Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. In Vitro Cytotoxicity Assays
2.3. Tumor Cell Lysis Assays
2.4. Binding of Ipilimumab and Nivolumab to Mouse CTLA-4 and Lymphocytes by ELISA Assays
2.5. Expression of Leukotriene B4 (LTB4)
2.6. Expression of Nuclear Factor NF-Kappa-B p65 Subunit (p65-NF-kB)
2.7. Expression of NLRP3 Inflammasome and MyD88
2.8. Confocal Laser Scanning Microscope (CLSM)
2.9. Secretion of Pro-Inflammatory Cytokines
2.10. Animal Models and In Vivo Studies
2.11. Echocardiographic Analysis of Ventricular Function
2.12. Effects of Ipilimumab Administration on Pro-Inflammatory Markers and Cytokine Profile in Heart Tissues
2.13. Statistical Analyses
3. Results
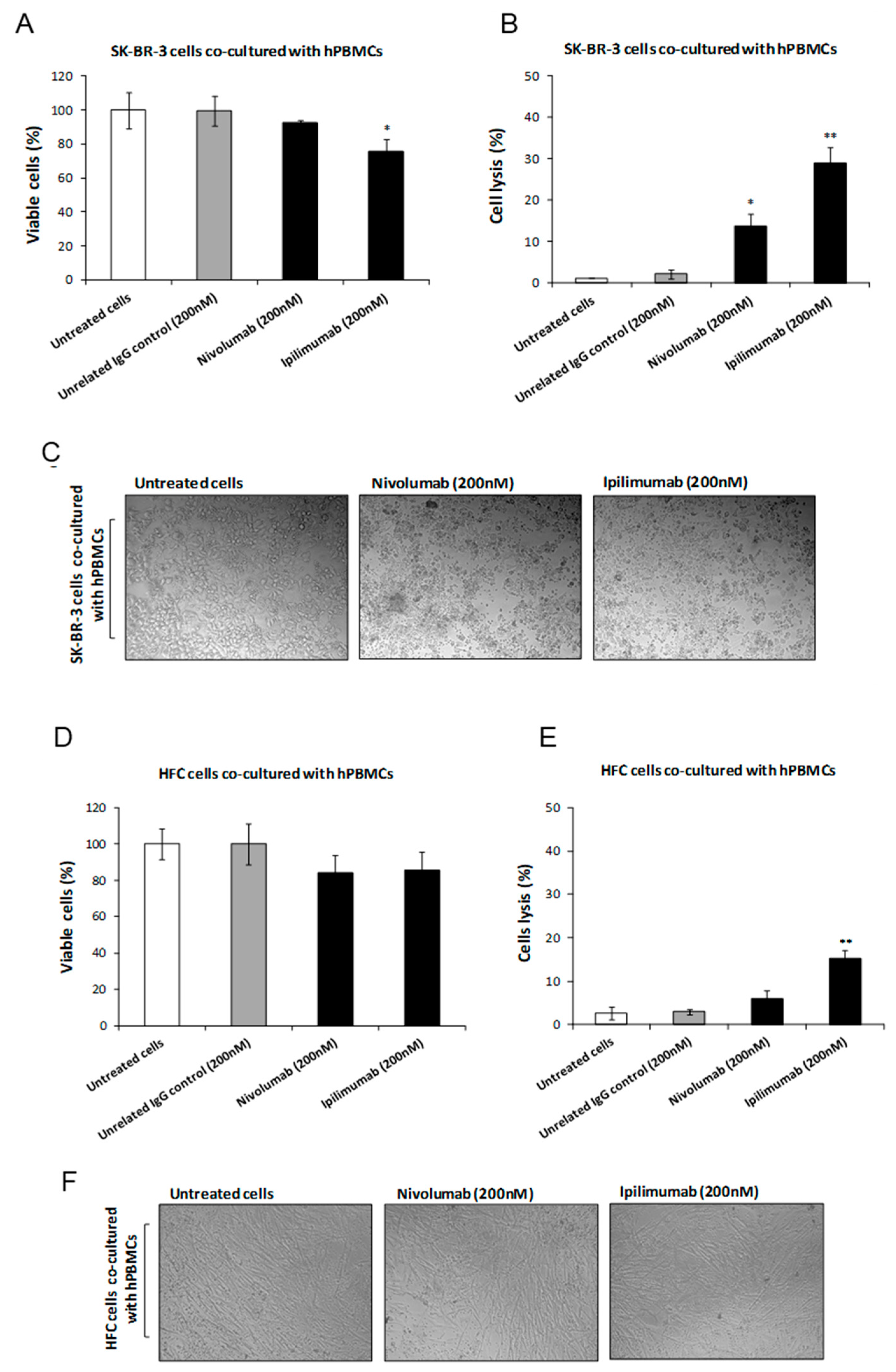
3.1. Effects of Ipilimumab and Nivolumab on Co-Cultures of hPBMCs and Tumor or Cardiac Cells
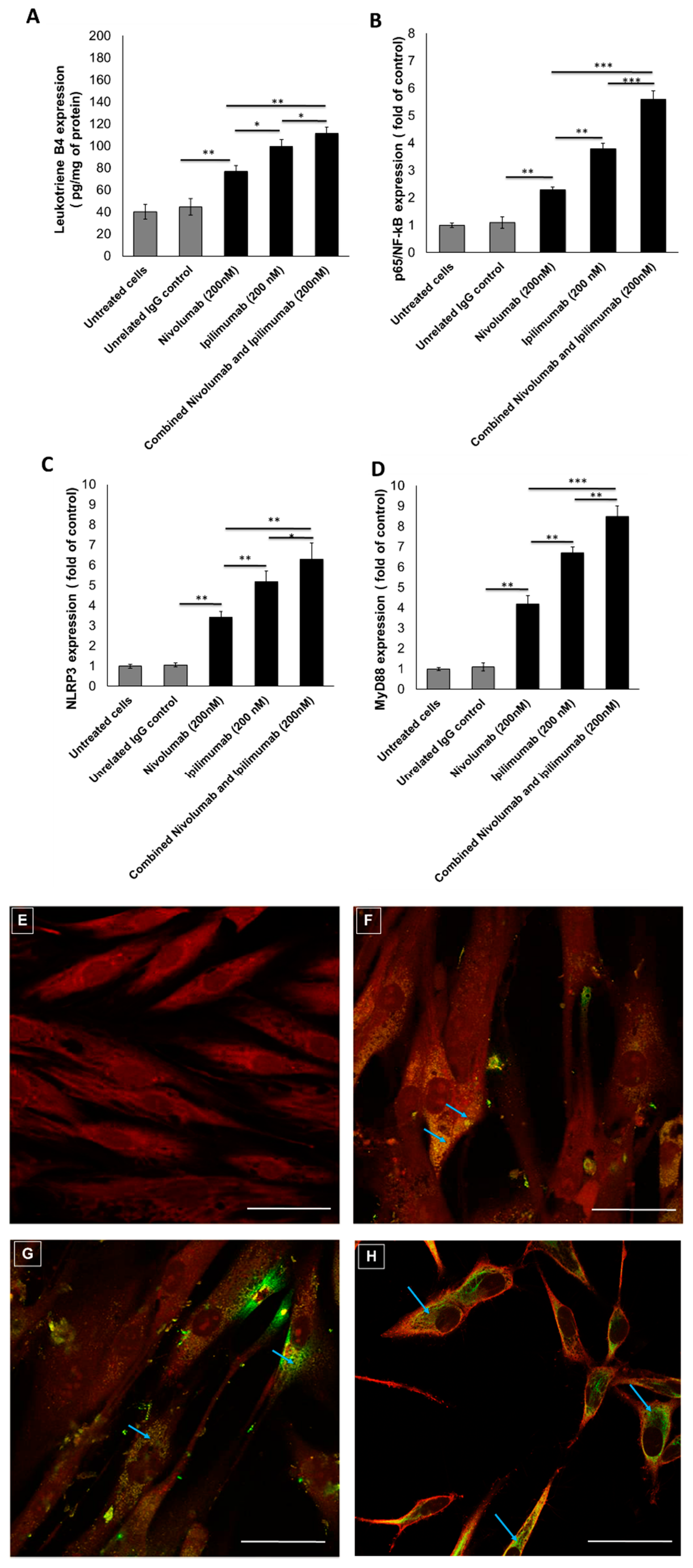
3.2. Pro-Inflammatory Markers after Treatments with Immune Checkpoint Inhibitors (ICIs)
3.3. NLRP3 Staining by Confocal Laser Scanning Microscope
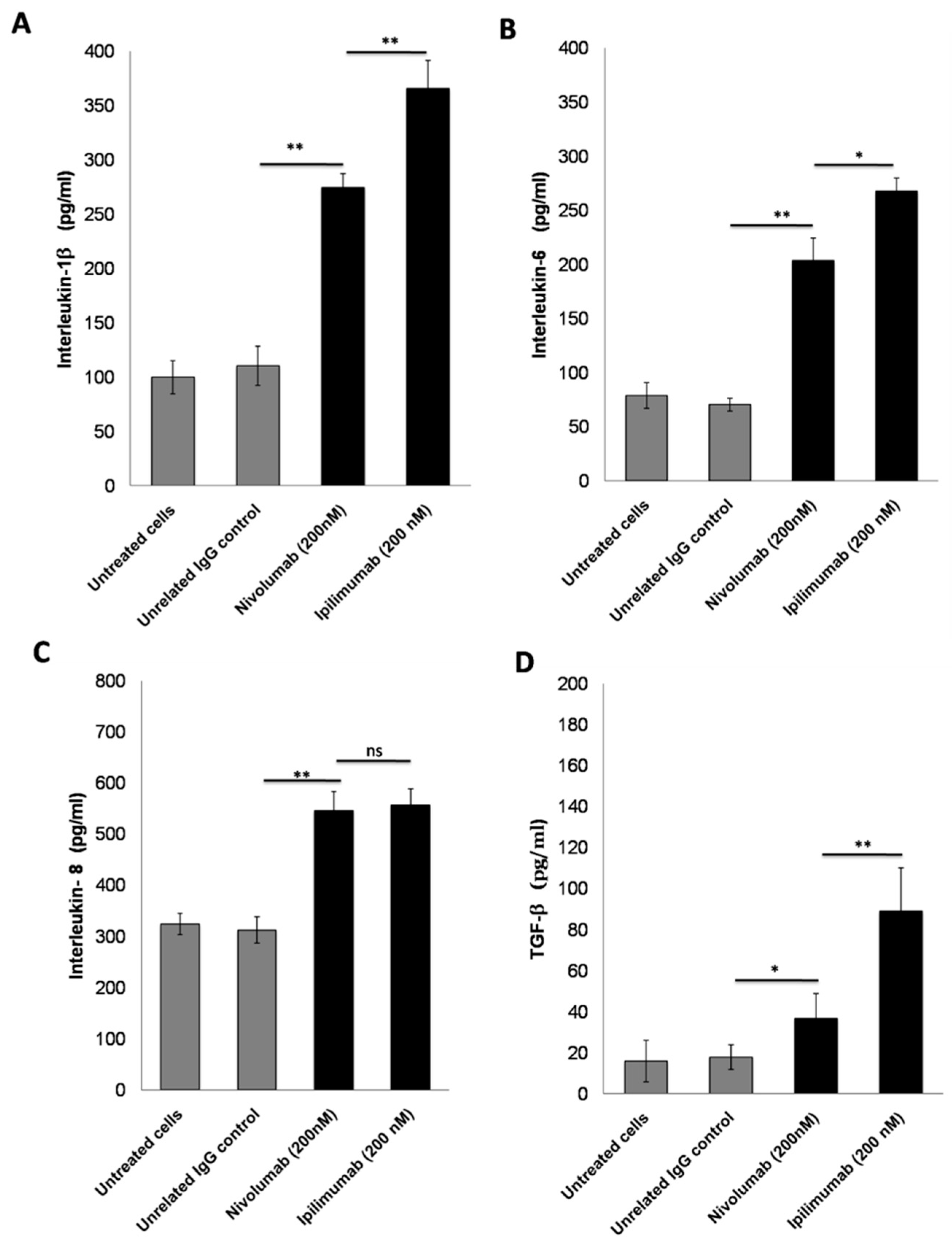
3.4. Secretion of Pro-Inflammatory Cytokines after Treatment with ICIs
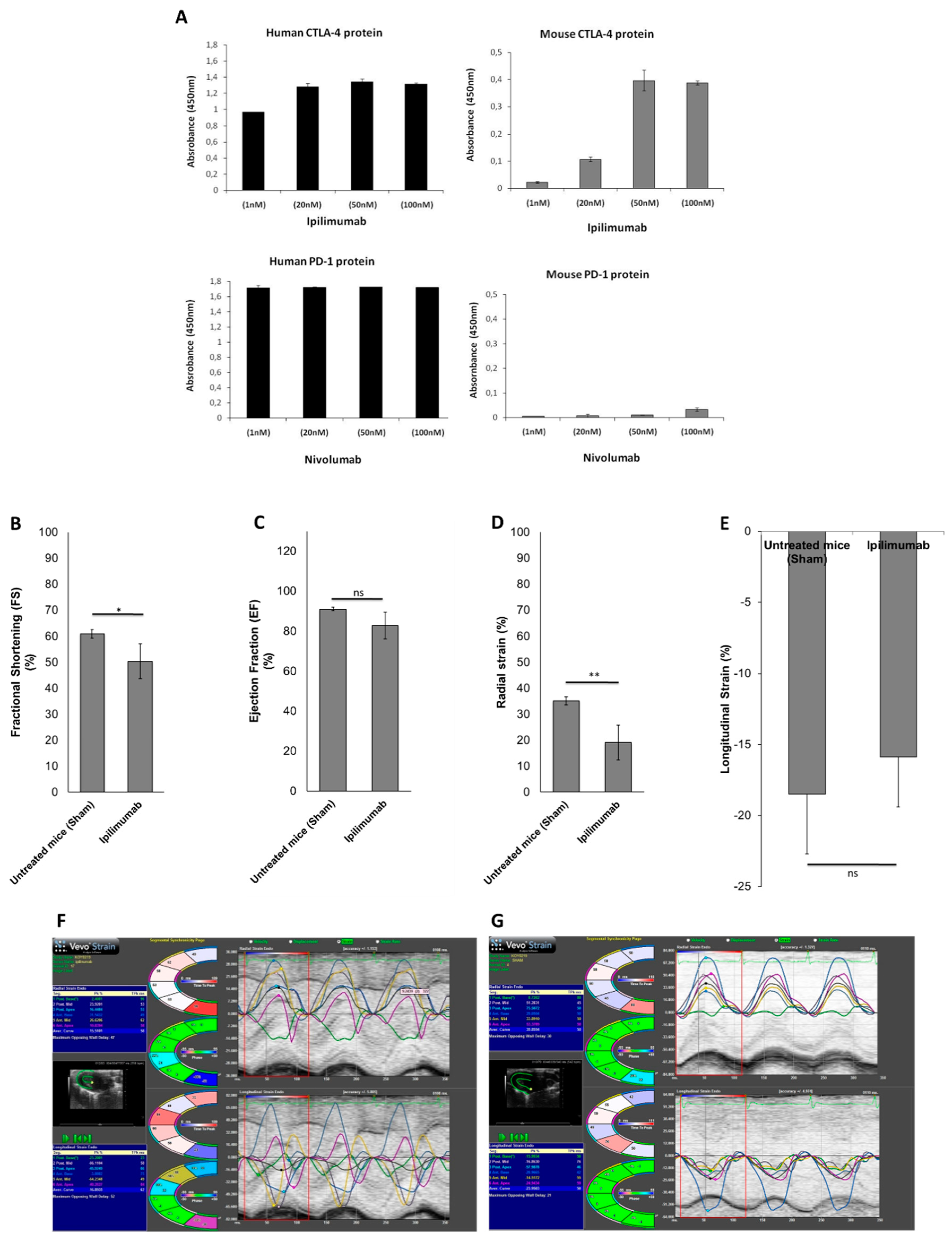
3.5. Preclinical Studies
3.5.1. Echocardiographic Evaluation of Ventricular Function in Mice
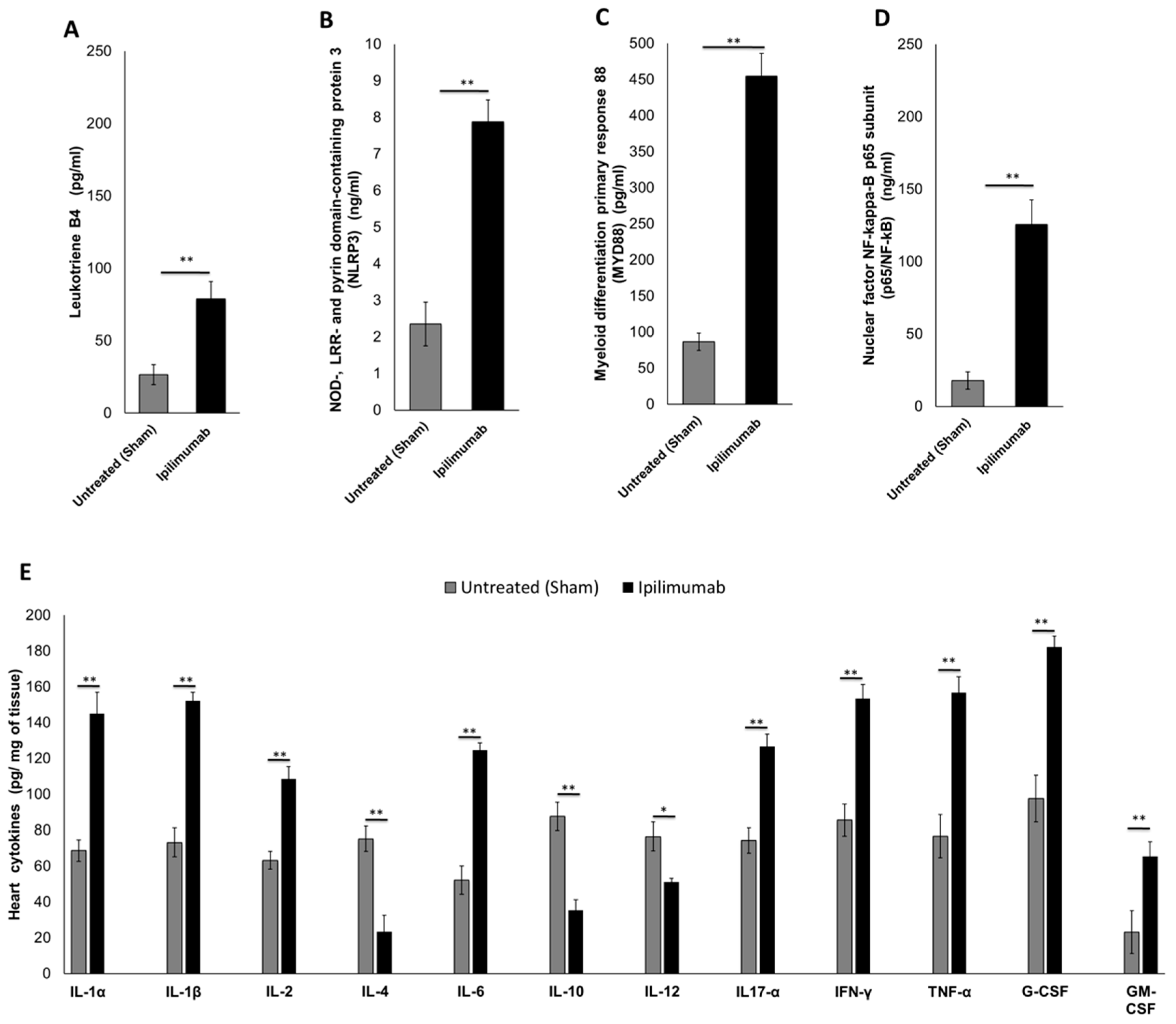
3.5.2. Pro-Inflammatory Markers in Cardiac Tissue (Leukotrienes B4, p65/NF-κB, NLRP3 Inflammasome and MyD88 Complex)
3.5.3. 12-Cytokines Multiplex Assay
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Soyano, A.E.; Dholaria, B.; Knutson, K.L.; Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Nivolumab: A Review in advanced nonsquamous non-small cell lung cancer. Drugs 2016, 76, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Du Rusquec, P.; De Calbiac, O.; Robert, M.; Campone, M.; Frenel, J.S. Clinical utility of pembrolizumab in the management of advanced solid tumors: An evidence-based review on the emerging new data. Cancer Manag. Res. 2019, 11, 4297–4312. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Hui, R.; Özgüroğlu, M.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): A randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1670–1680. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: An emerging issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.L.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Zhou, Y.-W.; Zhu, Y.-J.; Wang, M.-N.; Xie, Y.; Chen, C.-Y.; Zhang, T.; Xia, F.; Ding, Z.-Y.; Liu, J.-Y. Immune checkpoint inhibitor-associated cardiotoxicity: Current understanding on its mechanism, diagnosis and management. Front. Pharmacol. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, C.; Paciello, R.; Riccio, G.; Rea, D.; Barbieri, A.; Coppola, C.; Maurea, N. Cardiotoxic effects of the novel approved anti-ErbB2 agents and reverse cardioprotective effects of ranolazine. OncoTargets Ther. 2018, 11, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, T.; Damiano, V.; Rosa, R.; Bianco, R.; Cozzolino, R.; Tortora, G.; Laccetti, P.; D’Alessio, G.; De Lorenzo, C. Two novel human anti-ErbB2 immunoagents are active on trastuzumab-resistant tumours. Br. J. Cancer 2010, 102, 513–519. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Coppola, C.; Rea, D.; Barbieri, A.; Scherillo, M.; Monti, M.; Iaffaioli, R.; De Laurentiis, M.; Ascierto, P.; et al. Cardiotoxicity and pro-inflammatory effects of the immune checkpoint inhibitor Pembrolizumab associated to Trastuzumab. Int. J. Cardiol. 2019, 292, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Camorani, S.; Vetrei, C.; Ricci, S.; Cerchia, L.; De Lorenzo, C. Ipilimumab and its derived EGFR aptamer-based conjugate induce efficient NK cell activation against cancer cells. Cancers 2020, 12, 331. [Google Scholar] [CrossRef]
- Borriello, M.; Laccetti, P.; Terrazzano, G.; D’Alessio, G.; De Lorenzo, C. A novel fully human antitumour immunoRNase targeting ErbB2-positive tumours. Br. J. Cancer 2011, 104, 1716–1723. [Google Scholar] [CrossRef][Green Version]
- Sasso, E.; D’Avino, C.; Passariello, M.; D’Alise, A.M.; Siciliano, D.; Esposito, M.L.; Froechlich, G.; Cortese, R.; Scarselli, E.; Zambrano, N.; et al. Massive parallel screening of phage libraries for the generation of repertoires of human immunomodulatory monoclonal antibodies. MAbs 2018, 10, 1060–1072. [Google Scholar] [CrossRef]
- Barbarisi, M.; Barbarisi, A.; De Sena, G.; Armenia, E.; Aurilio, C.; Libutti, M.; Iaffaioli, R.V.; Botti, G.; Maurea, N.; Quagliariello, V. Boswellic acid has anti-inflammatory effects and enhances the anticancer activities of Temozolomide and Afatinib, an irreversible ErbB family blocker, in human glioblastoma cells. Phytother. Res. 2019, 33, 1670–1682. [Google Scholar] [CrossRef]
- Poulaki, V.; Mitsiades, C.S.; Joussen, A.M.; Lappas, A.; Kirchhof, B.; Mitsiades, N. Constitutive nuclear factor-κB activity is crucial for human retinoblastoma cell viability. Am. J. Pathol. 2002, 161, 2229–2240. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, D.; Sun, L. Clinical Significance of High-Mobility Group Box 1 Protein (HMGB1) and Nod-Like Receptor Protein 3 (NLRP3) in patients with ulcerative colitis. Med. Sci. Monit. 2020, 26, e919530. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, B.; Zhang, L.; Zhang, Z.; Wang, L.; Tang, L.; Li, S.; Yang, Y.; Yang, F.; Zhang, P.; et al. MyD88-dependent Toll-like receptor 4 signal pathway in intervertebral disc degeneration. Exp. Ther. Med. 2016, 12, 611–618. [Google Scholar] [CrossRef]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic acid nanohydrogel loaded with quercetin alone or in combination to a macrolide derivative of Rapamycin RAD001 (Everolimus) as a new treatment for hormone-responsive human breast cancer. J. Cell. Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, X.; Zhang, B.; Zhang, R.; Ji, H.; Wang, X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J. Transl. Med. 2014, 12, 36. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Woods, A.; Fu, T.; He, Q.; Nielsen, M.C.; Hasan, F.; Roszik, J.; Xiao, Z.; Vianden, C.; Khong, H.; et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J. Clin. Investig. 2018, 128, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Ageing Australian Government. Australian Public Assessment Report for IPILIMUMAB; Bristol-Myers Squibb Australia Pty Ltd.: Mulgrave, Australia, 2011.
- Hanaizi, Z.; Van Zwieten-Boot, B.; Calvo, G.; Lopez, A.S.; Van Dartel, M.; Camarero, J.; Abadie, E.; Pignatti, F. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: Summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur. J. Cancer 2012, 48, 237–242. [Google Scholar] [CrossRef]
- Pmda. Available online: https://www.pmda.go.jp/files/000215223.pdf (accessed on 3 June 2015).
- Selby, M.J.; Engelhardt, J.J.; Johnston, R.J.; Lu, L.-S.; Han, M.; Thudium, K.; Yao, D.; Quigley, M.; Valle, J.; Wang, C.; et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: Mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS ONE 2016, 11, e0161779. [Google Scholar] [CrossRef]
- Rea, D.; Coppola, C.; Barbieri, A.; Monti, M.G.; Misso, G.; Palma, G.; Bimonte, S.; Zarone, M.R.; Luciano, A.; Liccardo, D.; et al. Strain analysis in the assessment of a mouse model of cardiotoxicity due to chemotherapy: Sample for preclinical research. In Vivo 2016, 30, 279–290. [Google Scholar]
- Peng, Y.; Popovic, Z.B.; Sopko, N.; Drinko, J.; Zhang, Z.; Thomas, J.D.; Penn, M.S. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am. J. Physiol. Circ. Physiol. 2009, 297, H811–H820. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Coppola, C.; Mita, D.; Piscopo, G.; Iaffaioli, R.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Vecchione, R.; Quagliariello, V.; Calabria, D.; Calcagno, V.; De Luca, E.; Iaffaioli, R.V.; Netti, P.A. Curcumin bioavailability from oil in water nano-emulsions: In vitro and in vivo study on the dimensional, compositional and interactional dependence. J. Control. Release 2016, 233, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, M.; Su, J.; Zhang, P.; Tang, F.; Ye, P.; Devenport, M.; Wang, X.; Zhang, Y.; Liu, Y.; et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018, 28, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Toldo, S.; Chojnacki, J.; Mezzaroma, E.; Liu, K.; Salloum, F.N.; Nordio, A.; Carbone, S.; Mauro, A.G.; Das, A.; et al. Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J. Cardiovasc. Pharmacol. 2015, 66, 1–8. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, C.; Chen, Z.; Liu, L.; Jiang, J.; Wu, Z.; Zhao, M.; Chen, Y. NLRP3: A novel mediator in cardiovascular disease. J. Immunol. Res. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, G.S.; Cho, W.K.; Park, H.C. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2416–2429. [Google Scholar] [CrossRef]
- Jannin, A.; Penel, N.; Ladsous, M.; Vantyghem, M.C.; Cao, C.D. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit. Rev. Oncol. 2019, 141, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Banna, G.L.; Metro, G.; Di Maio, M. Chemotherapy in combination with immune checkpoint inhibitors for the first-line treatment of patients with advanced non-small cell lung cancer: A systematic review and literature-based meta-analysis. Front. Oncol. 2019, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Myers, G. Immune-related adverse events of immune checkpoint inhibitors: A brief review. Curr. Oncol. 2018, 25, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Huertas, R.M.; Serrano, C.S.; Perna, C.; Gómez, A.F.; Alonso-Gordoa, T. Cardiac toxicity of immune-checkpoint inhibitors: A clinical case of nivolumab-induced myocarditis and review of the evidence and new challenges. Cancer Manag. Res. 2019, 11, 4541–4548. [Google Scholar] [CrossRef]
- Yun, S.; Vincelette, N.D.; Mansour, I.; Hariri, D.; Motamed, S. Late onset ipilimumab-induced pericarditis and pericardial effusion: A rare but life threatening complication. Case Rep. Oncol. Med. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Sakai, T.; Yahagi, K.; Hoshino, T.; Yokota, T.; Tanabe, K.; Mori, M.; Ikeda, S. Nivolumab-induced myocardial necrosis in a patient with lung cancer: A case report. Respir. Med. Case Rep. 2019, 27, 100839. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Keshino, E.; Makiyama, A.; Sasaguri, T.; Ohshima, K.; Katano, H.; Mohri, M. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ. Heart Fail. 2016, 9, 003514. [Google Scholar] [CrossRef]
- Geisler, B.P.; Raad, R.; Esaian, D.; Sharon, E.; Schwartz, D.R. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: A case of takotsubo-like syndrome. J. Immunother. Cancer 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.R.S.; Gonzalez, N.C.; Wood, J.G. Leukotriene B (4) promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J. Appl. Physiol. 2001, 91, 1160–1167. [Google Scholar] [CrossRef]
- Mijatovic, T.; Kruys, V.; Caput, D.; Defrance, P.; Huez, G. Interleukin-4 and -13 inhibit tumor necrosis factor-α mRNA translational activation in lipopolysaccharide-induced mouse macrophages. J. Biol. Chem. 1997, 272, 14394–14398. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Thomas, P.; Breit, S.; Dugas, M.; Mailhammer, R.; Van Eden, W.; Van Der Zee, R.; Biedermann, T.; Prinz, J.; Mack, M.; et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 2002, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Malefyt, W.R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, C.; Paciello, R.; Riccio, G.; Coppola, C.; Coppola, M.; Laccetti, P.; Maurea, N.; Raines, R.T.; De Lorenzo, C. Effects of a second-generation human anti-ErbB2 ImmunoRNase on trastuzumab-resistant tumors and cardiac cells. Protein Eng. Des. Sel. 2014, 27, 83–88. [Google Scholar] [CrossRef][Green Version]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. National comprehensive cancer network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.J.; Scemama, U.; Jacquier, A.; et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef]
- Pagès, C.; Gornet, J.M.; Monsel, G.; Allez, M.; Bertheau, P.; Bagot, M.; Lebbé, C.; Viguier, M. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013, 23, 227–230. [Google Scholar] [CrossRef]
- Tay, R.Y.; Blackley, E.; McLean, C.; Moore, M.; Bergin, P.; Gill, S.; Haydon, A. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br. J. Cancer 2017, 117, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef]
- Jain, V.; Bahia, J.; Mohebtash, M.; Barac, A. Cardiovascular complications associated with novel cancer immunotherapies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for severe immune checkpoint inhibitor–Associated myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 27, 546. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, C.; Khozin, S.; Suzman, D.; Zhang, L.; Tang, S.; Wahby, S.; Goldberg, K.B.; Kim, G.; Pazdur, R.U.S. food and drug administration approval summary: Atezolizumab for metastatic non–small cell lung cancer. Clin. Cancer Res. 2017, 23, 4534–4539. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Schwartz, R.S. Immunosuppression—The promise of specificity. N. Engl. J. Med. 2005, 353, 836–839. [Google Scholar] [CrossRef]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef]
- Gabay, C.; Emery, P.; Van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Winiarska, M.; Olszewska, M.; Rudnicka, L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Adv. Dermatol. Allergol. 2016, 4, 247–252. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Passariello, M.; Rea, D.; Barbieri, A.; Iovine, M.; Bonelli, A.; Caronna, A.; Botti, G.; De Lorenzo, C.; Maurea, N. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. J. Pers. Med. 2020, 10, 179. https://doi.org/10.3390/jpm10040179
Quagliariello V, Passariello M, Rea D, Barbieri A, Iovine M, Bonelli A, Caronna A, Botti G, De Lorenzo C, Maurea N. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. Journal of Personalized Medicine. 2020; 10(4):179. https://doi.org/10.3390/jpm10040179
Chicago/Turabian StyleQuagliariello, Vincenzo, Margherita Passariello, Domenica Rea, Antonio Barbieri, Martina Iovine, Annamaria Bonelli, Antonietta Caronna, Gerardo Botti, Claudia De Lorenzo, and Nicola Maurea. 2020. "Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models" Journal of Personalized Medicine 10, no. 4: 179. https://doi.org/10.3390/jpm10040179
APA StyleQuagliariello, V., Passariello, M., Rea, D., Barbieri, A., Iovine, M., Bonelli, A., Caronna, A., Botti, G., De Lorenzo, C., & Maurea, N. (2020). Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. Journal of Personalized Medicine, 10(4), 179. https://doi.org/10.3390/jpm10040179







