Resting-State Isolated Effective Connectivity of the Cingulate Cortex as a Neurophysiological Biomarker in Patients with Severe Treatment-Resistant Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessments
2.3. Measurement and Preprocessing of Resting-State EEG
2.4. iCoh Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinico-Demographics Data
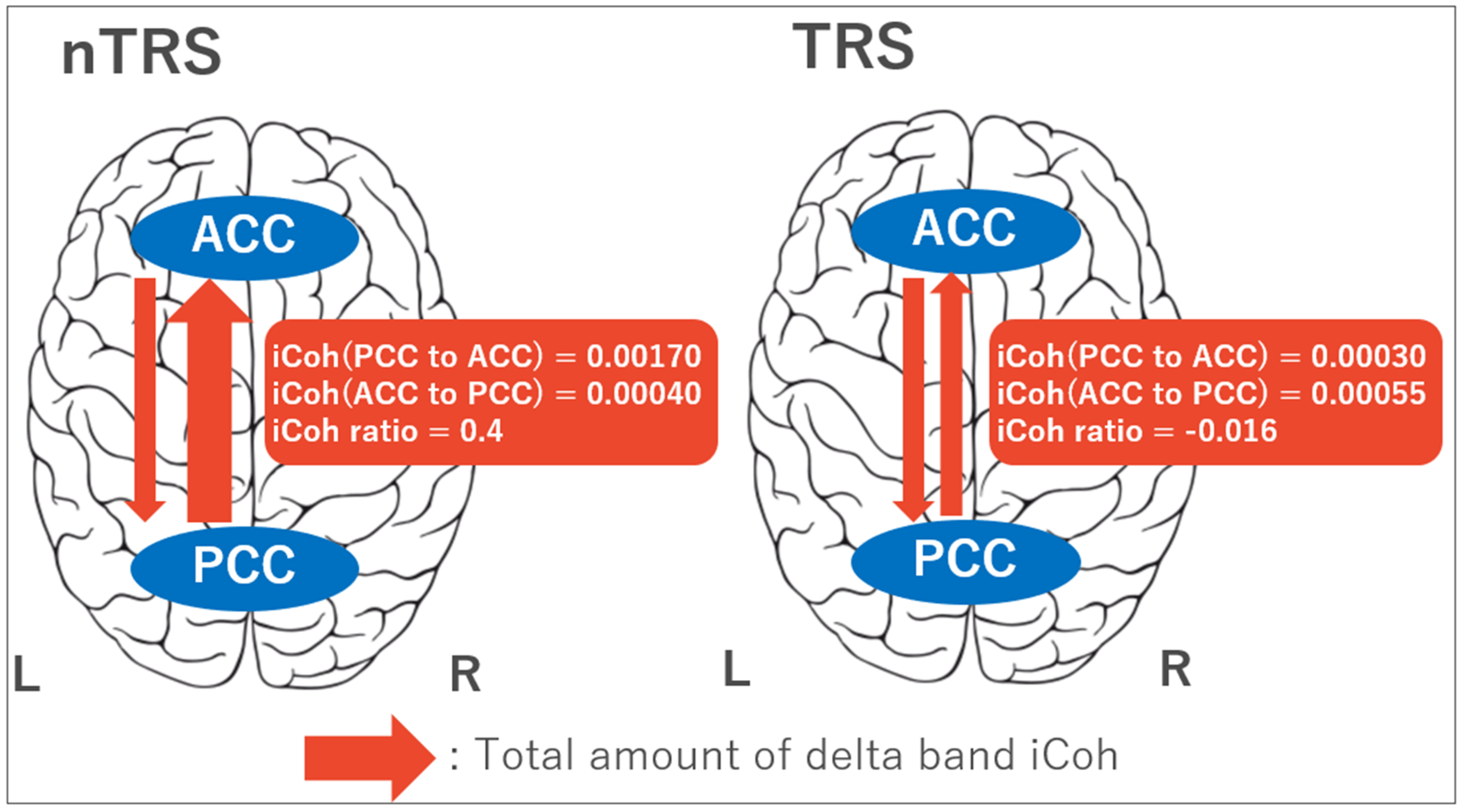
3.2. Four-Way ANOVA for iCoh Values
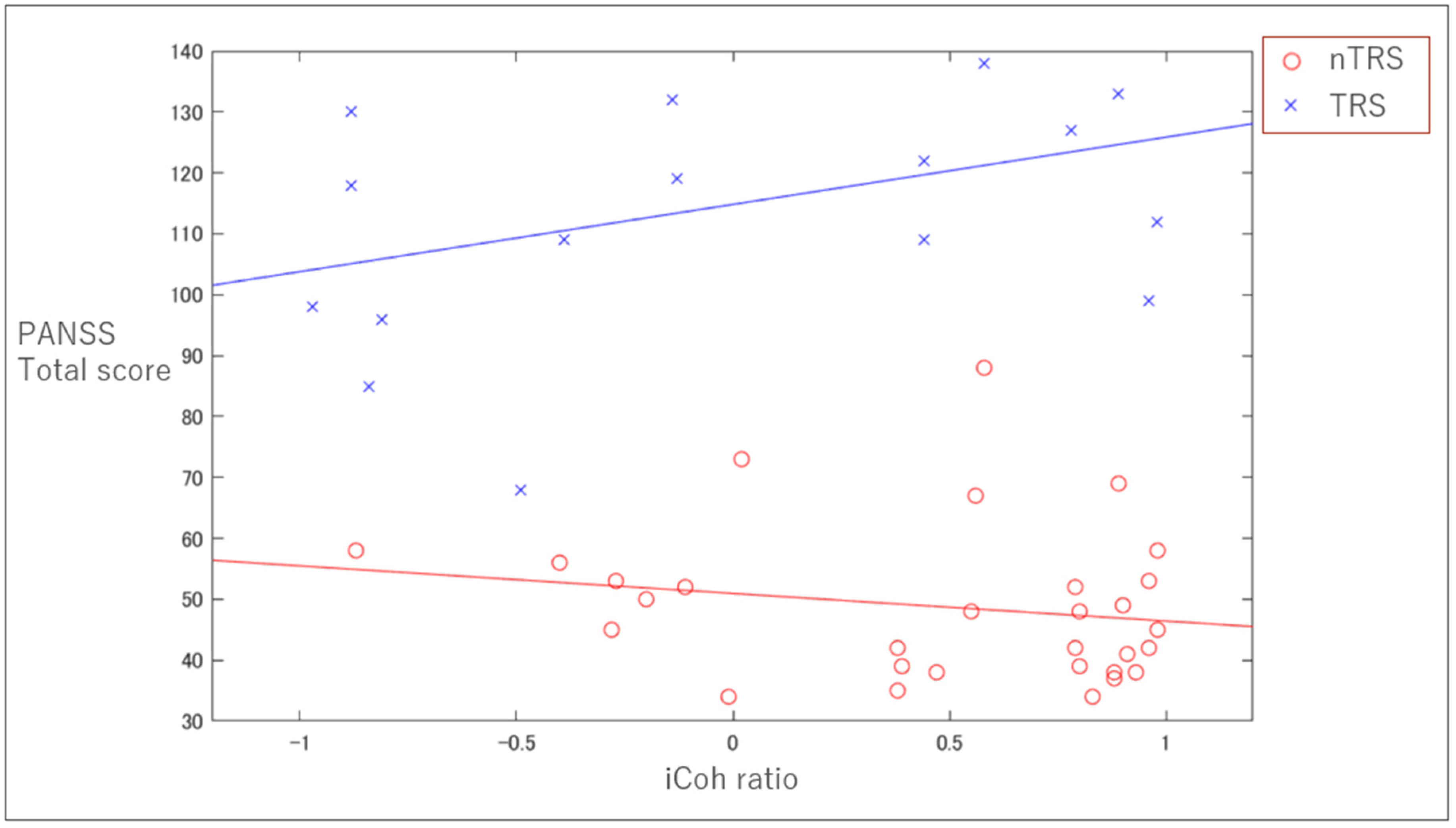
3.3. Clinical Correlation with iCoh
3.4. ROC Analysis of the iCoh Ratio between TRS and nTRS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | anterior cingulate cortex |
| CPZ | chlorpromazine |
| DMN | default mode network |
| DBS | deep brain stimulation |
| EEG | electroencephalography |
| fMRI | functional magnetic resonance imaging |
| iCoh | isolated effective coherence |
| IPL | inferior parietal lobe |
| ITL | inferior temporal lobe |
| mPFC | medial prefrontal cortex |
| MNI | Montreal Neurological Institute |
| PANSS | positive and negative syndrome scale |
| PCC | posterior cingulate cortex |
| rm-ANOVA | repeated-measures analysis of variance |
| ROC | receiver operating characteristic |
| ROIs | regions of interest |
| sLORETA | standardized low-resolution brain electromagnetic tomography |
| TRRIP | treatment response and resistance in psychosis |
| TMS | transcranial magnetic stimulation |
| TRS | treatment-resistant schizophrenia |
| nTRS | non treatment-resistant schizophrenia |
References
- Hasan, A.; Falkai, P.; Wobrock, T.; Lieberman, J.; Glenthoj, B.; Gattaz, W.F.; Thibaut, F.; Möller, H.-J. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 2012, 13, 318–378. [Google Scholar] [CrossRef] [PubMed]
- Samara, M.T.; Dold, M.; Gianatsi, M.; Nikolakopoulou, A.; Helfer, B.; Salanti, G.; Leucht, S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia: A network meta-analysis. JAMA Psychiatry 2016, 73, 199. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Takeuchi, H.; Plitman, E.; Fervaha, G.; Gerretsen, P.; Caravaggio, F.; Chung, J.K.; Iwata, Y.; Remington, G.; Graff-Guerrero, A. Neuroimaging findings in treatment-resistant schizophrenia: A systematic review: Lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr. Res. 2015, 164, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, C.S.; Macdonald, A.; Ross, L.L.; Stenger, V.A. Anterior Cingulate Cortex Activity and Impaired Self-Monitoring of Performance in Patients with Schizophrenia: An Event-Related fMRI Study. Am. J. Psychiatry 2001, 158, 1423–1428. [Google Scholar] [CrossRef]
- Walter, H.; Ciaramidaro, A.; Adenzato, M.; Vasic, N.; Ardito, R.B.; Erk, S.; Bara, B.G. Dysfunction of the social brain in schizophrenia is modulated by intention type: An fMRI study. Soc. Cogn. Affect. Neurosci. 2009, 4, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Tian, L.; Yan, J.; Sun, W.; Liu, Q.; Zhang, Y.-B.; Li, X.-M.; Zang, Y.-F.; Zhang, D. Functional and Anatomical Connectivity Abnormalities in Cognitive Division of Anterior Cingulate Cortex in Schizophrenia. PLoS ONE 2012, 7, e45659. [Google Scholar] [CrossRef]
- Salgado-Pineda, P.; Landin-Romero, R.; Fakra, E.; Delaveau, P.; Amann, B.L.; Blin, O. Structural Abnormalities in Schizophrenia: Further Evidence on the Key Role of the Anterior Cingulate Cortex. Neuropsychobiology 2014, 69, 52–58. [Google Scholar] [CrossRef]
- Mouchlianitis, E.; Bloomfield, M.A.P.; Law, V.; Beck, K.; Selvaraj, S.; Rasquinha, N.; Waldman, A.D.; Turkheimer, F.E.; Egerton, A.; Stone, J.; et al. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr. Bull. 2015, 42, 744–752. [Google Scholar] [CrossRef]
- Iwata, Y.; Nakajima, S.; Plitman, E.; Caravaggio, F.; Kim, J.; Shah, P.; Mar, W.; Chavez, S.; De Luca, V.; Mimura, M.; et al. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol. Psychiatry 2019, 85, 596–605. [Google Scholar] [CrossRef]
- Demjaha, A.; Egerton, A.; Murray, R.M.; Kapur, S.; Howes, O.D.; Stone, J.; McGuire, P. Antipsychotic Treatment Resistance in Schizophrenia Associated with Elevated Glutamate Levels but Normal Dopamine Function. Boil. Psychiatry 2014, 75, e11–e13. [Google Scholar] [CrossRef]
- Tarumi, R.; Tsugawa, S.; Noda, Y.; Plitman, E.; Honda, S.; Matshusita, K.; Chavez, S.; Sawada, K.; Wada, M.; Matsui, M.; et al. Levels of glutamatergic neurometabolites in patients with severe treatment-resistant schizophrenia: A proton magnetic resonance spectroscopy study. Neuropsychopharmacology 2020, 46, S313. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.J.; Pearlson, G.D.; Stevens, M.C.; Calhoun, V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage 2008, 39, 1666–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skudlarski, P.; Jagannathan, K.; Anderson, K.; Stevens, M.C.; Calhoun, V.D.; Skudlarska, B.A.; Pearlson, G. Brain Connectivity Is Not Only Lower but Different in Schizophrenia: A Combined Anatomical and Functional Approach. Biol. Psychiatry 2010, 68, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.; Yu, M.; Tang, X.; Wang, X.; Zhang, X.; Zhang, X.R.; Chen, J. Convergent and divergent altered patterns of default mode network in deficit and non-deficit schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2019, 89, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Schacter, D.L.; Andrews-Hanna, J.R. The Brain’s Default Network. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wójcik, M.; Karakuła-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.-K.; Park, K.; Kim, C.-E.; Ryu, S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. NeuroImage Clin. 2019, 22, 101805. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Abram, S.V.; Reilly, J.L.; Eack, S.; Goldman, M.B.; Csernansky, J.G.; Wang, L.; Smith, M.J. Default mode functional connectivity is associated with social functioning in schizophrenia. J. Abnorm. Psychol. 2017, 126, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, R.L.; Miller, J.; Lanius, R.A.; Osuch, E.A.; Boksman, K.; Neufeld, R.; Théberge, J.; Schaefer, B.; Williamson, P. Spontaneous Low-Frequency Fluctuations in the BOLD Signal in Schizophrenic Patients: Anomalies in the Default Network. Schizophr. Bull. 2007, 33, 1004–1012. [Google Scholar] [CrossRef]
- Garrity, A.G.; Pearlson, G.D.; McKiernan, K.; Lloyd, D.; Kiehl, K.A.; Calhoun, V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry 2007, 164, 450–457. [Google Scholar] [CrossRef]
- Hare, S.M.; Ford, J.M.; Mathalon, D.H.; Damaraju, E.; Bustillo, J.; Belger, A.; Lee, H.J.; A Mueller, B.; O Lim, K.; Brown, G.G.; et al. Salience–Default Mode Functional Network Connectivity Linked to Positive and Negative Symptoms of Schizophrenia. Schizophr. Bull. 2018, 45, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Solís, A.; Vives-Gilabert, Y.; Grasa, E.; Portella, M.J.; Rabella, M.; Sauras, R.B.; Roldán, A.; Núñez-Marín, F.; Gomez-Anson, B.; Pérez, V.; et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 2015, 161, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Whitford, T.J.; Lee, S.W.; Oh, J.S.; De Luis-García, R.; Savadjiev, P.; Alvarado, J.L.; Westin, C.-F.; Niznikiewicz, M.; Nestor, P.G.; McCarley, R.W.; et al. Localized abnormalities in the cingulum bundle in patients with schizophrenia: A Diffusion Tensor tractography study. NeuroImage Clin. 2014, 5, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaniyappan, L.; Al-Radaideh, A.; Mougin, O.; Das, T.; Gowland, P.A.; Liddle, P.F. Aberrant myelination of the cingulum and Schneiderian delusions in schizophrenia: A 7T magnetization transfer study. Psychol. Med. 2018, 49, 1890–1896. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Biscay, R.J.; Bosch-Bayard, J.; Lehmann, D.; Kochi, K.; Kinoshita, T.; Yamada, N.; Sadato, N. Assessing direct paths of intracortical causal information flow of oscillatory activity with the isolated effective coherence (iCoh). Front. Hum. Neurosci. 2014, 8, 448. [Google Scholar] [CrossRef]
- Schelter, B.; Timmer, J.; Eichler, M. Assessing the strength of directed influences among neural signals using renormalized partial directed coherence. J. Neurosci. Methods 2009, 179, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Plomp, G.; Quairiaux, C.; Michel, C.M.; Astolfi, L. The physiological plausibility of time-varying Granger-causal modeling: Normalization and weighting by spectral power. NeuroImage 2014, 97, 206–216. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.A.; Agid, O.; De Bartolomeis, A.; Van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.A.P.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Kitaura, Y.; Nishida, K.; Yoshimura, M.; Mii, H.; Katsura, K.; Ueda, S.; Ikeda, S.; Pascual-Marqui, R.D.; Ishii, R.; Kinoshita, T. Functional localization and effective connectivity of cortical theta and alpha oscillatory activity during an attention task. Clin. Neurophysiol. Pr. 2017, 2, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mouchlianitis, E.; McCutcheon, R.A.; Howes, O.D. Brain-imaging studies of treatment-resistant schizophrenia: A systematic review. Lancet Psychiatry 2016, 3, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Coito, A.; Michel, C.M.; Vulliemoz, S.; Plomp, G. Directed functional connections underlying spontaneous brain activity. Hum. Brain Mapp. 2019, 40, 879–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fransson, P.; Marrelec, G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage 2008, 42, 1178–1184. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, B.; Zhang, X.; Li, J.; Qin, W.; Yu, C.; Jiang, T. The Structural Connectivity Pattern of the Default Mode Network and Its Association with Memory and Anxiety. Front. Neuroanat. 2015, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Maddock, R.J.; Garrett, A.S.; Buonocore, M.H. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 2001, 104, 667–676. [Google Scholar] [CrossRef]
- Lefebvre, S.; Demeulemeester, M.; Leroy, A.; Delmaire, C.; Lopes, R.; Pins, D.; Thomas, P.; Jardri, R. Network dynamics during the different stages of hallucinations in schizophrenia. Hum. Brain Mapp. 2016, 37, 2571–2586. [Google Scholar] [CrossRef]
- Manoliu, A.; Riedl, V.; Zherdin, A.; Mühlau, M.; Schwerthöffer, D.; Scherr, M.; Peters, H.; Zimmer, C.; Förstl, H.; Bäuml, J.; et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014, 40, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Lagioia, A.; Van De Ville, D.; Debbané, M.; Lazeyras, F.; Eliez, S. Adolescent resting state networks and their associations with schizotypal trait expression. Front. Syst. Neurosci. 2010, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Tettamanti, M.; Vaghi, M.M.; Bara, B.G.; Cappa, S.F.; Enrici, I.; Adenzato, M. Effective connectivity gateways to the Theory of Mind network in processing communicative intention. NeuroImage 2017, 155, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Quidé, Y.; Wilhelmi, C.; Green, M.J. Structural brain morphometry associated with theory of mind in bipolar disorder and schizophrenia. PsyCh J. 2020, 9, 234–246. [Google Scholar] [CrossRef]
- Corcoran, R.; Mercer, G.; Frith, C.D. Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophr. Res. 1995, 17, 5–13. [Google Scholar] [CrossRef]
- Hakamata, Y.; Iwase, M.; Kato, T.; Senda, K.; Inada, T. The Neural Correlates of Mindful Awareness: A Possible Buffering Effect on Anxiety-Related Reduction in Subgenual Anterior Cingulate Cortex Activity. PLoS ONE 2013, 8, e75526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Deng, H.; Xiao, X.; Zuo, Y.; Sun, J.; Wang, Z. Persistent Neuronal Activity in Anterior Cingulate Cortex Correlates with Sustained Attention in Rats Regardless of Sensory Modality. Sci. Rep. 2017, 7, 43101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-S.; Kang, D.-H.; Kim, J.-J.; Ha, T.-H.; Roh, K.S.; Youn, T.; Kwon, J.S. Decreased caudal anterior cingulate gyrus volume and positive symptoms in schizophrenia. Psychiatry Res. Neuroimaging 2005, 139, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.D.; Bjorkquist, O.A.; Olsen, E.K.; Herbener, E.S. Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: An fMRI investigation. Psychiatry Res. 2015, 234, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Ohi, K.; Shimada, T.; Nemoto, K.; Kataoka, Y.; Yasuyama, T.; Kimura, K.; Okubo, H.; Uehara, T.; Kawasaki, Y. Cognitive clustering in schizophrenia patients, their first-degree relatives and healthy subjects is associated with anterior cingulate cortex volume. NeuroImage Clin. 2017, 16, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Oestreich, L.K.; Pasternak, O.; Shenton, M.E.; Kubicki, M.; Gong, X.; Whitford, T.J.; Australian Schizophrenia Research Bank; McCarthy-Jones, S.; Whitford, T.J. Abnormal white matter microstructure and increased extracellular free-water in the cingulum bundle associated with delusions in chronic schizophrenia. NeuroImage Clin. 2016, 12, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Sun, H.; Shi, S.; Jiang, D.; Tao, B.; Zhao, Y.; Zhang, W.; Gong, Q.; Sweeney, J.A.; Lui, S. White Matter Abnormalities in Never-Treated Patients With Long-Term Schizophrenia. Am. J. Psychiatry 2018, 175, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Ekstrom, A.; Watrous, A.J. Multifaceted roles for low-frequency oscillations in bottom-up and top-down processing during navigation and memory. NeuroImage 2014, 85, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Hlinka, J.; Alexakis, C.; Diukova, A.; Liddle, P.F.; Auer, D.P. Slow EEG pattern predicts reduced intrinsic functional connectivity in the default mode network: An inter-subject analysis. NeuroImage 2010, 53, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Neuner, I.; Arrubla, J.; Werner, C.J.; Hitz, K.; Boers, F.; Kawohl, W.; Shah, N.J. The Default Mode Network and EEG Regional Spectral Power: A Simultaneous fMRI-EEG Study. PLoS ONE 2014, 9, e88214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tik, M.; Hoffmann, A.; Sladky, R.; Tomova, L.; Hummer, A.; De Lara, L.I.N.; Bukowski, H.; Pripfl, J.; Biswal, B.B.; Lamm, C.; et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 2017, 162, 289–296. [Google Scholar] [CrossRef]
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Lehmann, D.; Faber, P.L.; Tei, S.; Pascual-Marqui, R.D.; Milz, P.; Kochi, K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. NeuroImage 2012, 60, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- A Ponomarev, V.; Kropotov, I.D. Improving source localization of ERPs in the GO/NOGO task by modeling of their cross-covariance structure. Fiziol. Cheloveka 2013, 39, 36–50. [Google Scholar]
- Coben, R.; Hammond, D.C.; Arns, M. 19 Channel Z-Score and LORETA Neurofeedback: Does the Evidence Support the Hype? Appl. Psychophysiol. Biofeedback 2019, 44, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Eugene, A.R.; Masiak, J. Identifying Treatment Response of Sertraline in a Teenager with Selective Mutism using Electrophysiological Neuroimaging. Int. J. Clin. Pharmacol. Toxicol. 2016, 5, 216–219. [Google Scholar] [CrossRef]
| nTRS (n = 30) | TRS (n = 17) | t-Value (Chi-Squared Value for Sex), p-Value | |
|---|---|---|---|
| Age, mean (SD), years | 41.2 (12.6) | 42.4 (13.4) | t45 = 0.29, p = 0.78 |
| Sex (number of male) (%) | 13 (43) | 5 (29) | χ245 = 0.89, p = 0.34 |
| Education, mean (SD), years | 13.3 (1.81) | 13.3 (2.42) | t45 = 0.06, p = 0.95 |
| Age of onset, mean (SD), years | 26.0 (9.47) | 26.6 (7.64) | t45 = 0.23, p = 0.82 |
| Treatment duration, mean (SD), years | 14.5 (12.0) | 15.5 (11.3) | t45 = 0.27, p = 0.79 |
| Chlorpromazine equivalents, mean (SD), mg | 406 (233.5) | 748 (319.0) | t45 = 4.22, p < 0.001 * |
| PANSS total, mean (SD) | 48.8 (12.7) | 114.6 (21.2) | t45 = 13.4, p < 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, M.; Nakajima, S.; Tarumi, R.; Masuda, F.; Miyazaki, T.; Tsugawa, S.; Ogyu, K.; Honda, S.; Matsushita, K.; Kikuchi, Y.; et al. Resting-State Isolated Effective Connectivity of the Cingulate Cortex as a Neurophysiological Biomarker in Patients with Severe Treatment-Resistant Schizophrenia. J. Pers. Med. 2020, 10, 89. https://doi.org/10.3390/jpm10030089
Wada M, Nakajima S, Tarumi R, Masuda F, Miyazaki T, Tsugawa S, Ogyu K, Honda S, Matsushita K, Kikuchi Y, et al. Resting-State Isolated Effective Connectivity of the Cingulate Cortex as a Neurophysiological Biomarker in Patients with Severe Treatment-Resistant Schizophrenia. Journal of Personalized Medicine. 2020; 10(3):89. https://doi.org/10.3390/jpm10030089
Chicago/Turabian StyleWada, Masataka, Shinichiro Nakajima, Ryosuke Tarumi, Fumi Masuda, Takahiro Miyazaki, Sakiko Tsugawa, Kamiyu Ogyu, Shiori Honda, Karin Matsushita, Yudai Kikuchi, and et al. 2020. "Resting-State Isolated Effective Connectivity of the Cingulate Cortex as a Neurophysiological Biomarker in Patients with Severe Treatment-Resistant Schizophrenia" Journal of Personalized Medicine 10, no. 3: 89. https://doi.org/10.3390/jpm10030089
APA StyleWada, M., Nakajima, S., Tarumi, R., Masuda, F., Miyazaki, T., Tsugawa, S., Ogyu, K., Honda, S., Matsushita, K., Kikuchi, Y., Fujii, S., Blumberger, D. M., Daskalakis, Z. J., Mimura, M., & Noda, Y. (2020). Resting-State Isolated Effective Connectivity of the Cingulate Cortex as a Neurophysiological Biomarker in Patients with Severe Treatment-Resistant Schizophrenia. Journal of Personalized Medicine, 10(3), 89. https://doi.org/10.3390/jpm10030089






