808. [Google Scholar] [CrossRef]
- Morash, M.; Mitchell, H.; Beltran, H.; Elemento, O.; Pathak, J. The role of next-generation sequencing in precision medicine: A review of outcomes in oncology. J. Pers. Med. 2018, 8, 30. [Google Scholar] [CrossRef]
- Singh, D.B. The Impact of Pharmacogenomics in Personalized Medicine. Adv. Biochem. Eng. Biotechnol. 2020, 171, 369–394. [Google Scholar] [CrossRef]
- Morganti, S.; Tarantino, P.; Ferraro, E.; D’Amico, P.; Duso, B.; Curigliano, G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. In Translational Research and Onco-Omics Applications in the Era of Cancer Personal Genomics; Springer: Cham, Switzerland, 2019; Volume 1168, pp. 9–30. [Google Scholar] [CrossRef]
- Relling, M.V.; Altman, R.B.; Goetz, M.P.; Evans, W.E. Clinical implementation of pharmacogenomics: Overcoming genetic exceptionalism. Lancet Oncol. 2010, 11, 507–509. [Google Scholar] [CrossRef][Green Version]
- Crews, K.R.; Hicks, J.K.; Pui, C.H.; Relling, M.V.; Evans, W.E. Pharmacogenomics and individualized medicine: Translating science into practice. Clin. Pharmacol. Ther. 2012, 92, 467–475. [Google Scholar] [CrossRef]
- Relling, M.V.; Klein, T.E. CPIC: Clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef]
- Cavallari, L.; Weitzel, K.; Elsey, A.; Liu, X.; Mosley, S.; Smith, D.; Staley, B.; Winterstein, A.; Mathews, C.; Franchi, F.; et al. Institutional profile: University of Florida Health Personalized Medicine Program. Pharmacogenomics 2017, 18, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.O.; Fei, K.; Levy, K.D.; Elsey, A.R.; Pollin, T.I.; Ramirez, A.H.; Weitzel, K.W.; Horowitz, C.R. Physician-reported benefits and barriers to clinical implementation of genomic medicine: A multi-site ignite-network survey. J. Pers. Med. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Swan, M. Health 2050: The realization of personalized medicine through crowdsourcing, the quantified self, and the participatory biocitizen. J. Pers. Med. 2012, 2, 93–118. [Google Scholar] [CrossRef]
- Mason-Suares, H.; Sweetser, D.A.; Lindeman, N.I.; Morton, C.C. Training the future leaders in personalized medicine. J. Pers. Med. 2016, 6, 1. [Google Scholar] [CrossRef]
- Feero, W.G.; Eric, D. Green Genomics education for health care professionals in the 21st century. JAMA J. Am. Med. Assoc. 2011, 306, 989–990. [Google Scholar] [CrossRef]
- Nagy, M.; Lynch, M.; Kamal, S.; Mohamed, S.; Hadad, A.; Abouelnaga, S.; Aquilante, C.L. Assessment of healthcare professionals’ knowledge, attitudes, and perceived challenges of clinical pharmacogenetic testing in Egypt. Per. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Júnior, L.C.; Carvalho Júnior, P.M.; de Faria Ferraz, V.E.; Nascimento, L.C.; Van Riper, M.; Flória-Santos, M. Genetic education, knowledge and experiences between nurses and physicians in primary care in Brazil: A cross-sectional study. Nurs. Health Sci. 2017, 19, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.A.; Mohamed Shaqran, T.; Elfoutoh Eltobgy, A.A.; Albalawi, A.R.; Alnawmasi, W.S. Physicians’ perspective on diabetes mellitus management within the context of personalized medicine era in Tabuk governorate, Saudi Arabia. Open Access Maced. J. Med. Sci. 2019, 7, 1706–1711. [Google Scholar] [CrossRef]
- Ya’u, A.; Husain, R.B.; Haque, M. A systematic review of knowledge, attitude and practice towards pharmacogenomics among doctors. Int. J. Pharm. Res. 2015, 7, 9–16. [Google Scholar]
- Rahawi, S.; Naik, H.; Blake, K.V.; Owusu Obeng, A.; Wasserman, R.M.; Seki, Y.; Funanage, V.L.; Oishi, K.; Scott, S.A. Knowledge and attitudes on pharmacogenetics among pediatricians. J. Hum. Genet. 2020, 65, 437–444. [Google Scholar] [CrossRef]
- De Denus, S.; Letarte, N.; Hurlimann, T.; Lambert, J.P.; Lavoie, A.; Robb, L.; Sheehan, N.L.; Turgeon, J.; Vadnais, B. An evaluation of pharmacists expectations towards pharmacogenomics. Pharmacogenomics 2013, 14, 165–175. [Google Scholar] [CrossRef]
- Bernhardt, B.A.; Zayac, C.; Gordon, E.S.; Wawak, L.; Pyeritz, R.E.; Gollust, S.E. Incorporating direct-to-consumer genomic information into patient care: Attitudes and experiences of primary care physicians. Per. Med. 2012, 9, 683–692. [Google Scholar] [CrossRef]
- Powell, K.P.; Cogswell, W.A.; Christianson, C.A.; Dave, G.; Verma, A.; Eubanks, S.; Henrich, V.C. Primary care physicians’ awareness, experience and opinions of direct-to-consumer genetic testing. J. Genet. Couns. 2012, 21, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, J.; Schwartz, C.R.; Esteve, A. The reversal of the gender gap in education and its consequences for family life. Annu. Rev. Sociol. 2018, 44, 341–360. [Google Scholar] [CrossRef]
- Quenzel, G.; Hurrelmann, K. The growing gender gap in education. Int. J. Adolesc. Youth 2013, 18, 69–84. [Google Scholar] [CrossRef]
- Tayoun, A.A.; Loney, T.; Khansaheb, H.; Ramaswamy, S.; Aldabal, L.M.; Uddin, M.; Hamoudi, R.; Halwani, R.; Senok, A.; Hamid, Q.; et al. Whole genome sequencing and phylogenetic analysis of SARS-CoV-2 strains from the index and early patients with COVID-19 in Dubai, United Arab Emirates, 29 January to 18 March 2020. bioRxiv 2020, 1–11. [Google Scholar] [CrossRef]
- AlSafar, H.S.; Al-Ali, M.; Elbait, G.D.; Al-Maini, M.H.; Ruta, D.; Peramo, B.; Henschel, A.; Tay, G.K. Introducing the first whole genomes of nationals from the United Arab Emirates. Sci. Rep. 2019, 9, 14725. [Google Scholar] [CrossRef]
- Al-Ali, M.; Osman, W.; Tay, G.K.; Alsafar, H.S. A 1000 Arab genome project to study the Emirati population. J. Hum. Genet. 2018, 63, 533–536. [Google Scholar] [CrossRef]
- Al-Mahayri, Z.N.; Al Jaibeji, H.S.; Saab, Y.; Soliman, K.; Al-Gazali, L.; Patrinos, G.P.; Ali, B.R. Vkorc1 variants as significant predictors of Warfarin dose in Emiratis. Pharmgenomics. Pers. Med. 2019, 12, 47–57. [Google Scholar] [CrossRef]
- Ali Alhmoudi, O.; Jones, R.J.; Tay, G.K.; Alsafar, H.; Hadi, S. Population genetics data for 21 autosomal STR loci for United Arab Emirates (UAE) population using next generation multiplex STR kit. Forensic Sci. Int. Genet. 2015, 19, 190–191. [Google Scholar] [CrossRef]
- Akawi, N.A.; Al-Gazali, L.; Ali, B.R. Clinical and molecular analysis of UAE fibrochondrogenesis patients expands the phenotype and reveals two COL11A1 homozygous null mutations. Clin. Genet. 2012, 82, 147–156. [Google Scholar] [CrossRef]
- Osman, W.; Tay, G.K.; Alsafar, H. Multiple genetic variations confer risks for obesity and type 2 diabetes mellitus in arab descendants from UAE. Int. J. Obes. 2018, 42, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Shaya, B.; Al Homsi, N.; Eid, K.; Haidar, Z.; Khalil, A.; Merheb, K.; Honein-Abou Haidar, G.; Akl, E.A. Factors associated with the public’s trust in physicians in the context of the Lebanese healthcare system: A qualitative study. BMC Health Serv. Res. 2019, 19, 525. [Google Scholar] [CrossRef]
- Gupta, N.; Thiele, C.M.; Daum, J.I.; Egbert, L.K.; Chiang, J.S.; Kilgore, A.E.; Johnson, C.D. Building Patient-Physician Trust: A Medical Student Perspective. Acad. Med. 2020, 95, 980–983. [Google Scholar] [CrossRef]
- Bonter, K.; Desjardins, C.; Currier, N.; Pun, J.; Ashbury, F.D. Personalised medicine in Canada: A survey of adoption and practice in oncology, cardiology and family medicine. BMJ Open 2011, 1. [Google Scholar] [CrossRef]
- Abu-Elmagd, M.; Assidi, M.; Schulten, H.J.; Dallol, A.; Pushparaj, P.N.; Ahmed, F.; Scherer, S.W.; Al-Qahtani, M. Individualized medicine enabled by genomics in Saudi Arabia. BMC Genom. 2015, 8, S3. [Google Scholar] [CrossRef] [PubMed]
- Nembaware, V.; Mulder, N. The African Genomic Medicine Training Initiative (AGMT): Showcasing a Community and Framework Driven Genomic Medicine Training for Nurses in Africa. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Korf, B.R.; Berry, A.B.; Limson, M.; Marian, A.J.; Murray, M.F.; O’Rourke, P.P.; Passamani, E.R.; Relling, M.V.; Tooker, J.; Tsongalis, G.J.; et al. Framework for development of physician competencies in genomic medicine: Report of the competencies working group of the inter-society coordinating committee for physician education in genomics. Genet. Med. 2014, 16, 804–809. [Google Scholar] [CrossRef] [PubMed]
- McClaren, B.J.; King, E.A.; Crellin, E.; Gaff, C.; Metcalfe, S.A.; Nisselle, A. Development of an Evidence-Based, Theory-Informed National Survey of Physician Preparedness for Genomic Medicine and Preferences for Genomics Continuing Education. Front. Genet. 2020, 11, 59. [Google Scholar] [CrossRef]
- Stark, Z.; Nisselle, A.; McClaren, B.; Lynch, F.; Best, S.; Long, J.C.; Martyn, M.; Patel, C.; Schlapbach, L.J.; Barnett, C.; et al. Attitudes of Australian health professionals towards rapid genomic testing in neonatal and paediatric intensive care. Eur. J. Hum. Genet. 2019, 27, 1493–1501. [Google Scholar] [CrossRef]
- Baroncini, A.; Olga, C.; Marco, C.; Elisabetta, P.; Francesca, T.; Stefania, B. Knowledge and attitude of general pratictioners towards direct-to-consumer genomic tests: A survey conducted in Italy. Epidemiol. Biostat. Public Health 2015, 12, 11613. [Google Scholar] [CrossRef]
- Goldsmith, L.; Jackson, L.; O’Connor, A.; Skirton, H. Direct-to-consumer genomic testing from the perspective of the health professional: A systematic review of the literature. J. Community Genet. 2013, 4, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.B.; Carrig, M.M.; O’Daniel, J.M.; Orlando, L.A.; Killeya-Jones, L.A.; Ginsburg, G.S.; Cho, A. Genomic risk profiling: Attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J. Gen. Intern. Med. 2011, 26, 834–840. [Google Scholar] [CrossRef]
- Petersen, K.E.; Prows, C.A.; Martin, L.J.; Maglo, K.N. Personalized medicine, availability, and group disparity: An inquiry into how physicians perceive and rate the elements and barriers of personalized medicine. Public Health Genom. 2014, 17, 209–220. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Lynd, L.D.; Davis, J.C.; Bryan, S.; Anis, A.; Marra, M.; Marra, C.A. Barriers to integrating personalized medicine into clinical practice: A best-worst scaling choice experiment. Genet. Med. 2012, 14, 520–526. [Google Scholar] [CrossRef]
- Bain, K.T.; Schwartz, E.J.; Knowlton, O.V.; Knowlton, C.H.; Turgeon, J. Implementation of a pharmacist-led pharmacogenomics service for the Program of All-Inclusive Care for the Elderly (PHARM-GENOME-PACE). J. Am. Pharm. Assoc. 2018, 58, 281–289.e1. [Google Scholar] [CrossRef]
- Bank, P.C.D.; Swen, J.J.; Schaap, R.D.; Klootwijk, D.B.; Baak–Pablo, R.; Guchelaar, H.J. A pilot study of the implementation of pharmacogenomic pharmacist initiated pre-emptive testing in primary care. Eur. J. Hum. Genet. 2019, 27, 1532–1541. [Google Scholar] [CrossRef]
- Brown, J.T.; Gregornik, D.; Kennedy, M.J. The role of the pediatric pharmacist in precision medicine and clinical pharmacogenomics for children. J. Pediatr. Pharmacol. Ther. 2018, 23, 499–501. [Google Scholar] [CrossRef]
- van der Wouden, C.H.; Bank, P.C.D.; Özokcu, K.; Swen, J.J.; Guchelaar, H.J. Pharmacist-initiated pre-emptive pharmacogenetic panel testing with clinical decision support in primary care: Record of PGx results and real-world impact. Genes 2019, 10, 416. [Google Scholar] [CrossRef]
- Knapp, K.; Ignoffo, R. Oncology Pharmacists Can Reduce the Projected Shortfall in Cancer Patient Visits: Projections for Years 2020 to 2025. Pharmacy 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Schuh, M.J.; Crosby, S. Description of an Established, Fee-for-Service, Office-Based, Pharmacist-Managed Pharmacogenomics Practice. Sr. Care Pharm. 2019, 34, 660–668. [Google Scholar] [CrossRef]
- Rahmani, A.; Afandi, B. Improving neonatal complications with a structured multidisciplinary approach to gestational diabetes mellitus management. J. Neonatal. Perinatal. Med. 2016, 8, 359–362. [Google Scholar] [CrossRef]
- Antoniak, J. Handwashing compliance. Can. Nurse 2004, 100, 21–25. [Google Scholar]
- Haleeqa, M.A.; Alshamsi, I.; Al Habib, A.; Noshi, M.; Abdullah, S.; Kamour, A.; Ibrahim, H. Optimizing supportive care in COVID-19 patients: A multidisciplinary approach. J. Multidiscip. Healthc. 2020, 13, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, T.; Kassie, S.; Ali, O.M.; Mozahem, K.; Al Fardan, S.; Ahmed, A.M. Psychiatric comorbidity in neurological disorders: Towards a multidisciplinary approach to illness management in the United Arab Emirates. Front. Psychiatry 2019, 10, 263. [Google Scholar] [CrossRef]
- Hawamdeh, S.; Almakhzoomy, I.; Hayajneh, Y. Screening and Correlates of Depression and HbA1C in United Arab Emirates (UAE) Women With Diabetes. Perspect. Psychiatr. Care 2013, 49, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rowland-Jones, R. The efqm concepts of excellence approach to management development within the uae healthcare industry utilizing action modalities. Hum. Resour. Dev. Int. 2012, 15, 501–514. [Google Scholar] [CrossRef]
- Manda, V.; Sreedharan, J.; Muttappallymyalil, J.; Das, R.; Hisamatsu, E. Foot ulcers and risk factors among diabetic patients visiting Surgery Department in a University Teaching Hospital in Ajman, UAE. Int. J. Med. Public Health 2012, 2, 34–38. [Google Scholar] [CrossRef]
- Rahma, A.T.; Elbarazi, I.; Ali, B.R.; Patrinos, G.P.; Ahmed, L.A.; Maskari, F. Al Genomics and pharmacogenomics knowledge, attitudes and practice of pharmacists working in United Arab Emirates: Findings from focus group discussions—A qualitative study. J. Pers. Med. 2020, 10, 134. [Google Scholar] [CrossRef]
| Count (Percentage) | |
|---|---|
| Gender | |
| Female | 350 (63.4%) |
| Male | 202 (36.6%) |
| Age Group | |
| 20–30 | 148 (26.9%) |
| 31–41 | 225 (40.8%) |
| 42–52 | 124 (22.5%) |
| 53–63 | 53 (9.6%) |
| 64–74 | 1 (0.2%) |
| Occupation | |
| Pharmacy-Related | 232 (42%) |
| Nurse | 153 (27.7%) |
| Medicine | 134 (24.3%) |
| Business & Management | 14 (2.5%) |
| Administration | 5 (0.9%) |
| Allied Health | 5 (0.9%) |
| Governmental | 5 (0.9%) |
| Intern | 2 (0.4%) |
| Years of Experience | |
| <10 years | 265 (52.2%) |
| >10 years | 149 (29.3%) |
| Nationality | |
| Middle East | 226 (40.9%) |
| Asia | 179 (32.4%) |
| United Arab Emirates (UAE) | 68 (12.3%) |
| Africa | 34 (6.2%) |
| Europe & Australia | 16 (2.9%) |
| North America | 14 (2.5%) |
| Gulf Cooperation Council (GCC) countries | 8 (1.4%) |
| Choose the Correct Answer: | Correct Answer | True n (%) | False n (%) | Do Not Know n (%) |
|---|---|---|---|---|
| 1. Humans have 48 chromosomes. | False | 196 (38.8%) | 281 (55.6%) | 28 (5.5%) |
| 2. Adenine (A) only pairs with cytosine (C) and Thymine (T) only pairs with Guanine (G). | False | 148 (29.3%) | 183 (36.2%) | 174 (34.5%) |
| 3. Pharmacogenomics seeks to individualize therapy based on the patient’s genetic profile. | True | 407 (80.6%) | 32 (6.3%) | 66 (13.1%) |
| 4. Genetic changes can cause adverse reactions. | True | 395 (78.2%) | 45 (8.9%) | 65 (12.9%) |
| 5. Pharmacogenomics testing is recommended by FDA for certain drugs. | True | 335 (66.3%) | 16 (3.2%) | 154 (30.5%) |
| 6. Genetic changes can affect the patient’s response to certain drugs. | True | 451 (89.3%) | 16 (3.2%) | 38 (7.5%) |
| 7. Genes can be activated or deactivated by other genes. | True | 379 (75.0%) | 38 (7.5%) | 88 (17.4%) |
| 8. Every cell of the body contains the whole genome. | False | 338 (66.9%) | 67 (13.3%) | 100 (19.8%) |
| 9. Environmental factors, such as cigarette smoke, can affect gene activity. | True | 379 (75.0%) | 52 (10.3%) | 74 (14.7%) |
| Level of Knowledge | ||||
|---|---|---|---|---|
| Good | Fair | Poor | p-Value | |
| Gender | 0.01 * | |||
| Female | 95 (27.1%) | 196 (56.0%) | 59 (16.9%) | |
| Male | 74 (36.6%) | 87 (43.1%) | 41 (20.3%) | |
| Age Group | 0.12 ** | |||
| 20–30 | 46 (31.1%) | 73 (49.3%) | 29 (19.6%) | |
| 31–41 | 63 (28.0%) | 119 (52.9%) | 43 (19.1%) | |
| 42–52 | 34 (27.4%) | 71 (57.3%) | 19 (15.3%) | |
| 53–63 | 25 (47.2%) | 19 (35.8%) | 9 (17.0%) | |
| 64–74 | 1 (100%) | 0 (0.0%) | 0 (0.0%) | |
| Years of Experience | 0.88 | |||
| <10 | 72 (30.0%) | 126 (52.5%) | 42 (17.5%) | |
| >10 | 97 (31.1%) | 157 (50.3%) | 58 (18.6%) | |
| Occupation Category | 0.00 ** | |||
| Pharmacy-Related | 69 (29.7%) | 117 (50.4%) | 46 (19.8%) | |
| Nurse | 31 (20.3%) | 88 (57.5%) | 34 (22.2%) | |
| Medicine | 61 (45.5%) | 59 (44.0%) | 14 (10.4%) | |
| Business & Management | 5 (35.7%) | 7 (50.0%) | 2 (14.3%) | |
| Administration | 0 (0.0%) | 3 (60.0%) | 2 (40.0%) | |
| Allied Health | 1 (20.0%) | 4 (80.0%) | 0 (0.0%) | |
| Governmental | 0 (0.0%) | 3 (60.0%) | 2 (40.0%) | |
| Intern | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | |
| Previous Exposure to Genetic Issues | 0.30 | |||
| Yes | 54 (35.3%) | 75 (49.0%) | 24 (15.7%) | |
| No | 115 (28.8%) | 208 (52.1%) | 76 (19.0%) | |
| Completed PGX/ Pharmacogenetics Training or Education | 0.01 * | |||
| Yes | 51 (41.5%) | 55 (44.7%) | 17 (13.8%) | |
| No | 118 (27.5%) | 228 (53.1%) | 83 (19.3%) | |
| Have you ever advised any of your patients to undertake a genetic test? | 0.31 | |||
| Yes | 57 (38.0%) | 83 (55.3%) | 10 (6.7%) | |
| No | 71 (34.6%) | 112 (54.6%) | 22 (10.7%) | |
| Have you had any patients who asked about undertaking a genetic test in the last two years? | 0.02 * | |||
| Yes | 59 (45.7%) | 62 (48.1%) | 8 (6.2%) | |
| No | 74 (31.6%) | 132 (56.4%) | 28 (12.0%) | |
| Have you had any patients who asked your advice about the results of a genetic test in the last two years? | 0.28 | |||
| Yes | 50 (41.7%) | 57 (47.5%) | 13 (10.8%) | |
| No | 85 (34.1%) | 140 (56.2%) | 24 (9.6%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahma, A.T.; Elsheik, M.; Ali, B.R.; Elbarazi, I.; Patrinos, G.P.; Ahmed, L.A.; Al Maskari, F. Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study. J. Pers. Med. 2020, 10, 216. https://doi.org/10.3390/jpm10040216
Rahma AT, Elsheik M, Ali BR, Elbarazi I, Patrinos GP, Ahmed LA, Al Maskari F. Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study. Journal of Personalized Medicine. 2020; 10(4):216. https://doi.org/10.3390/jpm10040216
Chicago/Turabian StyleRahma, Azhar T., Mahanna Elsheik, Bassam R. Ali, Iffat Elbarazi, George P. Patrinos, Luai A. Ahmed, and Fatma Al Maskari. 2020. "Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study" Journal of Personalized Medicine 10, no. 4: 216. https://doi.org/10.3390/jpm10040216
APA StyleRahma, A. T., Elsheik, M., Ali, B. R., Elbarazi, I., Patrinos, G. P., Ahmed, L. A., & Al Maskari, F. (2020). Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study. Journal of Personalized Medicine, 10(4), 216. https://doi.org/10.3390/jpm10040216