Failure of Achieving Tacrolimus Target Blood Concentration Might Be Avoided by a Wide Genotyping of Transplanted Patients: Evidence from a Retrospective Study
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
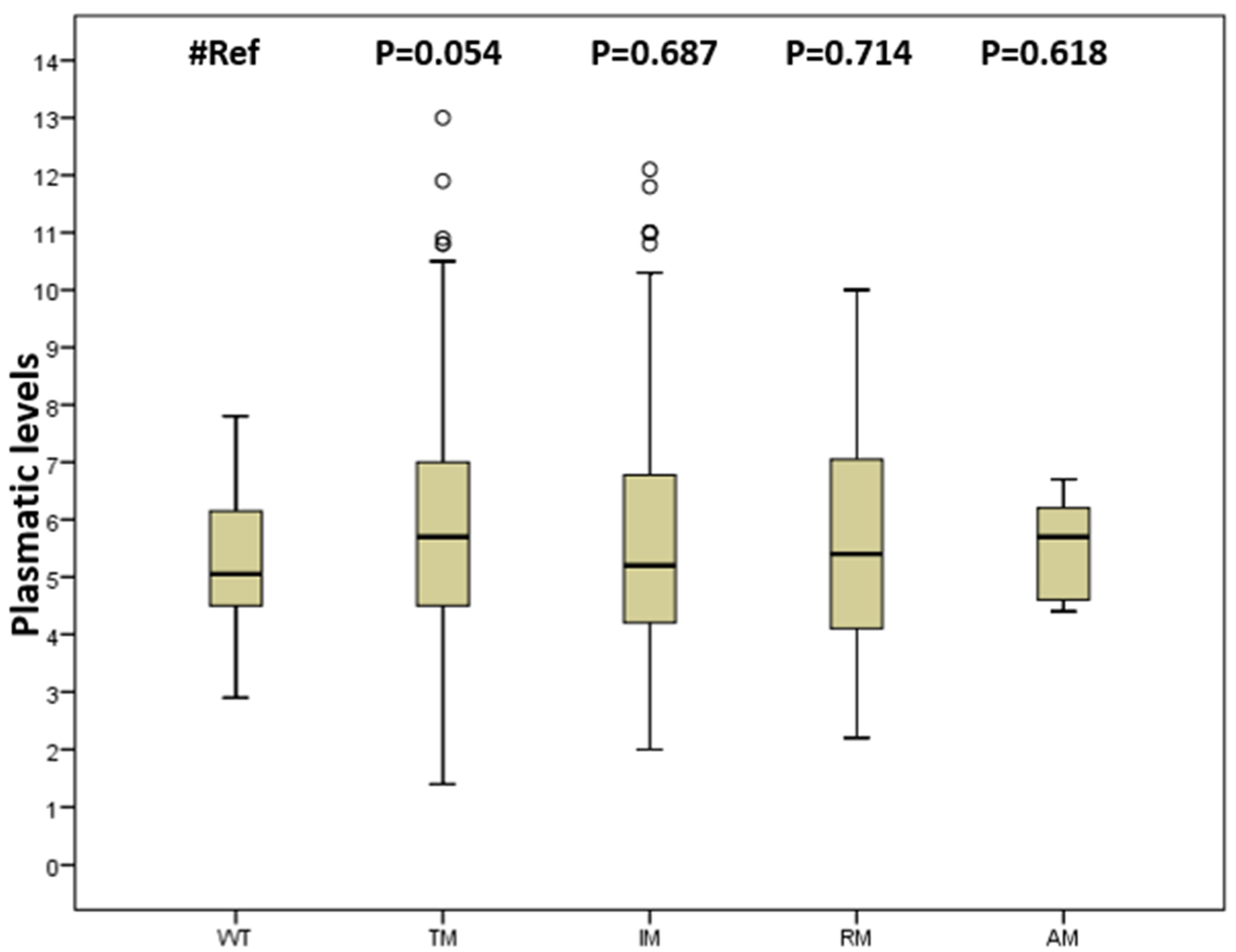
2.2. Tacrolimus Blood Levels
2.3. Tacrolimus Dose
3. Discussion
4. Materials and Methods
4.1. Patients and Data Collection
4.2. Tacrolimus Dosage, DNA Extraction and Real-Time PCR
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bowman, L.J.; Brennan, D.C. The role of tacrolimus in renal transplantation. Expert Opin. Pharmacother. 2008, 9, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kershner, R.P.; Fitzsimmons, W.E. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996, 62, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Le Meur, Y.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Schiff, J.; Cole, E.; Cantarovich, M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin. J. Am. Soc. Nephrol. 2007, 2, 374–384. [Google Scholar] [CrossRef]
- Monchaud, C.; de Winter, B.C.; Knoop, C.; Estenne, M.; Reynaud-Gaubert, M.; Pison, C.; Stern, M.; Kessler, R.; Guillemain, R.; Marquet, P.; et al. Population pharmacokinetic modelling and design of a Bayesian estimator for therapeutic drug monitoring of tacrolimus in lung transplantation. Clin. Pharmacokinet. 2012, 51, 175–186. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar]
- Pauli-Magnus, C.; Kroetz, D.L. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1). Pharm. Res. 2004, 21, 904–913. [Google Scholar] [CrossRef]
- Shuker, N.; Bouamar, R.; Weimar, W.; van Schaik, R.H.; van Gelder, T.; Hesselink, D.A. ATP-binding cassette transporters as pharmacogenetic biomarkers for kidney transplantation. Clin. Chim. Acta 2012, 413, 1326–1337. [Google Scholar]
- Hesselink, D.A.; Bouamar, R.; Elens, L.; van Schaik, R.H.; van Gelder, T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2013, 53, 123–139. [Google Scholar] [CrossRef]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin. Pharmacol. Ther. 2003, 74, 245–254. [Google Scholar] [CrossRef]
- Elens, L.; Van Schaik, R.H.; Panin, N.; de Meyer, M.; Wallemacq, P.; Lison, D.; Mourad, M.; Haufroid, V. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 2011, 12, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Sinues, B.; Vicente, J.; Fanlo, A.; Vasquez, P.; Medina, J.C.; Mayayo, E.; Conde, B.; Arenaz, I.; Martinez-Jarreta, B. CYP3A5*3 and CYP3A4*1B allele distribution and genotype combinations: Differences between Spaniards and Central Americans. Ther. Drug Monit. 2007, 29, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Gervasini, G.; Vizcaino, S.; Gasiba, C.; Carrillo, J.A.; Benitez, J. Differences in CYP3A5*3 genotype distribution and combinations with other polymorphisms between Spaniards and Other Caucasian populations. Ther. Drug Monit. 2005, 27, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Staatz, D.C.E.; Goodman, L.K.; Tett, S.E. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin. Pharmacokinet. 2010, 49, 141–175. [Google Scholar] [CrossRef] [PubMed]
- Elens, L.; Capron, A.; Van Kerckhove, V.R.; Lerut, J.; Mourad, M.; Lison, D.; Wallemacq, P.; Haufroid, V. 1199 G>A and 2677 G> T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharm. Genom. 2007, 17, 873–883. [Google Scholar] [CrossRef]
- Kroetz, D.L.; Pauli-Magnus, C.; Hodges, L.M.; Huang, C.C.; Kawamoto, M.; Johns, S.J.; Stryke, D.; Ferrin, T.E.; DeYoung, J.; Taylor, T.; et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics 2003, 13, 481–494. [Google Scholar] [CrossRef]
- Mancinelli, L. The pharmacokinetics and metabolic disposition of tacrolimus: A comparison across ethnic groups. Clin. Pharmacol. Ther. 2001, 69, 24–31. [Google Scholar] [CrossRef]
- Glowacki, F.; Lionet, A.; Buob, D.; Labalette, M.; Allorge, D.; Provôt, F.; Hazzan, M.; Noël, C.; Broly, F.; Cauffiez, C. CYP3A5 and ABCB1 polymorphisms in donor and recipient: Impact on Tacrolimus dose requirements and clinical outcome after renal transplantation. Nephrol. Dial. Transplant. 2011, 26, 3046–3050. [Google Scholar] [CrossRef]
- Soria, M.L.-M.; Berga, J.K.; Catalan, S.B.; Payà, J.M.; Mateu, L.P.; Torres, N.J. Genetic polymorphisms and individualized tacrolimus dosing. Transpl. Proc. 2010, 42, 3031–3033. [Google Scholar] [CrossRef] [PubMed]
- Macphee, I.A.; Fredericks, S.; Mohamed, M.; Moreton, M.; Carter, N.D.; Johnston, A.; Goldberg, L.; Holt, D.W. Tacrolimus pharmacogenetics: The CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation 2005, 79, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Spierings, N.; Holt, D.W.; MacPhee, I.A. CYP3A5 genotype had no impact on intrapatient variability of tacrolimus clearance in renal transplant recipients. Ther. Drug Monit. 2013, 35, 328–331. [Google Scholar] [CrossRef]
- Ball, S.E.; Scatina, J.; Kao, J.; Ferron, G.M.; Fruncillo, R.; Mayer, P.; Weinryb, I.; Guida, M.; Hopkins, P.J.; Warner, N.; et al. Population distribution and effects on drug metabolism of a genetic vari- ant in the 5’ promoter region of CYP3A4. Clin. Pharmacol. Ther. 1999, 66, 288–294. [Google Scholar] [CrossRef]
- García-Martín, E.; Martínez, C.; Pizarro, R.M.; García-Gamito, F.J.; Gullsten, H.; Raunio, H.; Agúndez, J.A. CYP3A4 variant alleles in white individuals with low CYP3A4 enzyme activity. Clin. Pharmacol. Ther. 2002, 71, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Wandel, C.; Witte, J.S.; Hall, J.M.; Stein, C.M.; Wood, A.J.; Wilkinson, G.R. CYP3A activity in African American and European American men: Population differences and functional effect of the CYP3A4*1B 5‘-promoter region polymorphism. Clin. Pharmacol. Ther. 2000, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.N.; Barama, A.; Poirier, C.; Vinet, B.; Roger, M. CYP3A4, CYP3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharm. Genom. 2006, 16, 659–665. [Google Scholar] [CrossRef]
- Hesselink, D.A.; van Schaik, R.H.; van Agteren, M.; de Fijter, J.W.; Hartmann, A.; Zeier, M.; Budde, K.; Kuypers, D.R.; Pisarski, P.; Le Meur, Y.; et al. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharm. Genom. 2008, 18, 339–348. [Google Scholar] [CrossRef]
- Elens, L.; Bouamar, R.; Hesselink, D.A.; Haufroid, V.; van der Heiden, I.P.; van Gelder, T.; van Schaik, R.H. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin. Chem. 2011, 57, 1574–1583. [Google Scholar] [CrossRef]
- Hoffmeyer, S.; Burk, O.; von Richter, O.; Arnold, H.P.; Brockmöller, J.; Johne, A.; Cascorbi, I.; Gerloff, T.; Roots, I.; Eichelbaum, M.; et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- MacPhee, I.A.; Fredericks, S.; Tai, T.; Syrris, P.; Carter, N.D.; Johnston, A.; Goldberg, L.; Holt, D.W. Tacrolimus pharmacogenetics: Polymorphisms associated with expression of cytochrome p450A5 and P-glycoprotein correlate with dose requirement. Transplantation 2002, 74, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Anglicheau, D.; Verstuyft, C.; Laurent-Puig, P.; Becquemont, L.; Schlageter, M.-H.; Cassinat, B.; Beaune, P.; Legendre, C.; Thervet, E. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J. Am. Soc. Nephrol. 2003, 14, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Gervasini, G.; Garcia, M.; Macias, R.M.; Cubero, J.J.; Caravaca, F.; Benitez, J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl. Int. 2012, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R.; Naesens, M.; de Jonge, H.; Lerut, E.; Verbeke, K.; Vanrenterghem, Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther. Drug Monit. 2010, 32, 394–404. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Cui, G.; Wang, H.; Wang, D.W. Genotyping on ALDH2: Comparison of four different technologies. PLoS ONE 2015, 24, e0122745. [Google Scholar] [CrossRef]
| Patients | Adults | Children | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n (75) | (%) | n (55) | (%) | n (20) | (%) | ||
| TDM Median [Q1–Q3] | 5.4 (4.4–6.8) | 5.7 (4.6–7.0) | 5.0 (4.0–6.1) | <0.001 | |||
| Dose Median [Q1–Q3] | 3.0 (1.5–4.5) | 3.0 (2.0–5.5) | 2.0 (1.0–4.0) | <0.001 | |||
| Female | 25 | 33.3 | 17 | 30.9 | 8 | 40.0 | 0.663 |
| Male | 50 | 66.7 | 38 | 69.1 | 12 | 60.0 | -- |
| CYP3A5 (G6986A) Mut | 9 | 12.0 | 7 | 12.7 | 2 | 10.0 | 0.748 |
| CYP3A4 (A392G) Mut | 17 | 22.7 | 12 | 21.8 | 5 | 25.0 | 0.771 |
| ABCB1 (C3435T) Mut | 55 | 73.3 | 40 | 72.7 | 15 | 75.5 | 0.844 |
| ABCB1 (C1236T) Mut | 22 | 29.3 | 15 | 27.3 | 7 | 35.0 | 0.516 |
| ABCB1 (G2677T/A) Mut | 47 | 62.7 | 32 | 58.2 | 15 | 75.0 | 0.183 |
| CYP3A4*22 Mut | 5 | 6.7 | 4 | 7.3 | 1 | 5.0 | 0.727 |
| SLCO1B1 (T521C) Mut | 19 | 25.3 | 13 | 23.6 | 6 | 30.0 | 0.575 |
| WT | 5 | 6.7 | 4 | 7.3 | 1 | 5.0 | -- |
| TM | 45 | 60.0 | 33 | 60.0 | 12 | 60.0 | 0.747 |
| IM | 20 | 26.7 | 14 | 25.5 | 6 | 30.0 | 0.656 |
| RM | 4 | 5.3 | 3 | 5.45 | 1 | 5.0 | 0.858 |
| AM | 1 | 1.3 | 1 | 1.8 | - | - | |
| N Patients | At Least Once Out of Range | (%) | N of Samples | N of Samples Out of Range | % of Samples Out of Range | p-Value vs. WT | p-Value vs. TM | |
|---|---|---|---|---|---|---|---|---|
| WT | 5 | 1 | 20.0 | 56 | 3 | 5.4 | -- | |
| TM | 45 | 25 | 55.6 | 361 | 54 | 15.0 | 0.052 | -- |
| IM | 20 | 14 | 70.0 | 143 | 37 | 25.9 | 0.001 | 0.004 |
| RM | 4 | 3 | 75.0 | 28 | 5 | 17.9 | 0.066 | 0.169 |
| AM | 1 | 0 | 0.0 | 5 | 0 | 0.0 |
| Not Normalized by Dose | Normalized by Dose | |||
|---|---|---|---|---|
| Variable | B [95% CI] | p-Value | B [95% CI] | p-Value |
| Sex (M) | 0.003 (−0.087/0.093) | 0.949 | 0.005 (−0.087/0.097) | 0.918 |
| Age (Child) | −0.096 (−0.192/0.001) | 0.051 | −0.094 (−0.208/0.020) | 0.104 |
| TM | 0.100 (−0.060/0.259) | 0.221 | 0.113 (−0.047/0.274) | 0.167 |
| IM | 0.213 (0.041/0.384) | 0.015 | 0.206 (0.310/0.381) | 0.021 |
| RM | 0.123 (−0.118/0.363) | 0.317 | 0.099 (−0.167/0.365) | 0.467 |
| Dose | -- | -- | −0.011 (−0.034/0.012) | 0.364 |
| Variable | B [95% CI] | p-Value |
|---|---|---|
| Sex (M) | −0.086 (−0.213/0.041) | 0.183 |
| Age | 0.287 (0.141/0.433) | <0.001 |
| TM | −0.015 (−0.236/0.205) | 0.891 |
| IM | 0.096 (−0.143/0.335) | 0.433 |
| RM | −0.513 (−0.876/-0.150) | 0.006 |
| RS | Type of Primer | Sequence |
|---|---|---|
| CYP3A4*1B (A392G) RS2740574 | WT special primer | 5’ CAGCCATAGAGACAAGGGGAA 3’ |
| MUT special primer | 5’ AGCCATAGAGACAAGGGGAG 3’ | |
| Common primer | 5’ GGAAGAGGCTTCTCCACCTTG 3’ | |
| CYP3A5*3 (A698G) RS776746 | WT special primer | 5’ GGTCCAAACAGGGAAGAGAAAT 3’ |
| MUT special primer | 5’ TGGTCCAAACAGGGAAGAGAAAC 3’ | |
| Common primer | 5’ AGTCCTTGTGAGCACTTGATG 3’ | |
| ABCB1 (C3435T) RS1045642 | WT special primer | 5’ TGGTGTCACAGGAAGAGTTC 3’ |
| MUT special primer | 5’ GTGGTGTCACAGGAAGAGTTT 3’ | |
| Common primer | 5’ AACCCAAACAGGAAGTGTGG 3’ | |
| ABCB1 (C1236T) RS1128503 | WT special primer | 5’ TCCTGGTAGATCTTGAAGCGC 3’ |
| MUT special primer | 5’ TCCTGGTAGATCTTGAAGCGT 3’ | |
| Common primer | 5’ AGATGTGCAATGTGACTGCTG 3’ | |
| ABCB1 (G2677T) RS2032582 | WT special primer | 5’ AGTTTGACTCACCTTCCCTGC 3’ |
| MUT special primer | 5’ AGTTTGACTCACCTTCCCTGA 3’ | |
| MUT special primer | 5’ GTTTGACTCACCTTCCCTGT 3’ | |
| Common primer | 5’ GGTTCCAGGCTTGCTGTAATT 3’ | |
| CYP3A4*22 (C15389T) RS35599367 | WT special primer | 5’ AGTGTCTCCATCACACCCTGC 3’ |
| MUT special primer | 5’ CAGTGTCTCCATCACACCCTGT 3’ | |
| Common primer | 5’ CCCAAAGACGCTGAGTGGAGAA 3’ | |
| SLCO1B1 (T521C) RS4149056 | WT special primer | 5’ TCCACGAAGCATATTACCCATGATCG 3’ |
| MUT special primer | 5’ CCACGAAGCATATTACCCATGATCA 3’ | |
| Common primer | 5’ CAGCCATGAGGAACTATGAGTCCA 3’ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallio, G.; Irrera, N.; Bitto, A.; Mannino, F.; Minutoli, L.; Rottura, M.; Pallio, S.; Altavilla, D.; Alibrandi, A.; Marciano, M.C.; et al. Failure of Achieving Tacrolimus Target Blood Concentration Might Be Avoided by a Wide Genotyping of Transplanted Patients: Evidence from a Retrospective Study. J. Pers. Med. 2020, 10, 47. https://doi.org/10.3390/jpm10020047
Pallio G, Irrera N, Bitto A, Mannino F, Minutoli L, Rottura M, Pallio S, Altavilla D, Alibrandi A, Marciano MC, et al. Failure of Achieving Tacrolimus Target Blood Concentration Might Be Avoided by a Wide Genotyping of Transplanted Patients: Evidence from a Retrospective Study. Journal of Personalized Medicine. 2020; 10(2):47. https://doi.org/10.3390/jpm10020047
Chicago/Turabian StylePallio, Giovanni, Natasha Irrera, Alessandra Bitto, Federica Mannino, Letteria Minutoli, Michelangelo Rottura, Socrate Pallio, Domenica Altavilla, Angela Alibrandi, Maria Concetta Marciano, and et al. 2020. "Failure of Achieving Tacrolimus Target Blood Concentration Might Be Avoided by a Wide Genotyping of Transplanted Patients: Evidence from a Retrospective Study" Journal of Personalized Medicine 10, no. 2: 47. https://doi.org/10.3390/jpm10020047
APA StylePallio, G., Irrera, N., Bitto, A., Mannino, F., Minutoli, L., Rottura, M., Pallio, S., Altavilla, D., Alibrandi, A., Marciano, M. C., Righi, M., Mannucci, C., Arcoraci, V., & Squadrito, F. (2020). Failure of Achieving Tacrolimus Target Blood Concentration Might Be Avoided by a Wide Genotyping of Transplanted Patients: Evidence from a Retrospective Study. Journal of Personalized Medicine, 10(2), 47. https://doi.org/10.3390/jpm10020047







