Dysregulation of Endothelin-1: Implications for Health Disparities in Alzheimer’s Disease
Abstract
1. Background/Introduction
2. Underserved Minority Populations and AD
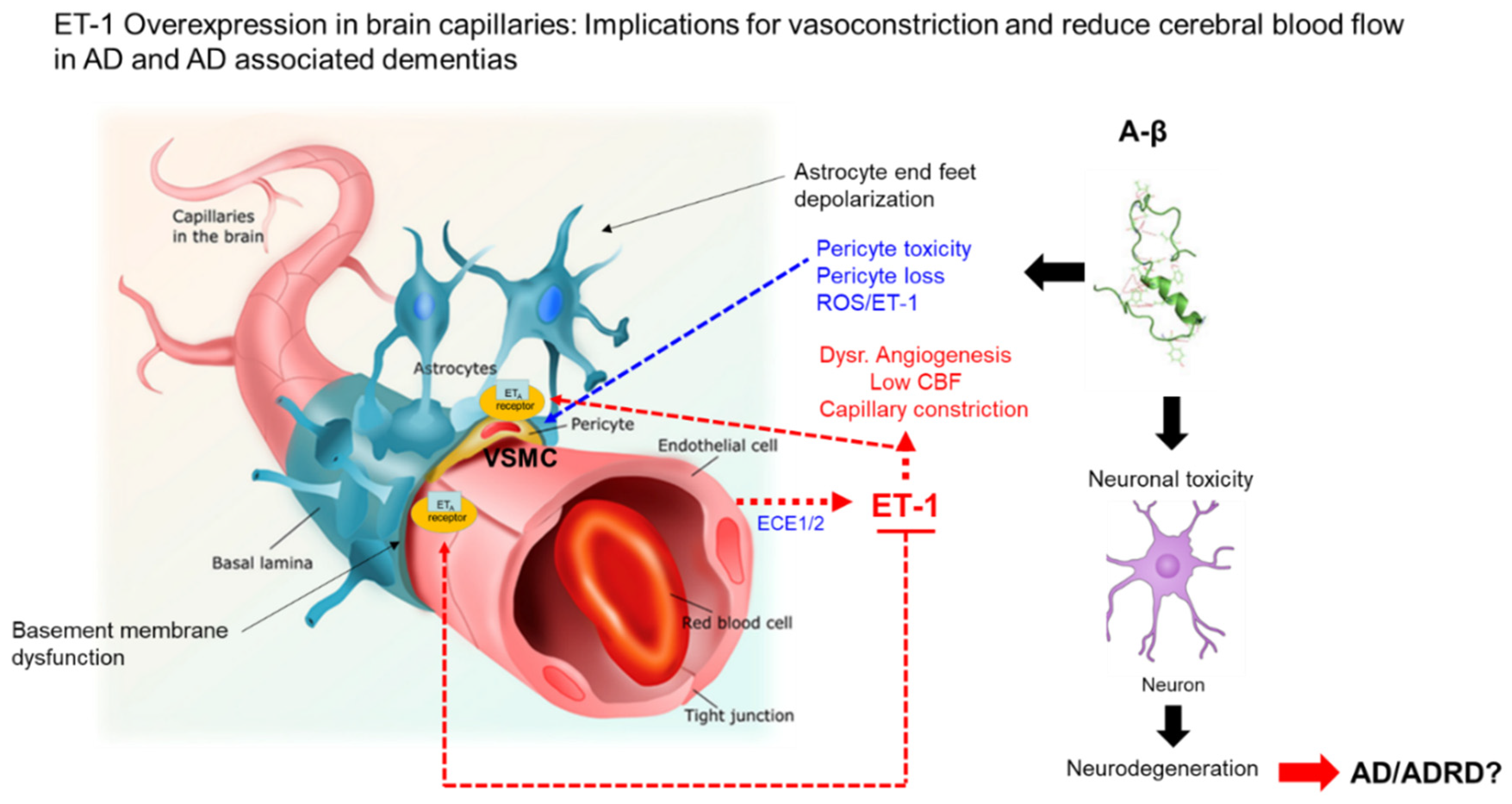
3. ET-1 System and Regulation
4. ET-1 System and AD in Minority Populations
5. ET-1 and Aβ Interactions
6. Genetics and ET-1 Dysregulation and Racial Disparities in AD
7. Potential Therapeutics for ET-1 Dysregulation in AD
7.1. Endothelin Receptor Agonist and Antagonists
7.2. Endothelin Vaccines
8. Potential Role of Endothelin-1 and Other Endothelin-1-Related Molecules as Biomarkers in Biofluids for AD
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAs | African Americans |
| Aβ | Amyloid beta protein |
| AD | Alzheimer’s disease |
| ADRD | Alzheimer’s disease and related dementias |
| APPsw AD | Transgenic mouse mode for Alzheimer’s disease |
| CT-pro-ET-1 | C-terminal-pro-ET-1 |
| DM | Diabetes mellitus |
| ECE-1 | Endothelin-converting enzyme-1 |
| ECE-2 | Endothelin-converting enzyme-2 |
| EDN-1 | Endothelin-1 gene locus |
| EDN-2 | Endothelin-2 gene Locus |
| EDN-3 | Endothelin-3 gene Locus |
| ERAs | Endothelin receptor antagonists |
| ETA | Endothelin receptor type A |
| ETB | Endothelin receptor type B |
| ET-1 | Endothelin-1 |
| ET-2 | Endothelin-2 |
| ET-3 | Endothelin-3 |
| H/L | Hispanic/Latino |
| NHWs | Non-Hispanic Whites |
| NS-398 | COX-2 inhibitor |
| PS1/APPsw | Transgenic mouse mode for Alzheimer’s disease |
| ROS | Reactive oxygen species |
| SB202190 | Specific inhibitor of p38 MAPK signaling pathway |
| SCUBE2 | Signal peptide-CUB-EGF-like-containing protein 2 |
| tagSNPs | Tagged single nucleotide polymorphisms |
| T2DM | Type 2 diabetes mellitus |
References
- Novak, P.; Chu, J.; Ali, M.M.; Chen, J. Racial and Ethnic Disparities in Serious Psychological Distress among Those with Alzheimer’s Disease and Related Dementias. Am. J. Geriatr. Psychiatry 2019, 19, 30473–30477. [Google Scholar]
- Clark, L.R.; Norton, D.; Berman, S.E.; Johnson, S.C.; Bendlin, B.B.; Wieben, O.; Turski, P.; Carlsson, C.; Asthana, S.; Gleason, C.E.; et al. Association of Cardiovascular and Alzheimer’s Disease Risk Factors with Intracranial Arterial Blood Flow in Whites and African Americans. J. Alzheimers Dis. 2019, 72, 919–929. [Google Scholar] [CrossRef]
- Potter, G.G.; Plassman, B.L.; Burke, J.R.; Kabeto, M.U.; Langa, K.M.; Llewellyn, D.J.; Rogers, M.A.; Steffens, D.C. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009, 6, 445–453. [Google Scholar]
- Gianattasio, K.Z.; Prather, C.; Glymour, M.M.; Ciarleglio, A.; Power, M.C. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement. 2019, 5, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Mayeda, E.R.; Glymour, M.M.; Quesenberry, C.P.; Whitmer, R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016, 3, 216–224. [Google Scholar]
- Treiber, F.A.; Jackson, R.W.; Davis, H.; Pollock, J.S.; Kapuku, G.; Mensah, G.A.; Pollock, D.M. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension 2000, 3, 722–735. [Google Scholar] [CrossRef]
- Ahmed, M.; Rghigh, A. Polymorphism in Endothelin-1 Gene: An Overview. Curr. Clin. Pharmacol. 2016, 3, 191–210. [Google Scholar]
- Tsui, J.C.; Dashwood, M.R. A role for endothelin-1 in peripheral vascular disease. Curr. Vasc. Pharmacol. 2005, 4, 325–332. [Google Scholar] [CrossRef]
- Wager, O.F.; Chirst, G.; Wojta, J.; Vierhapper, H.; Parzer, S.; Nowotny, P.J. Polar secretion of endothelin-1 by cultured endothelial cells. J. Biol. Chem. 1992, 267, 16066–16068. [Google Scholar]
- Dehouck, M.P.; Vigne, P.; Torpier, G.; Breittmayer, J.P.; Cecchelli, R.; Frelin, C. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. J. Cereb. Blood Flow Metab. 1997, 4, 464–469. [Google Scholar]
- Ergul, A.; Portik-Dobos, V.; Giulumian, A.D.; Molero, M.M.; Fuchs, L.C. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am. J. Physiol. Heart Circ. Physiol. 2003, 5, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, D.; Leng, S.X. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clin. Interv. Aging 2015, 10, 549–560. [Google Scholar]
- Ali, H.; Rustam, R.; Aprilia, D.; Arizal, C.; Gusadri, I.B.; Utami, P.R. Upregulation of SCUBE2 expression in dyslipidemic type 2 diabetes mellitus is associated with endothelin-1. Diabetes Metab. Syndr. 2019, 5, 2869–2872. [Google Scholar] [CrossRef]
- Kostov, K.; Blazhev, A.; Atanasova, M.; Dimitrova, A. Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2016, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Shabnam, B.; Kehoe, P.G.; Love, S. Endothelin-converting enzyme-2 is increased in Alzheimer’s disease and up-regulated by Aβ. Am. J. Pathol. 2009, 1, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jin, H.; Zhu, Y.; Wan, Y.; Opoku, E.N.; Zhu, L.; Hu, B. Diverse Functions and Mechanisms of Pericytes in Ischemic Stroke. Curr. Neuropharmacol. 2017, 6, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Barnes, L.L.; Bennett, D.A.; Li, Y.; Bienias, J.L.; De Leon, C.M.; Evans, D.A. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology 2005, 64, 380–382. [Google Scholar] [CrossRef]
- Barnes, L.L.; Bennett, D.A. Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff. 2014, 4, 580–586. [Google Scholar] [CrossRef]
- Krogstad, J.N. Fact Tank, News in the Numbers. Pew Research Center; Key Facts about How the U.S. Hispanic Population is Changing. Available online: http://www.pewresearch.org/fact-tank/2016/09/08/key-facts-about-how-the-u-s-hispanic-population-is-changing/ (accessed on 12 November 2016).
- US Census Bureau. Available online: https://www.census.gov/topics/population/data.html (accessed on 25 November 2016).
- Xiong, C.; Luo, J.; Coble, D.; Folasade, A.; Kukull, W.; Morris, J.C. Complex interactions underlie racial disparity in the risk of developing Alzheimer’s disease dementia. Alzheimers Dement. 2020, 4, 589–597. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 4, 459–569. [Google Scholar]
- Mehta, K.M.; Yeo, G.W. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017, 1, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.L.; Negash, S.; Hamilton, R. Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011, 3, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Vega, W.A.; Resendez, J.; Jin, H. Latinos and Alzheimer’s Disease: New Numbers Behind the Crisis. Projection of the Costs for US Latinos Living with Alzheimer’s Disease through 2060. Available online: http://www.usagainstalzheimers.org/sites/default/files/Latinos-and-AD_USC_UsA2-Impact-Report.pdf (accessed on 3 December 2016).
- Livney, M.G.; Clark, C.M.; Karlawish, J.H.; Cartmell, S.; Negrón, M.; Nuñez, J.; Xie, S.X.; Entenza-Cabrera, F.; Vega, I.E.; Arnold, S.E. Ethnoracial differences in the clinical characteristics of Alzheimer’s disease at initial presentation at an urban Alzheimer’s disease center. Am. J. Geriatr. Psychiatry 2011, 5, 430–439. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Johnson, L.; Balldin, V.; Edwards, M.; Barber, R.; Williams, B.; Devous, M.; Cushings, B.; Knebl, J.; Hall, J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2013, 2, 373–379. [Google Scholar] [CrossRef]
- Vega, I.E.; Cabrera, L.Y.; Wygant, C.M.; Velez-Ortiz, D.; Counts, S.E. Alzheimer’s Disease in the Latino Community: Intersection of Genetics and Social Determinants of Health. J. Alzheimers Dis. 2017, 4, 979–992. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Hama, H.; Kasuya, Y. Molecular pharmacology and pathophysiological significance of endothelin. Jpn. J. Pharmacol. 1996, 4, 261–290. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Masaki, T. Pathophysiology of endothelin in the cardiovascular system. Annu. Rev. Physiol. 1999, 61, 391–415. [Google Scholar] [CrossRef]
- Thorin, E.; Clozel, M. The cardiovascular physiology and pharmacology of endothelin-1. Adv. Pharmacol. 2010, 60, 1–26. [Google Scholar]
- Kitada, K.; Ohkita, M.; Matsumura, Y. Pathological Importance of the Endothelin-1/ETB Receptor System on Vascular Diseases. Cardiol. Res. Pract. 2012, 2012, 731970. [Google Scholar]
- Hirata, Y.; Takagi, Y.; Fukuda, Y.; Marumo, F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis 1989, 3, 225–228. [Google Scholar] [CrossRef]
- Clozel, M.; Gray, G.A.; Breu, V.; Löffler, B.M.; Osterwalder, R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem. Biophys. Res. Commun. 1992, 186, 867–873. [Google Scholar] [CrossRef]
- Takahashi, M.; Matsushita, Y.; Iijima, Y.; Tanzawa, K. Purification and characterization of endothelin-converting enzyme from rat lung. J. Biol. Chem. 1993, 268, 21394–21398. [Google Scholar] [PubMed]
- Houde, M.; Desbiens, L.; D’Orléans-Juste, P. Endothelin-1: Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar]
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 gene regulation. FASEB J. 2011, 1, 16–28. [Google Scholar] [CrossRef]
- Inoue, A.; Yanagisawa, M.; Takuwa, Y.; Mitsui, Y.; Kobayashi, M.; Masaki, T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J. Biol. Chem. 1989, 264, 14954–14959. [Google Scholar]
- Arai, H.; Hori, S.; Aramori, I.; Ohkubo, H.; Nakanishi, S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990, 348, 730–732. [Google Scholar] [CrossRef]
- Sakurai, T.; Yanagisawa, M.; Takuwa, Y.; Miyazaki, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef]
- Levin, E.R. Endothelins. N. Engl. J. Med. 1995, 333, 356–363. [Google Scholar] [CrossRef]
- Huggins, J.P.; Pelton, J.T.; Miller, R.C. The structure and specificity of endothelin receptors: Their importance in physiology and medicine. Pharmacol. Ther. 1993, 59, 55–123. [Google Scholar] [CrossRef]
- Janes, R.W.; Peapus, D.H.; Wallace, B.A. The crystal structure of human endothelin. Nat. Struct. Biol. 1994, 1, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.A.; Janes, R.W. The crystal structure of human endothelin-1 and how it relates to receptor binding. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 3), S250–S253. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.D.; Coppell, A.L.; Davenport, A.P. In vitro enzymatic processing of radiolabelled big ET-1 in human kidney. Biochem. Pharmacol. 1998, 55, 697–701. [Google Scholar] [CrossRef]
- Peto, H.; Corder, R.; Janes, R.W.; Wallace, B.A. A molecular model for human Big-Endothelin-1 (Big ET-1). FEBS Lett. 1996, 394, 191–195. [Google Scholar] [CrossRef]
- Xu, D.; Emoto, N.; Giaid, A.; Slaughter, C.; Kaw, S.; deWit, D.; Yanagisawa, M. ECE-1: A membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 1994, 78, 473–485. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- Shah, R. Endothelins in health and disease. Eur. J. Intern. Med. 2007, 4, 272–282. [Google Scholar] [CrossRef]
- Haynes, W.G.; Ferro, C.J.; O’Kane, K.P.; Somerville, D.; Lomax, C.C.; Webb, D.J. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 1996, 93, 1860–1870. [Google Scholar] [CrossRef]
- Haynes, W.G.; Webb, D.J. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet 1994, 344, 852–854. [Google Scholar] [CrossRef]
- Alcendor, D.J. Interactions between Amyloid-Β Proteins and Human Brain Pericytes: Implications for the Pathobiology of Alzheimer’s Disease. J. Clin. Med. 2020, 5, 1490. [Google Scholar] [CrossRef]
- Evans, R.R.; Phillips, B.G.; Singh, G.; Bauman, J.L.; Gulati, A. Racial and gender differences in endothelin-1. Am. J. Cardiol. 1996, 78, 486–488. [Google Scholar] [CrossRef]
- Grubbs, A.L.; Anstadt, M.P.; Ergul, A. Saphenous vein endothelin system expression and activity in African American patients. Arterioscler. Thromb. Vasc. Biol. 2002, 7, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Treiber, F.A.; Kapuku, G.K.; Davis, H.; Pollock, J.S.; Pollock, D.M. Plasma endothelin-1 release during acute stress: Role of ethnicity and sex. Psychosom. Med. 2002, 5, 707–713. [Google Scholar]
- Nortley, R.; Korte, N.; Izquierdo, P.; Hirunpattarasilp, C.; Mishra, A.; Jaunmuktane, Z.; Kyrargyri, V.; Pfeiffer, T.; Khennouf, L.; Madry, C.; et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019, 6450, eaav9518. [Google Scholar] [CrossRef] [PubMed]
- Campia, U.; Cardillo, C.; Panza, J.A. Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients. Circulation 2004, 25, 3191–3195. [Google Scholar] [CrossRef]
- Manly, J.J.; Jacobs, D.; Mayeux, R. Alzheimer’s disease among different ethnic and racial groups. In Alzheimer’s Disease, 2nd ed.; Terry, R.D., Katzman, R., Bick, K.L., Sisodia, S.S., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999; pp. 117–131. [Google Scholar]
- Chui, H.C.; Gatz, M. Cultural diversity in Alzheimer disease: The interface between biology, belief, and behavior. Alzheimer Dis. Assoc. Disord. 2005, 4, 250–255. [Google Scholar] [CrossRef]
- Gilligan, A.M.; Malone, D.C.; Warholak, T.L.; Armstrong, E.P. Racial and ethnic disparities in Alzheimer’s disease pharmacotherapy exposure: An analysis across four state Medicaid populations. Am. J. Geriatr. Pharmacother. 2012, 5, 303–312. [Google Scholar] [CrossRef]
- Rovner, B.W.; Casten, R.J.; Harris, L.F. Cultural diversity and views on Alzheimer disease in older African Americans. Alzheimer Dis. Assoc. Disord. 2013, 2, 133–137. [Google Scholar] [CrossRef]
- Yammine, L.; Kang, D.H.; Baun, M.M.; Meininger, J.C. Endothelin-1 and psychosocial risk factors for cardiovascular disease: A systematic review. Psychosom. Med. 2014, 76, 109–121. [Google Scholar] [CrossRef]
- Cooper, D.C.; Mills, P.J.; Bardwell, W.A.; Ziegler, M.G.; Dimsdale, J.E. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. Am. J. Hypertens. 2009, 7, 698–704. [Google Scholar] [CrossRef]
- Miners, J.S.; Palmer, J.C.; Tayler, H.; Palmer, L.E.; Ashby, E.; Kehoe, P.G.; Love, S. Aβ degradation or cerebral perfusion? Divergent effects of multifunctional enzymes. Front. Aging Neurosci. 2014, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Barker, R.; Kehoe, P.G.; Love, S. Endothelin-1 is elevated in Alzheimer’s disease and upregulated by amyloid-β. J. Alzheimers Dis. 2012, 4, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Tayler, H.M.; Love, S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer’s disease. J. Alzheimers Dis. 2013, 36, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Kehoe, P.G.; Love, S. Endothelin-converting enzyme-1 in Alzheimer’s disease and vascular dementia. Neuropathol. Appl. Neurobiol. 2010, 36, 487–497. [Google Scholar] [CrossRef]
- Paris, D.; Humphrey, J.; Quadros, A.; Patel, N.; Crescentini, R.; Crawford, F.; Mullan, M. Vasoactive effects of Aβ in isolated human cerebrovessels and inatransgenic mouse model of Alzheimer’s disease: Role of inflammation. Neurol. Res. 2003, 25, 642–651. [Google Scholar] [CrossRef]
- Liang, L.L.; Chen, L.; Zhou, M.Y.; Cai, M.Y.; Cheng, J.; Chen, Y.; You, S.K.; Chen, L.B.; Tang, Z.B.; Yang, X.L.; et al. Genetic susceptibility of five tagSNPs in the endothelin-1 (EDN1) gene to coronary artery disease in a Chinese Han population. Biosci. Rep. 2018, 5, BSR20171320. [Google Scholar] [CrossRef]
- Verweij, N.; Mahmud, H.; Mateo Leach, I.; de Boer, R.A.; Brouwers, F.P.; Yu, H.; Asselbergs, F.W.; Struck, J.; Bakker, S.J.; Gansevoort, R.T.; et al. Genome-wide association study on plasma levels of midregional-proadrenomedullin and C-terminal-pro-endothelin-1. Hypertension 2013, 3, 602–608. [Google Scholar] [CrossRef]
- Palmer, J.; Love, S. Endothelin receptor antagonists: Potential in Alzheimer’s disease. Pharmacol. Res. 2011, 6, 525–531. [Google Scholar] [CrossRef]
- Pulido, T.; Adzerikho, I.; Channick, R.N.; Delcroix, M.; Galiè, N.; Ghofrani, H.A.; Jansa, P.; Jing, Z.C.; Le Brun, F.O.; Mehta, S.; et al. SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 9, 809–818. [Google Scholar] [CrossRef]
- Widlitz, A.C.; Barst, R.J.; Horn, E.M. Sitaxsentan: A novel endothelin-A receptor antagonist for pulmonary arterial hypertension. Expert Rev. Cardiovasc. Ther. 2005, 6, 985–991. [Google Scholar] [CrossRef]
- Pacheco-Quinto, J.; Eckman, C.B.; Eckman, E.A. Major amyloid-β-degrading enzymes, endothelin-converting enzyme-2 and neprilysin, are expressed by distinct populations of GABAergic interneurons in hippocampus and neocortex. Neurobiol. Aging 2016, 48, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quinto, J.; Herdt, A.; Eckman, C.B.; Eckman, E.A. Endothelin converting enzymes and related metalloproteases in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33 (Suppl. 1), S101–S110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguiz, W.; Gadnidze, R.M.; Ragnauth, K.; Dorr, N.; Yanagisawa, M.; Wetsel, W.C.; Devi, L.A. Animals lacking endothelin-converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2008, 7, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Nguyen, C.; Leonard, M.; Gulati, A. Stimulation of endothelin B receptors by IRL-1620 decreases the progression of Alzheimer’s disease. Neuroscience 2015, 301, 1–11. [Google Scholar] [CrossRef]
- Nagiri, C.; Shihoya, W.; Inoue, A.; Kadji, F.M.N.; Aoki, J.; Nureki, O. Crystal structure of human endothelin ETB receptor in complex with peptide inverse agonist IRL2500. Commun. Biol. 2019, 2, 236. [Google Scholar] [CrossRef]
- Shihoya, W.; Izume, T.; Inoue, A.; Yamashita, K.; Kadji, F.M.N.; Hirata, K.; Aoki, J.; Nishizawa, T.; Nureki, O. Crystal structures of human ETB receptor provide mechanistic insight into receptor activation and partial activation. Nat. Commun. 2018, 9, 4711. [Google Scholar] [CrossRef]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years from Discovery to Therapy. Hypertension 2019, 6, 1232–1265. [Google Scholar] [CrossRef]
- Dai, Q.Y.; Chen, X.; Song, X.; Chen, X.; Ma, W.; Lin, J.; Wu, H.; Hu, X.; Zhou, Y.; Zhang, H.; et al. Immunotherapy of Endothelin-1 Receptor Type A for Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2019, 20, 2567–2580. [Google Scholar] [CrossRef]
- Zhang, V.C.; Wang, X.; Zhang, H.; Yao, C.; Pan, H.; Guo, Y.; Fan, K.; Jing, S. Therapeutic Monoclonal Antibody Antagonizing Endothelin Receptor A for Pulmonary Arterial Hypertension. J. Pharmacol. Exp. Ther. 2019, 1, 54–61. [Google Scholar] [CrossRef]
- Raina, R.; Lou, L.; Berger, B.; Vogt, B.; Sao-Mai Do, A.; Cunningham, R.; Vasavada, P.; Herrmann, K.; Dell, K.; Simonson, M. Relationship of urinary endothelin-1 with estimated glomerular filtration rate in autosomal dominant polycystic kidney disease: A pilot cross-sectional analysis. BMC Nephrol. 2016, 17, 22. [Google Scholar] [CrossRef]
- Gurusankar, R.; Kumarathasan, P.; Saravanamuthu, A.; Thomson, E.M.; Vincent, R. Correlation between Saliva and Plasma Levels of Endothelin Isoforms ET-1, ET-2, and ET-3. Int. J. Pept. 2015, 2015, 828759. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.C.; Takahashi, K.; Ghatei, M.A.; Warrens, A.N.; Rees, A.J.; Bloom, S.R. Immunoreactive endothelin in human plasma, urine, milk, and saliva. J. Cardiovasc. Pharmacol. 1991, 17 (Suppl. 7), S390–S393. [Google Scholar] [CrossRef]
- Ken-Dror, S.; Weintraub, Z.; Yechiely, H.; Kahana, L. Atrial natriuretic peptide and endothelin concentrations in human milk during postpartum lactation. Acta Paediatr. 1997, 8, 793–795. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcendor, D.J. Dysregulation of Endothelin-1: Implications for Health Disparities in Alzheimer’s Disease. J. Pers. Med. 2020, 10, 199. https://doi.org/10.3390/jpm10040199
Alcendor DJ. Dysregulation of Endothelin-1: Implications for Health Disparities in Alzheimer’s Disease. Journal of Personalized Medicine. 2020; 10(4):199. https://doi.org/10.3390/jpm10040199
Chicago/Turabian StyleAlcendor, Donald J. 2020. "Dysregulation of Endothelin-1: Implications for Health Disparities in Alzheimer’s Disease" Journal of Personalized Medicine 10, no. 4: 199. https://doi.org/10.3390/jpm10040199
APA StyleAlcendor, D. J. (2020). Dysregulation of Endothelin-1: Implications for Health Disparities in Alzheimer’s Disease. Journal of Personalized Medicine, 10(4), 199. https://doi.org/10.3390/jpm10040199



