Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia
Abstract
1. Introduction
2. EEG Biomarkers
2.1. EEG Findings in Dementia (including AD)
- -
- -
- -
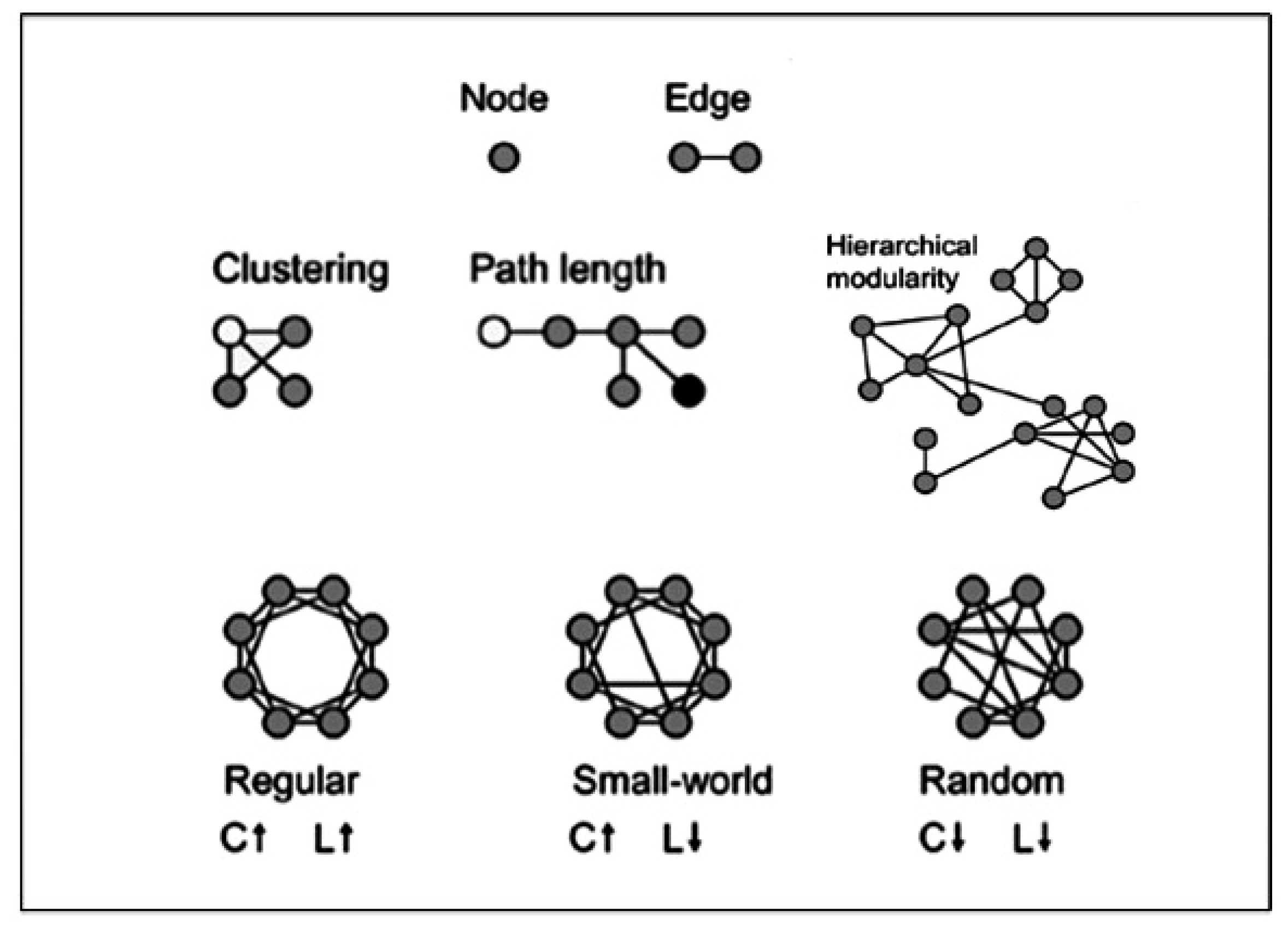
2.2. Brain Connectivity Methods including Graph-Theory
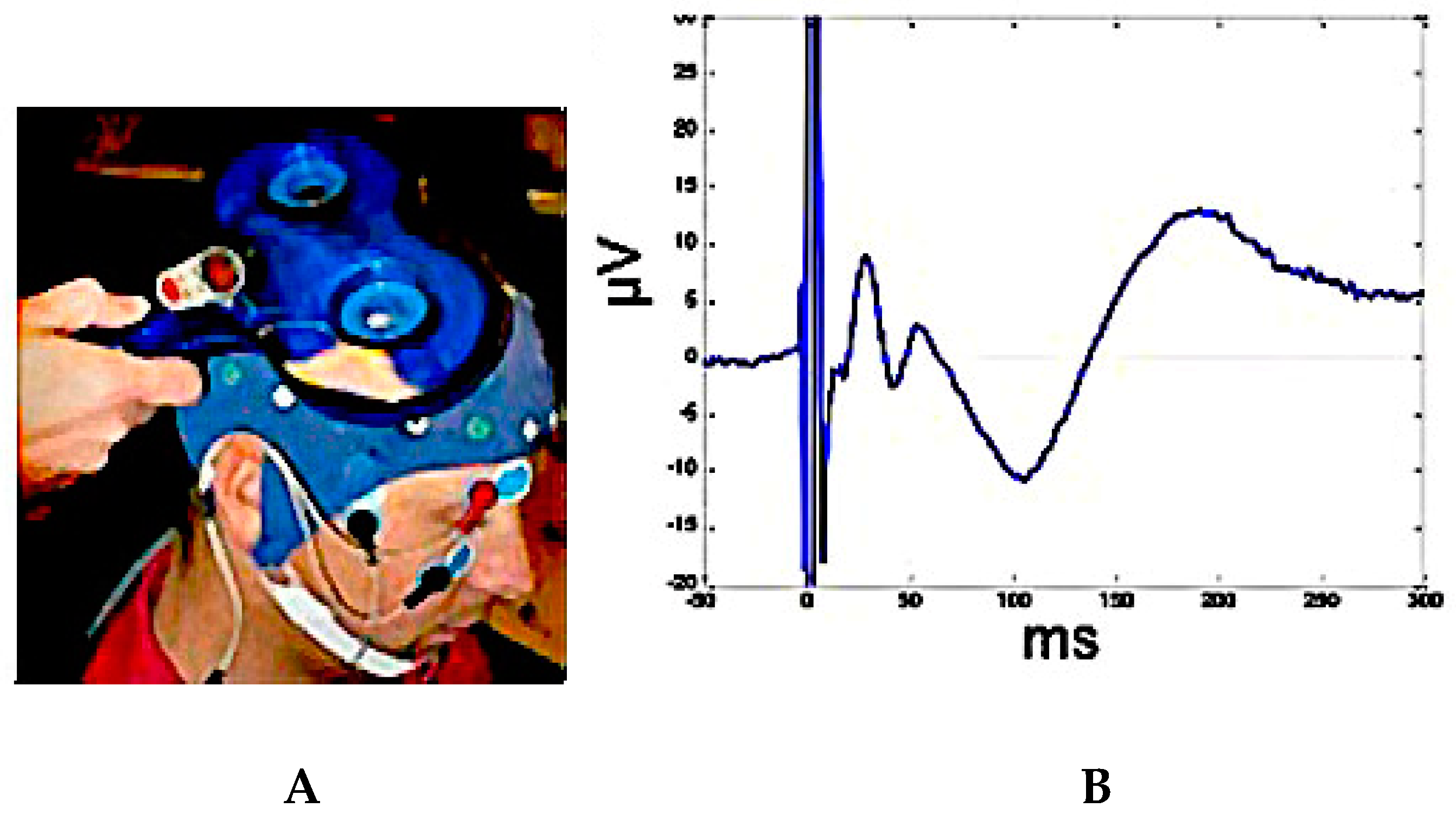
2.3. TMS-EEG Co-Registration for Testing Brain Connectivity
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Author (Year) | Methods | Biomarkers | Subjects | Main Findings | Reference |
|---|---|---|---|---|---|
| Babiloni C (2016) | EEG, FDG-PET | spectral analysis (power) | AD | 19 AD patients were compared with a group of 40 Nold. The AD group performed FDG-PET. In the AD patients, there was a positive correlation between the Alzheimer’s discrimination analysis tool (PALZ) score and the activity of delta sources in the cortical region of interest (p < 0.05) suggesting a relationship between resting-state cortical hypometabolism and synchronization of cortical neurons at delta rhythms in AD patients with dementia. | [22] |
| Rossini PM (2006) | EEG | spectral analysis (linear coupling) | AD, MCI | In 69 MCI, baseline fronto-parietal midline coherence, delta (temporal), theta (parietal, occipital and temporal), and alpha 1 (central, parietal, occipital, temporal, limbic) sources were stronger in MCI Converted than stable subjects (p < 0.05). Low midline coherence and weak temporal source were associated with a 10% annual rate AD conversion, while this rate increased up to 40% and 60% when strong temporal delta source and high midline gamma coherence were observed respectively. | [31] |
| Jelic V (2000) | EEG | spectral analysis (power) | MCI | In 27 MCI patients, progression to AD in a follow up of 21 months was associated with a significantly higher theta relative power and lower beta relative power and mean frequency at the temporal and temporo - occipital derivations | [34] |
| Adler G (2003) | EEG | spectral analysis (power) | AD | A study with 31 AD compared with 17 Nold. AD patients showed a widespread increase in delta and theta power density and posterior decrease in α and β power density with a lowering of α power density peak. | [35] |
| Stam CJ (2007) | EEG | spectral analysis (graph theory) | AD | In a study with 15 AD vs. 13 Nold, the characteristic path length L was significantly longer in the AD patients, whereas the cluster coefficient C showed no significant changes. This pattern was still present when L and C were computed as a function of K. A longer path length with a relatively preserved cluster coefficient suggests a loss of complexity and a less optimal organization. | [42] |
| Jelles B (1999) | EEG | spectral analysis (power) | AD | In a study with 24 probable AD vs. 22 Nold, the correlation dimension (D2) was significantly lower in the Alzheimer patients compared to controls | [43] |
| Dauwels J (2010) | EEG | spectral analysis (nonlinear coupling) | MCI | Two synchrony measures, Granger casuality, and stochastic event synchrony are able to distinguish MCI patients from age-matched control subjects. | [44] |
| Azami H (2016) | MEG | entropy | AD | In 36 AD vs. 26 Nold, multiscale dispersion entropy (MDE) values in AD compared with multiscale permutation entropy (MPE) and multiscale entropy (MSE) was significantly lower than their corresponding MSE- and MPE-based values. | [45] |
| Babiloni C (2009) | EEG, MRI | spectral analysis (power) | AD, MCI | In a study with 35 AD, 80 MCI and 60 Nold, the EEG sources showed a significant linear correlation with hippocampal volume also supported a non-linear correlation with hippocampal volume strongly for the logarithmic one, suggesting that progressive atrophy of hippocampus correlates with decreased cortical alpha power, as estimated by using LORETA source modeling, in the continuum, along MCI and AD conditions. | [47] |
| Babiloni C (2009) | EEG, MRI | spectral analysis (power) | MCI | Study with 54 MCI subjects with follow-up of 1-year vs. 45 Nold and 50 AD. In MCI, the EEG recordings showed a decreased power of posterior alpha1 and alpha2 sources, suggesting that the resting state EEG alpha sources were sensitive-at least at the group level-to the cognitive decline occurring in the amnesic MCI group over 1 year. | [51] |
| Jeong J (2004) | EEG | spectral analysis (nonlinear) | AD | EEG in AD showed a lower correlation dimension (D2) and the largest Lyapunov exponent (L1) values than in the healthy. Despite their different focus on static and dynamic properties of the EEGs, the results of both D2 and L1 were associated with a reduction of complexity in EEG activity due to AD | [54] |
| Smits FM (2016) | EEG | fractal dimension | AD | A comparision between 67 AD vs. 41 Nold showed a reduced fractal dimension in AD compared to healthy especially in temporal-occipital regions | [61] |
| Escudero J (2006) | EEG | entropy | AD, MCI | In a study with 11 AD and 11 Nold, entropy metrics of spontaneous EEGs in AD and in MCI showed reduced irregularity in AD patients’ EEG activity | [69] |
| Vecchio F (2015) | EEG, MRI/DTI | spectral analysis (graph theory) | AD, MCI | 40 subjects, including 9 Nold, 10 MCI, 10 mild AD, 11 moderate AD. Callosal fractional anisotropy (FA) reduction, observed in subjects with Alzheimer’s disease (AD) and mild cognitive impairment (MCI), is associated with a loss of brain interhemispheric functional connectivity characterized by increased delta and decreased alpha path length. | [97] |
| Vecchio F (2014) | EEG | spectral analysis (graph theory) | AD, MCI | Analysis of a database of 378 participants, including AD, MCI, and Nold. Path Length showed a different pattern between normal cognition and dementia as observed in the theta band (MCI subjects are found similar to healthy subjects), while for the normalized Clustering coefficient a significant increment was found for AD group in delta, theta, and alpha 1 bands; the small world parameter presented a significant interaction between AD and MCI groups showing a theta increase in MCI. | [98] |
| Miraglia F (2016) | EEG | spectral analysis (graph theory) | AD, MCI | 30 Nold, 30 aMCI, and 30 AD during eyes closed EC and eyes open EO. In Nold, in EO condition, the brain network is characterized by higher SW in alpha bands and lower SW in beta2 and gamma bands. In aMCI, SW has the same trend, except for delta and theta bands where the network shows less SW. AD shows a similar trend of Nold, but with less fluctuations between EO/EC conditions. aMCI presents SW midway between AD and Nold. In delta and theta bands, in EC, the aMCI group presents network’s architecture similar to Nold, while in EO aMCI, SW similar to AD | [112] |
| de Hann W (2012) | MEG | spectral analysis (graph theory) | AD | In 18 AD vs. 18 Nold, graph spectral analysis confirmed the hub status of the parietal areas and demonstrated a low centrality of the left temporal region in the theta band in AD patients that was strongly related to the MMSE. In AD, impaired network synchronization and a clinically relevant left temporal centrality loss were found | [115] |
| Vecchio F (2017) | EEG | spectral analysis (graph theory) | AD | In 110 AD and 34 healthy Nold, Alpha band connectivity was negatively correlated, while slow (delta) and fast-frequency (beta, gamma) bands positively correlated with the hippocampal volume of Alzheimer subjects. The larger the hippocampal volume, the lower the alpha, and the higher the delta, beta, and gamma Small World characteristics of connectivity. | [126] |
| Vecchio F (2018) | EEG, Apo-E allele | spectral analysis (graph theory) | aMCI | 145 aMCI classified as Converted to AD (C-MCI, 71) or Stable (S-MCI, 74) according to follow up. Small-World EEG analysis, in combination with an Apo-E allele testing, evaluate on an individual basis with great precision the risk of MCI progression (96.7% sensitivity, 86% specificity and 91.7% accuracy (AUC = 0.97)) | [127] |
| Julkunen P (2011) | TMS-EEG | Cortical Excitability (P30 amplitude) | AD, MCI | In this study with 4 control subjects, 5 MCI and 5 AD, the TMS–EEG response P30 amplitude correlated with cognitive decline and showed good specificity and sensitivity in identifying healthy subjects from those with MCI or AD. | [156] |
| Casarotto S (2011) | TMS-EEG | Cortical Excitability | AD | In this study with 9 healthy young, 9 healthy elderly, and 9 AD, frontal cortex excitability was not significantly different between healthy young and elderly individuals while was clearly reduced in AD patients. | [157] |
| Ferreri F (2016) | TMS-EEG | M1 Cortical Excitability and Connectivity | AD | In this study with 12 mild AD patients, the sensorimotor system was found hyperexcitable, and its connectivity disrupted with respect of 12 healthy elderly, despite the lack of clinically evident motor manifestations. | [158] |
| Bagattini C (2019) | TMS-EEG | Cortical Excitability (P30 amplitude) | AD | In this study with 26 AD patients, the TMS–EEG response P30 amplitude predicted MMSE and face-name memory scores. Particularly higher P30 amplitude predicted poorer cognitive and memory performances. | [159] |
| Koch G (2018) | rTMS, TEM-EEG | Cortical Excitability and Connectivity | AD | In 14 early AD, a 2-week treatment with rTMS on the precuneus induced a selective improvement in episodic memory. TMS-EEG recording revealed a precuneus enhanced activity and a modification of its functional connectivity within the DMN | [160] |
| Ferreri F (2003) | TMS | M1 Cortical Excitability (MEP amplitude) | AD | In 16 AD, motor cortex excitability, measured with TMS, was increased, and the center of gravity of motor cortical output, as represented by excitable scalp sites, showed a frontal and medial shift, without correlated changes in the site of maximal excitability (hot-spot). | [161] |
| Ferreri F (2011) | TMS | M1 Cortical Excitability (MEP amplitude) | AD | In 10 AD patients before and after long-term AchEIs therapy, M1 excitability was found to be unchanged in patients with stabilized cognitive performance during the therapy. | [162] |
References
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bak, T.; Caffarra, P.; Caltagirone, C.; Ceccaldi, M.; Collette, F.; Crutch, S.; Della Sala, S.; Démonet, J.F.; Dubois, B.; et al. The need for harmonisation and innovation of neuropsychological assessment in neurodegenerative dementias in Europe: Consensus document of the Joint Program for Neurodegenerative Diseases Working Group. Alzheimers Res. Ther. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Dubois, B.; Boccardi, M.; Monsch, A.U.; Demonet, J.F.; Cappa, S.F. Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers. Clinical validity of delayed recall tests as a gateway biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 2017, 52, 153–166. [Google Scholar] [CrossRef]
- Geldmacher, D.S. Cost-effectiveness of drug therapies for Alzheimer’s disease: A brief review. Neuropsychiatr. Dis. Treat. 2008, 4, 549–555. [Google Scholar]
- D’Amelio, M.; Rossini, P.M. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: From animal models to human findings. Prog. Neurobiol. 2012, 99, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Getsios, D.; Blume, S.; Ishak, K.J.; Maclaine, G.; Hernández, L. An economic evaluation of early assessment for Alzheimer’s disease in the United Kingdom. Alzheimers Dement. 2012, 8, 22–30. [Google Scholar] [CrossRef]
- Barnett, J.H.; Lewis, L.; Blackwell, A.D.; Taylor, M. Early intervention in Alzheimer’s disease: A health economic study of the effects of diagnostic timing. BMC Neurol. 2014, 14, 101. [Google Scholar] [CrossRef]
- Teipel, S.J.; Kurth, J.; Krause, B.; Grothe, M.J.; Alzheimer’s Disease Neuroimaging Initiative. The relative importance of imaging markers for the prediction of Alzheimer’s disease dementia in mild cognitive impairment—Beyond classical regression. Neuroimage Clin. 2015, 8, 583–593. [Google Scholar] [CrossRef]
- Petersen, R.C. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology 2018, 91, 395–402. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin. Neurophysiol. 2006, 117, 2424–2435. [Google Scholar] [CrossRef]
- Srinivasan, R.; Winter, W.R.; Ding, J.; Nunez, P.L. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 2007, 166, 41–52. [Google Scholar] [CrossRef]
- Hyvarinen, A. Blind source separation by nonstationarity of variance: A cumulant-based approach. IEEE Trans. Neural Netw. 2001, 12, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, F.; Porcaro, C.; Barbati, G.; Zappasodi, F. Functional source separation and hand cortical representation for a brain-computer interface feature extraction. J. Physiol. 2007, 580, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, C.; Medaglia, M.T.; Thai, N.J.; Seri, S.; Rotshtein, P.; Tecchio, F. Contradictory reasoning network: An EEG and FMRI study. PLoS ONE 2014, 9, e92835. [Google Scholar] [CrossRef] [PubMed]
- Scherg, M.; Berg, P. Use of prior knowledge in brain electromagnetic source analysis. Brain Topogr. 1991, 4, 143–150. [Google Scholar] [CrossRef]
- Mosher, J.C.; Lewis, P.S.; Leahy, R.M. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans. Biomed. Eng. 1992, 39, 541–557. [Google Scholar] [CrossRef]
- Mosher, J.C.; Baillet, S.; Leahy, R.M. EEG source localization and imaging using multiple signal classification approaches. J. Clin. Neurophysiol. 1999, 16, 225–238. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Vrba, J.; Robinson, S.E. Signal processing in magnetoencephalography. Methods 2001, 25, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Caroli, A.; Salvatore, E.; Nicolai, E.; Marzano, N.; Lizio, R.; Cavedo, E.; Landau, S.; Chen, K.; et al. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer’s disease: An EEG-PET study. Neurobiol. Aging 2016, 48, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Boccaletti, S.; Valladares, D.L.; Pecora, L.M.; Geffert, H.P.; Carroll, T. Reconstructing embedding spaces of coupled dynamical systems from multivariate data. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002, 65, 035204. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Sosa, P.A.; Sanchez-Bornot, J.M.; Sotero, R.C.; Iturria-Medina, Y.; Aleman-Gomez, Y.; Bosch-Bayard, J.; Carbonell, F.; Ozaki, T. Model driven EEG/fMRI fusion of brain oscillations. Hum. Brain Mapp. 2009, 30, 2701–2721. [Google Scholar] [CrossRef]
- Gramfort, A.; Strohmeier, D.; Haueisen, J.; Hämäläinen, M.S.; Kowalski, M. Time-frequency mixed-norm estimates: Sparse M/EEG imaging with non-stationary source activations. Neuroimage 2013, 70, 410–422. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme, G., Ed.; George Thieme: Stuttgard, Germany, 1988. [Google Scholar]
- Yao, D.; He, B. A self-coherence enhancement algorithm and its application to enhancing three-dimensional source estimation from EEGs. Ann. Biomed. Eng. 2001, 29, 1019–1027. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find Exp. Clin. Pharmacol. 2002, 24 (Suppl. C), 91–95. [Google Scholar]
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Pascual-Marqui, R.D. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: Frequency decomposition. arXiv 2007, arXiv:0711.1455. [Google Scholar]
- Rossini, P.M.; Del Percio, C.; Pasqualetti, P.; Cassetta, E.; Binetti, G.; Dal Forno, G.; Ferreri, F.; Frisoni, G.; Chiovenda, P.; Miniussi, C.; et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 2006, 143, 793–803. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jelic, V.; Johansson, S.E.; Almkvist, O.; Shigeta, M.; Julin, P.; Nordberg, A.; Winblad, B.; Wahlund, L.O. Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging 2000, 21, 533–540. [Google Scholar] [CrossRef]
- Adler, G.; Brassen, S.; Jajcevic, A. EEG coherence in Alzheimer’s dementia. J. Neural Transm. 2003, 110, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Van der Hiele, K.; Vein, A.A.; Reijntjes, R.H.A.M.; Westendorp, R.G.J.; Bollen, E.L.E.M.; van Buchem, M.A.; van Dijk, J.G.; Middelkoop, H.A.M. EEG correlates in the spectrum of cognitive decline. Clin. Neurophysiol. 2007, 118, 1931–1939. [Google Scholar] [CrossRef]
- Nishida, K.; Yoshimura, M.; Isotani, T.; Yoshida, T.; Kitaura, Y.; Saito, A.; Mii, H.; Kato, M.; Takekita, Y.; Suwa, A.; et al. Differences in quantitative EEG between frontotemporal dementia and Alzheimer’s disease as revealed by LORETA. Clin. Neurophysiol. 2011, 122, 1718–1725. [Google Scholar] [CrossRef]
- Scheeringa, R.; Petersson, K.M.; Kleinschmidt, A.; Jensen, O.; Bastiaansen, M.C.M. EEG Alpha Power Modulation of fMRI Resting-State Connectivity. Brain Connect 2012, 2, 254–264. [Google Scholar] [CrossRef]
- Pritchard, W.S.; Duke, D.W.; Krieble, K.K. Dimensional analysis of resting human EEG II: Surrogate-data testing indicates nonlinearity but not low-dimensional chaos. Psychophysiology 1995, 32, 486–491. [Google Scholar] [CrossRef]
- Woyshville, M.J.; Calabrese, J.R. Quantification of occipital EEG changes in Alzheimer’s disease utilizing a new metric: The fractal dimension. Biol. Psychiatry 1994, 35, 381–387. [Google Scholar] [CrossRef]
- Stam, C.J.; Jones, B.F.; Nolte, G.; Breakspear, M.; Scheltens, P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb. Cortex 2007, 17, 92–99. [Google Scholar] [CrossRef]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Jelles, B.; van Birgelen, J.H.; Slaets, J.P.; Hekster, R.E.; Jonkman, E.J.; Stam, C.J. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin. Neurophysiol. 1999, 110, 1159–1167. [Google Scholar] [CrossRef]
- Dauwels, J.; Vialatte, F.; Musha, T.; Cichocki, A. A comparative study of synchrony measures for the early diagnosis of Alzheimer’s disease based on EEG. Neuroimage 2010, 49, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Kinney-Lang, E.; Ebied, A.; Fernandez, A.; Escudero, J. Multiscale dispersion entropy for the regional analysis of resting-state magnetoencephalogram complexity in Alzheimer’s disease. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3182–3185. [Google Scholar] [PubMed]
- Dierks, T.; Ihl, R.; Frölich, L.; Maurer, K. Dementia of the alzheimer type: Effects on the spontaneous EEG described by dipole sources. Psychiatry Res. Neuroimaging 1993, 50, 151–162. [Google Scholar] [CrossRef]
- Babiloni, C.; Frisoni, G.B.; Pievani, M.; Vecchio, F.; Lizio, R.; Buttiglione, M.; Geroldi, C.; Fracassi, C.; Eusebi, F.; Ferri, R.; et al. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage 2009, 44, 123–135. [Google Scholar] [CrossRef]
- Babiloni, C.; Vecchio, F.; Lizio, R.; Ferri, R.; Rodriguez, G.; Marzano, N.; Frisoni, G.B.; Rossini, P.M. Resting state cortical rhythms in mild cognitive impairment and Alzheimer’s disease: Electroencephalographic evidence. J. Alzheimers Dis. 2011, 26 (Suppl. 3), 201–214. [Google Scholar] [CrossRef]
- Canuet, L.; Tellado, I.; Couceiro, V.; Fraile, C.; Fernandez-Novoa, L.; Ishii, R.; Takeda, M.; Cacabelos, R. Resting-state network disruption and APOE genotype in Alzheimer’s disease: A lagged functional connectivity study. PLoS ONE 2012, 7, e46289. [Google Scholar] [CrossRef]
- Hsiao, F.-J.; Wang, Y.-J.; Yan, S.-H.; Chen, W.-T.; Lin, Y.-Y. Altered Oscillation and Synchronization of Default-Mode Network Activity in Mild Alzheimer’s Disease Compared to Mild Cognitive Impairment: An Electrophysiological Study. PLoS ONE 2013, 8, e68792. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Del Percio, C.; Lizio, R.; Marzano, N.; Infarinato, F.; Soricelli, A.; Salvatore, E.; Ferri, R.; Bonforte, C.; Tedeschi, G.; et al. Cortical sources of resting state electroencephalographic alpha rhythms deteriorate across time in subjects with amnesic mild cognitive impairment. Neurobiol. Aging 2014, 35, 130–142. [Google Scholar] [CrossRef]
- Babiloni, C.; Lizio, R.; Marzano, N.; Capotosto, P.; Soricelli, A.; Triggiani, A.I.; Cordone, S.; Gesualdo, L.; Del Percio, C. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. 2016, 103, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Kouzuki, M.; Asaina, F.; Taniguchi, M.; Musha, T.; Urakami, K. The relationship between the diagnosis method of neuronal dysfunction (DIMENSION) and brain pathology in the early stages of Alzheimer’s disease. Psychogeriatrics 2013, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef] [PubMed]
- Hornero, R.; Abásolo, D.; Escudero, J.; Gómez, C. Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer’s disease. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 317–336. [Google Scholar] [CrossRef]
- Grassberger, P.; Procaccia, I. Measuring the strangeness of strange attractors. Phys. D Nonlinear Phenom. 1983, 9, 189–208. [Google Scholar] [CrossRef]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov exponents from a time series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017, 20, 340–352. [Google Scholar] [CrossRef]
- Garn, H.; Waser, M.; Deistler, M.; Benke, T.; Dal-Bianco, P.; Ransmayr, G.; Schmidt, H.; Sanin, G.; Santer, P.; Caravias, G.; et al. Quantitative EEG markers relate to Alzheimer’s disease severity in the Prospective Dementia Registry Austria (PRODEM). Clin. Neurophysiol. 2015, 126, 505–513. [Google Scholar] [CrossRef]
- Azami, H.; Escudero, J. Improved multiscale permutation entropy for biomedical signal analysis: Interpretation and application to electroencephalogram recordings. Biomed. Signal Process. Control 2016, 23, 28–41. [Google Scholar] [CrossRef]
- Smits, F.M.; Porcaro, C.; Cottone, C.; Cancelli, A.; Rossini, P.M.; Tecchio, F. Electroencephalographic Fractal Dimension in Healthy Ageing and Alzheimer’s Disease. PLoS ONE 2016, 11, e0149587. [Google Scholar] [CrossRef]
- Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Phys. D Nonlinear Phenom. 1988, 31, 277–283. [Google Scholar] [CrossRef]
- Katz, M.J. Fractals and the analysis of waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Humeau-Heurtier, A. The Multiscale Entropy Algorithm and Its Variants: A Review. Entropy 2015, 17, 3110–3123. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.-J. Is mental illness complex? From behavior to brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 253–257. [Google Scholar] [CrossRef]
- Escudero, J.; Abásolo, D.; Hornero, R.; Espino, P.; López, M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol. Meas. 2006, 27, 1091–1106. [Google Scholar] [CrossRef]
- Courtiol, J.; Perdikis, D.; Petkoski, S.; Müller, V.; Huys, R.; Sleimen-Malkoun, R.; Jirsa, V.K. The multiscale entropy: Guidelines for use and interpretation in brain signal analysis. J. Neurosci. Methods 2016, 273, 175–190. [Google Scholar] [CrossRef]
- Azami, H.; Abásolo, D.; Simons, S.; Escudero, J. Univariate and Multivariate Generalized Multiscale Entropy to Characterise EEG Signals in Alzheimer’s Disease. Entropy 2017, 19, 31. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Mandic, D.P. Multivariate multiscale entropy: A tool for complexity analysis of multichannel data. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011, 84, 061918. [Google Scholar] [CrossRef] [PubMed]
- Labate, D.; Foresta, F.L.; Morabito, G.; Palamara, I.; Morabito, F.C. Entropic Measures of EEG Complexity in Alzheimer’s Disease Through a Multivariate Multiscale Approach. IEEE Sens. J. 2013, 13, 3284–3292. [Google Scholar] [CrossRef]
- Azami, H.; Escudero, J.; Fernández, A. Refined composite multivariate multiscale entropy based on variance for analysis of resting-state magnetoencephalograms in Alzheimer’s disease. In Proceedings of the 2016 International Conference for Students on Applied Engineering (ICSAE), Qingdao, China, 29–30 May 2016; pp. 413–418. [Google Scholar]
- Morison, G.; Tieges, Z.; Kilborn, K. Analysis of Electroencephalography Activity in Early Stage Alzheimer’s Disease Using a Multiscale Statistical Complexity Measure. Adv. Sci. Lett. 2013, 19, 2414–2418. [Google Scholar] [CrossRef]
- Timothy, L.T.; Krishna, B.M.; Nair, U. Classification of mild cognitive impairment EEG using combined recurrence and cross recurrence quantification analysis. Int. J. Psychophysiol. 2017, 120, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. The formation of cooperative cell assemblies in the visual cortex. J. Exp. Biol. 1990, 153, 177–197. [Google Scholar] [PubMed]
- Jung, T.P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Analysis and visualization of single-trial event-related potentials. Hum. Brain Mapp. 2001, 14, 166–185. [Google Scholar] [CrossRef]
- Makeig, S.; Westerfield, M.; Jung, T.P.; Enghoff, S.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Dynamic brain sources of visual evoked responses. Science 2002, 295, 690–694. [Google Scholar] [CrossRef]
- Fuentemilla, L.; Marco-Pallarés, J.; Grau, C. Modulation of spectral power and of phase resetting of EEG contributes differentially to the generation of auditory event-related potentials. Neuroimage 2006, 30, 909–916. [Google Scholar] [CrossRef]
- Adrian, E.D.; Moruzzi, G. Impulses in the pyramidal tract. J. Physiol. 1939, 97, 153–199. [Google Scholar] [CrossRef]
- Buzsáki, G. Neuroscience: Neurons and navigation. Nature 2005, 436, 781–782. [Google Scholar] [CrossRef]
- Rossini, P.M.; Di Iorio, R.; Bentivoglio, M.; Bertini, G.; Ferreri, F.; Gerloff, C.; Ilmoniemi, R.J.; Miraglia, F.; Nitsche, M.A.; Pestilli, F.; et al. Methods for analysis of brain connectivity: An IFCN-sponsored review. Clin. Neurophysiol. 2019, 130, 1833–1858. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef]
- Buzsáki, G.; Schomburg, E.W. What does gamma coherence tell us about inter-regional neural communication? Nat. Neurosci. 2015, 18, 484–489. [Google Scholar] [PubMed]
- Blanco, S.; Quiroga, R.Q.; Rosso, O.A.; Kochen, S. Time-frequency analysis of electroencephalogram series. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 1995, 51, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L. Spatial-Temporal Structures of Human Alpha Rhythms: Theory, Microcurrent Sources, Multiscale Measurements, and Global Binding of Local Networks. Human Brain Mapping. 2001. Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.1030 (accessed on 26 March 2020).
- Kaplan, A.Y.; Fingelkurts, A.A.; Fingelkurts, A.A.; Borisov, S.V.; Darkhovsky, B.S. Nonstationary nature of the brain activity as revealed by EEG/MEG: Methodological, practical and conceptual challenges. Signal Process. 2005, 85, 2190–2212. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Rossini, P.M. Searching for signs of aging and dementia in EEG through network analysis. Behav. Brain Res. 2017, 317, 292–300. [Google Scholar]
- Worsley, K.J.; Chen, J.-I.; Lerch, J.; Evans, A.C. Comparing functional connectivity via thresholding correlations and singular value decomposition. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 913–920. [Google Scholar] [CrossRef]
- Canuet, L.; Ishii, R.; Pascual-Marqui, R.D.; Iwase, M.; Kurimoto, R.; Aoki, Y.; Ikeda, S.; Takahashi, H.; Nakahachi, T.; Takeda, M. Resting-state EEG source localization and functional connectivity in schizophrenia-like psychosis of epilepsy. PLoS ONE 2011, 6, e27863. [Google Scholar] [CrossRef]
- Barry, R.J.; De Blasio, F.M.; Borchard, J.P. Sequential processing in the equiprobable auditory Go/NoGo task: Children vs. adults. Clin. Neurophysiol. 2014, 125, 1995–2006. [Google Scholar] [CrossRef]
- Aoki, Y.; Ishii, R.; Pascual-Marqui, R.D.; Canuet, L.; Ikeda, S.; Hata, M.; Imajo, K.; Matsuzaki, H.; Musha, T.; Asada, T.; et al. Detection of EEG-resting state independent networks by eLORETA-ICA method. Front. Hum. Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef]
- Ikeda, S.; Mizuno-Matsumoto, Y.; Canuet, L.; Ishii, R.; Aoki, Y.; Hata, M.; Katsimichas, T.; Pascual-Marqui, R.D.; Hayashi, T.; Okamoto, E.; et al. Emotion Regulation of Neuroticism: Emotional Information Processing Related to Psychosomatic State Evaluated by Electroencephalography and Exact Low-Resolution Brain Electromagnetic Tomography. Neuropsychobiology 2015, 71, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ramyead, A.; Kometer, M.; Studerus, E.; Koranyi, S.; Ittig, S.; Gschwandtner, U.; Fuhr, P.; Riecher-Rössler, A. Aberrant Current Source-Density and Lagged Phase Synchronization of Neural Oscillations as Markers for Emerging Psychosis. Schizophr. Bull. 2015, 41, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Bramanti, P.; Rossini, P.M. Human brain networks in physiological aging: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimers Dis. 2014, 41, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Curcio, G.; Altavilla, R.; Scrascia, F.; Giambattistelli, F.; Quattrocchi, C.C.; Bramanti, P.; Vernieri, F.; Rossini, P.M. Cortical brain connectivity evaluated by graph theory in dementia: A correlation study between functional and structural data. J. Alzheimers Dis. 2015, 45, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Marra, C.; Quaranta, D.; Vita, M.G.; Bramanti, P.; Rossini, P.M. Human brain networks in cognitive decline: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimers Dis. 2014, 41, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Pellicciari, M.C.; Miraglia, F.; Brignani, D.; Miniussi, C.; Rossini, P.M. Effects of transcranial direct current stimulation on the functional coupling of the sensorimotor cortical network. Neuroimage 2016, 140, 50–56. [Google Scholar] [CrossRef]
- Engel, A.K.; Senkowski, D.; Schneider, T.R. Multisensory Integration through Neural Coherence. In The Neural Bases of Multisensory Processes; Murray, M.M., Wallace, M.T., Eds.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-1217-4. [Google Scholar]
- Singer, W.; Gray, C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995, 18, 555–586. [Google Scholar] [CrossRef]
- Gerloff, C.; Richard, J.; Hadley, J.; Schulman, A.E.; Honda, M.; Hallett, M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 1998, 121 Pt 8, 1513–1531. [Google Scholar] [CrossRef]
- Singer, W. Neuronal synchrony: A versatile code for the definition of relations? Neuron 1999, 24, 49–65. [Google Scholar] [CrossRef]
- Boenstrup, M.; Feldheim, J.; Heise, K.; Gerloff, C.; Hummel, F.C. The control of complex finger movements by directional information flow between mesial frontocentral areas and the primary motor cortex. Eur. J. Neurosci. 2014, 40, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating mutual information. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004, 69, 066138. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.; Blinowska, K.; Szelenberger, W. Investigation of coherence structure and EEG activity propagation during sleep. Acta Neurobiol. Exp. 1995, 55, 213–219. [Google Scholar]
- Friston, K.J.; Harrison, L.; Penny, W. Dynamic causal modelling. Neuroimage 2003, 19, 1273–1302. [Google Scholar] [CrossRef]
- Kiebel, S.J.; Garrido, M.I.; Moran, R.; Chen, C.-C.; Friston, K.J. Dynamic causal modeling for EEG and MEG. Hum. Brain Mapp. 2009, 30, 1866–1876. [Google Scholar] [CrossRef]
- Crossley, N.A.; Fox, P.T.; Bullmore, E.T. Meta-connectomics: Human brain network and connectivity meta-analyses. Psychol. Med. 2016, 46, 897–907. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Bramanti, P.; Rossini, P.M. Small-worldness characteristics and its gender relation in specific hemispheric networks. Neuroscience 2015, 310, 1–11. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Bramanti, P.; Rossini, P.M. EEG characteristics in “eyes-open” versus “eyes-closed” conditions: Small-world network architecture in healthy aging and age-related brain degeneration. Clin. Neurophysiol. 2016, 127, 1261–1268. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Sporns, O. The human connectome: Origins and challenges. Neuroimage 2013, 80, 53–61. [Google Scholar] [CrossRef]
- De Haan, W.; van der Flier, W.M.; Wang, H.; Van Mieghem, P.F.A.; Scheltens, P.; Stam, C.J. Disruption of functional brain networks in Alzheimer’s disease: What can we learn from graph spectral analysis of resting-state magnetoencephalography? Brain Connect 2012, 2, 45–55. [Google Scholar] [CrossRef]
- Onnela, J.-P.; Saramäki, J.; Kertész, J.; Kaski, K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 71, 065103. [Google Scholar] [CrossRef] [PubMed]
- Moharramipour, A.; Mostame, P.; Hossein-Zadeh, G.-A.; Wheless, J.W.; Babajani-Feremi, A. Comparison of statistical tests in effective connectivity analysis of ECoG data. J. Neurosci. Methods 2018, 308, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Alù, F.; Judica, E.; Cotelli, M.; Pellicciari, M.; Rossini, P. Human brain networks in physiological and pathological aging: Reproducibility of EEG graph theoretical analysis in cortical connectivity. (Unpublished).
- Zuo, X.-N.; Xing, X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014, 45, 100–118. [Google Scholar] [CrossRef] [PubMed]
- 120Colclough, G.L.; Woolrich, M.W.; Tewarie, P.K.; Brookes, M.J.; Quinn, A.J.; Smith, S.M. How reliable are MEG resting-state connectivity metrics? Neuroimage 2016, 138, 284–293. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Drakesmith, M.; Bells, S.; Parker, G.D.; Linden, D.E.; Jones, D.K. Improving the Reliability of Network Metrics in Structural Brain Networks by Integrating Different Network Weighting Strategies into a Single Graph. Front. Neurosci. 2017, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of “small-world” networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Newman, M.E.J. Properties of highly clustered networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003, 68, 026121. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Ferreri, F.; Vecchio, F.; Ponzo, D.; Pasqualetti, P.; Rossini, P.M. Time-varying coupling of EEG oscillations predicts excitability fluctuations in the primary motor cortex as reflected by motor evoked potentials amplitude: An EEG-TMS study. Hum. Brain Mapp. 2014, 35, 1969–1980. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Judica, E.; Cotelli, M.; Alù, F.; Rossini, P.M. Human brain networks: A graph theoretical analysis of cortical connectivity normative database from EEG data in healthy elderly subjects. Geroscience 2020. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Piludu, F.; Granata, G.; Romanello, R.; Caulo, M.; Onofrj, V.; Bramanti, P.; Colosimo, C.; Rossini, P.M. “Small World” architecture in brain connectivity and hippocampal volume in Alzheimer’s disease: A study via graph theory from EEG data. Brain Imaging Behav. 2017, 11, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Iberite, F.; Lacidogna, G.; Guglielmi, V.; Marra, C.; Pasqualetti, P.; Tiziano, F.D.; Rossini, P.M. Sustainable method for Alzheimer dementia prediction in mild cognitive impairment: Electroencephalographic connectivity and graph theory combined with apolipoprotein E. Ann. Neurol. 2018, 84, 302–314. [Google Scholar] [CrossRef]
- Schomer, D.L.; Silva, F.H.L.D. Niedermeyer’s ElectroencephalographyBasic Principles, Clinical Applications, and Related Fields; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- 130Giri, M.; Zhang, M.; Lü, Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging 2016, 11, 665–681. [Google Scholar]
- Miraglia, F.; Vecchio, F.; Marra, C.; Quaranta, D.; Alù, F.; Peroni, B.; Granata, G.; Judica, E.; Cotelli, M.; Rossini, P.M. Small World Index in Default Mode Network Predicts Progression from Mild Cognitive Impairment to Dementia. Int. J. Neural Syst. 2020, 30, 2050004. [Google Scholar] [CrossRef]
- Başar, E.; Güntekin, B.; Atagün, I.; Turp Gölbaşı, B.; Tülay, E.; Ozerdem, A. Brain’s alpha activity is highly reduced in euthymic bipolar disorder patients. Cogn. Neurodyn. 2012, 6, 11–20. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Kaiser, J.; Heidegger, T.; Lutzenberger, W. Behavioral relevance of gamma-band activity for short-term memory-based auditory decision-making. Eur. J. Neurosci. 2008, 27, 3322–3328. [Google Scholar] [CrossRef]
- Nikolić, D.; Fries, P.; Singer, W. Gamma oscillations: Precise temporal coordination without a metronome. Trends Cogn. Sci. 2013, 17, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Womelsdorf, T.; Buffalo, E.A.; Desimone, R.; Fries, P. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron 2013, 80, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Abeles, M. Corticonics: Neural Circuits of the Cerebral Cortex. Available online: /core/books/corticonics/7BF149062695412A32FFC1255C98B410 (accessed on 26 March 2020).
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Cassani, R.; Falk, T.H.; Fraga, F.J.; Kanda, P.A.M.; Anghinah, R. The effects of automated artifact removal algorithms on electroencephalography-based Alzheimer’s disease diagnosis. Front. Aging Neurosci. 2014, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Fraga, F.J.; Mamani, G.Q.; Johns, E.; Tavares, G.; Falk, T.H.; Phillips, N.A. Early diagnosis of mild cognitive impairment and Alzheimer’s with event-related potentials and event-related desynchronization in N-back working memory tasks. Comput. Methods Programs Biomed. 2018, 164, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Trambaiolli, L.R.; Lorena, A.C.; Fraga, F.J.; Kanda, P.A.M.; Anghinah, R.; Nitrini, R. Improving Alzheimer’s disease diagnosis with machine learning techniques. Clin. EEG Neurosci. 2011, 42, 160–165. [Google Scholar] [CrossRef]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 2018. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Alù, F.; Menna, M.; Judica, E.; Cotelli, M.; Rossini, P. Classification of Alzheimer’s Disease respect to physiological aging with innovative EEG Biomarkers in a machine learning implementation. J. Alzheimer Dis. 2020, in press. [Google Scholar]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Kicić, D. Methodology for combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Cracco, R.Q.; Amassian, V.E.; Maccabee, P.J.; Cracco, J.B. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1989, 74, 417–424. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef]
- Ferreri, F.; Rossini, P.M. TMS and TMS-EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev. Neurosci. 2013, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co-registration study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Pasqualetti, P.; Määttä, S.; Ponzo, D.; Ferrarelli, F.; Tononi, G.; Mervaala, E.; Miniussi, C.; Rossini, P.M. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 2011, 54, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Ponzo, D.; Hukkanen, T.; Mervaala, E.; Könönen, M.; Pasqualetti, P.; Vecchio, F.; Rossini, P.M.; Määttä, S. Human brain cortical correlates of short-latency afferent inhibition: A combined EEG-TMS study. J. Neurophysiol. 2012, 108, 314–323. [Google Scholar] [CrossRef]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef]
- Ferreri, F.; Guerra, A.; Vollero, L.; Ponzo, D.; Maatta, S.; Mervaala, E.; Iannello, G.; Di Lazzaro, V. Age-related changes of cortical excitability and connectivity in healthy humans: Non-invasive evaluation of sensorimotor network by means of TMS-EEG. Neuroscience 2017, 357, 255–263. [Google Scholar] [CrossRef][Green Version]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural frequencies of human corticothalamic circuits. J. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef]
- Assenza, G.; Capone, F.; di Biase, L.; Ferreri, F.; Florio, L.; Guerra, A.; Marano, M.; Paolucci, M.; Ranieri, F.; Salomone, G.; et al. Corrigendum: Oscillatory Activities in Neurological Disorders of Elderly: Biomarkers to Target for Neuromodulation. Front. Aging Neurosci. 2017, 9, 252. [Google Scholar] [CrossRef]
- Julkunen, P.; Jauhiainen, A.M.; Könönen, M.; Pääkkönen, A.; Karhu, J.; Soininen, H. Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer’s disease. Int. J. Alzheimers Dis. 2011, 2011, 654794. [Google Scholar] [CrossRef]
- Casarotto, S.; Määttä, S.; Herukka, S.-K.; Pigorini, A.; Napolitani, M.; Gosseries, O.; Niskanen, E.; Könönen, M.; Mervaala, E.; Rosanova, M.; et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport 2011, 22, 592–597. [Google Scholar] [CrossRef]
- Ferreri, F.; Vecchio, F.; Vollero, L.; Guerra, A.; Petrichella, S.; Ponzo, D.; Määtta, S.; Mervaala, E.; Könönen, M.; Ursini, F.; et al. Sensorimotor cortex excitability and connectivity in Alzheimer’s disease: A TMS-EEG Co-registration study. Hum. Brain Mapp. 2016, 37, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Bagattini, C.; Mutann, T.P.; Fracassi, C.; Manenti, R.; Cotelli, M.; Ilmoniemi, R.J.; Miniussi, C.; Bortoletto, M. Predicting Alzheimer’s disease severity by means of TMS-EEG coregistration. Neurobiol. Aging 2019, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; Di Lorenzo, F.; Serra, L.; et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018, 169, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Pauri, F.; Pasqualetti, P.; Fini, R.; Dal Forno, G.; Rossini, P.M. Motor cortex excitability in Alzheimer’s disease: A transcranial magnetic stimulation study. Ann. Neurol. 2003, 53, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Pasqualetti, P.; Määttä, S.; Ponzo, D.; Guerra, A.; Bressi, F.; Chiovenda, P.; Del Duca, M.; Giambattistelli, F.; Ursini, F.; et al. Motor cortex excitability in Alzheimer’s disease: A transcranial magnetic stimulation follow-up study. Neurosci. Lett. 2011, 492, 94–98. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, P.M.; Miraglia, F.; Alù, F.; Cotelli, M.; Ferreri, F.; Iorio, R.D.; Iodice, F.; Vecchio, F. Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia. J. Pers. Med. 2020, 10, 34. https://doi.org/10.3390/jpm10020034
Rossini PM, Miraglia F, Alù F, Cotelli M, Ferreri F, Iorio RD, Iodice F, Vecchio F. Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia. Journal of Personalized Medicine. 2020; 10(2):34. https://doi.org/10.3390/jpm10020034
Chicago/Turabian StyleRossini, Paolo Maria, Francesca Miraglia, Francesca Alù, Maria Cotelli, Florinda Ferreri, Riccardo Di Iorio, Francesco Iodice, and Fabrizio Vecchio. 2020. "Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia" Journal of Personalized Medicine 10, no. 2: 34. https://doi.org/10.3390/jpm10020034
APA StyleRossini, P. M., Miraglia, F., Alù, F., Cotelli, M., Ferreri, F., Iorio, R. D., Iodice, F., & Vecchio, F. (2020). Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia. Journal of Personalized Medicine, 10(2), 34. https://doi.org/10.3390/jpm10020034







