Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Protocol Registration
2.2. Eligibility Criteria
- Type of study: No restriction.
- Participants: Patients with clinical suspicion of DPE based on clinical complaints and/or physical examination.
- Index test: TVS, MRI, and RES were considered as index test.
- Reference test: Surgical procedure and histological examination were the reference standard of this meta-analysis according to literature [1].
- Outcome: According to our sections, we analyzed the diagnostic accuracy of each technique compared to one another (one-to-one comparison: TVS vs. MRI; TVS vs. RES, and MRI vs. RES) for each DPE location. The DPE locations reported and analyzed were: USLs, VW & VF, BL, RS, PoD and RVS.
- True positive (TP), false positive (FP), true negative (TN), and false negative (FN) were retrieved or calculated if necessary.
- Setting: Tertiary center hospitals
- Data required for extraction: Information sufficient to produce a 2 × 2 table for calculation of sensitivity and specificity.
- Language: No language restrictions were applied.
2.3. Information Sources and Search Strategies
2.4. Study Selection and Data Extraction
2.5. Risk of Bias in Individual Studies
2.6. Statistical Analysis
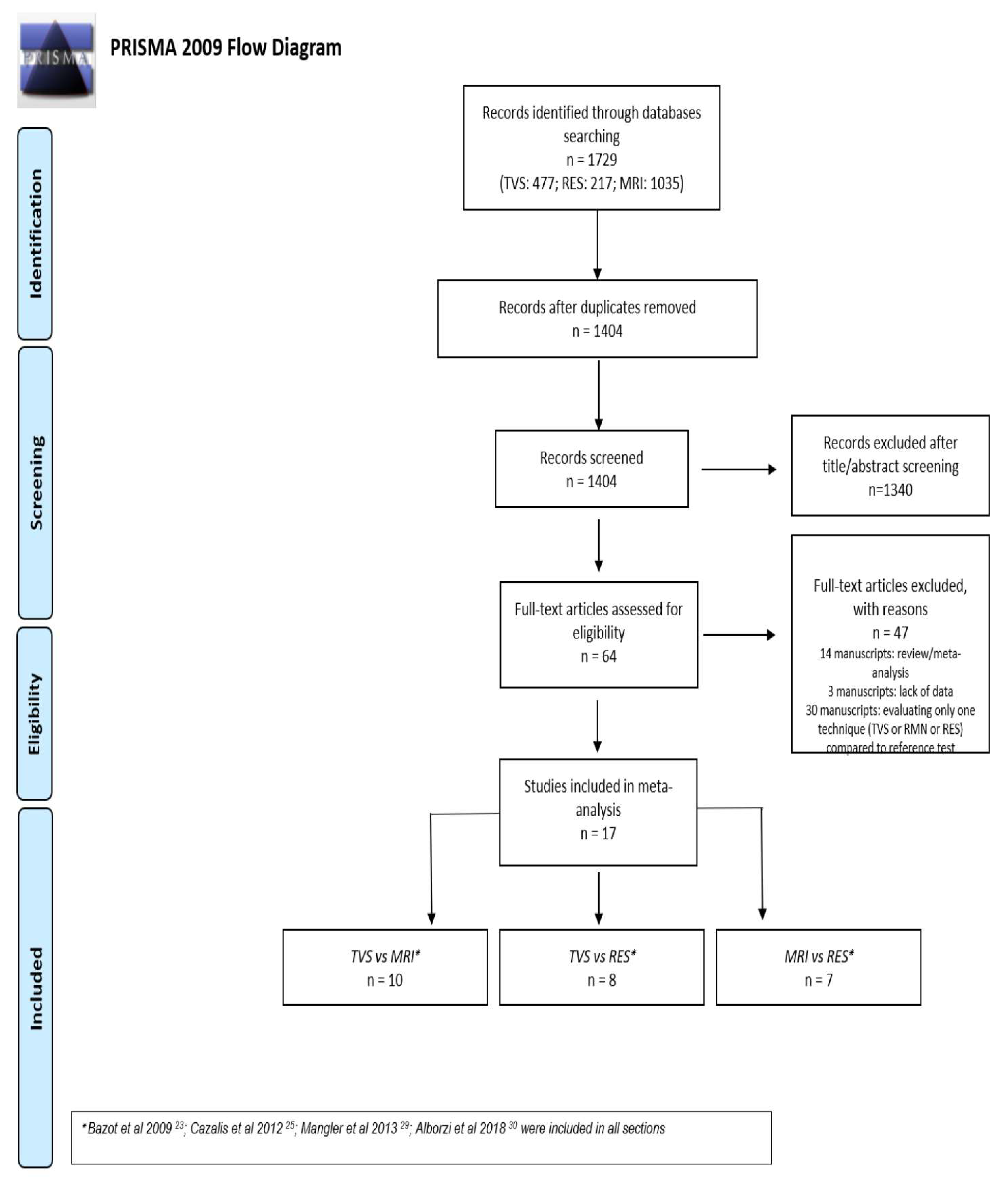
3. Results
3.1. Study Selection
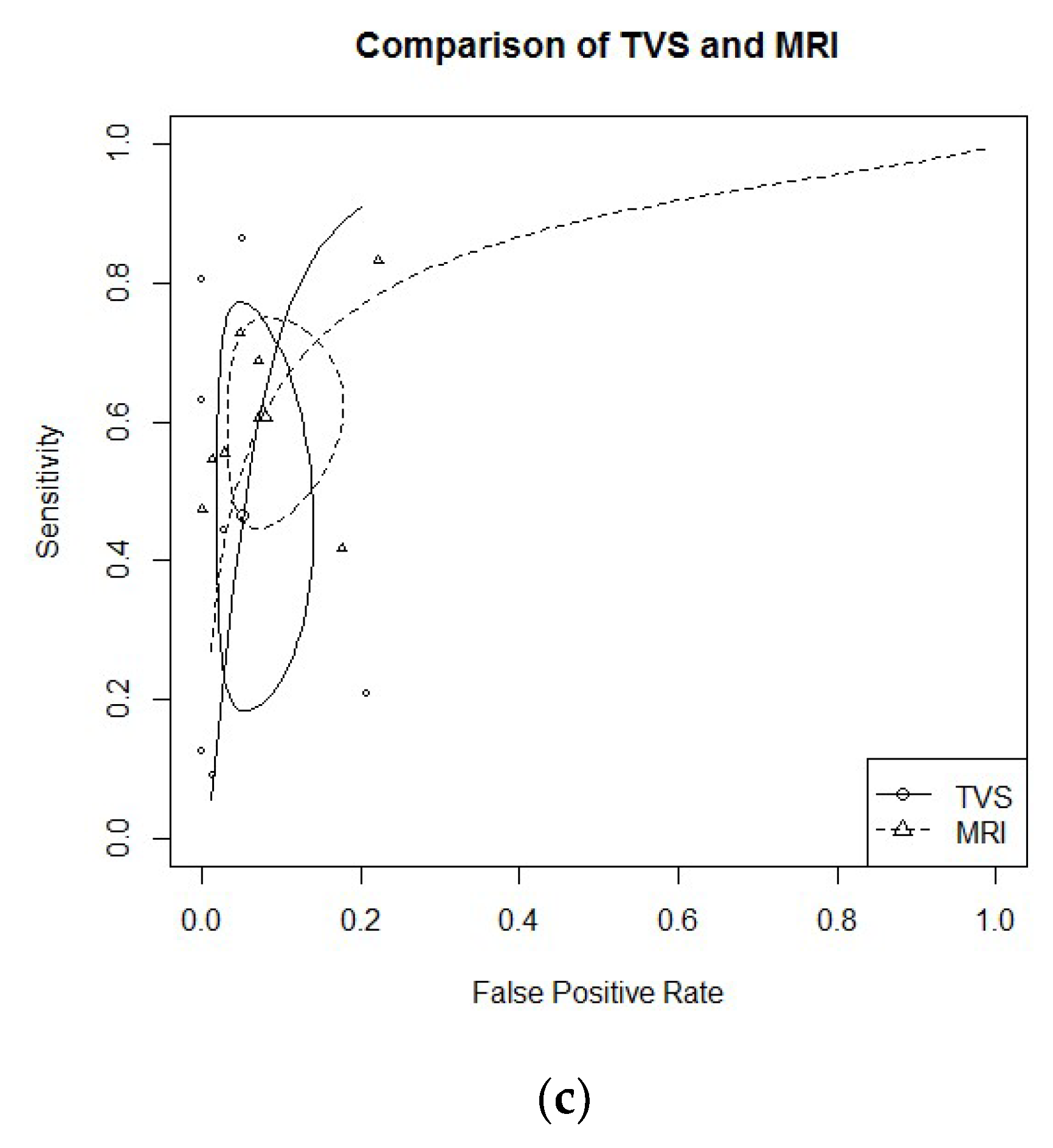
3.2. Diagnostic Performance of TVS Versus MRI
- USLs endometriosis: Six studies evaluated DTA of TVS and MRI for the detection of USLs endometriosis [21,24,25,26,27,30]. Pooled sensitivity and FPR were 0.71 (95% CI 0.65–0.77) and 0.11 (95% CI 0.06–0.19) for TVS, and 0.67 (95% CI 0.54–0.77) and 0.07 (95% CI 0.05–0.11) for MRI (HSROC in Figure 2a). Heterogeneity can be evaluated by predicted ellipses in Figure 2a. Bivariate meta-regression indicated similar accuracy for TVS and MRI (p = 0.65).
- RS endometriosis: Eight studies evaluated DTA of TVS and MRI for RS endometriosis detection [17,21,23,24,26,27,30,31]. Pooled sensitivity and FPR were 0.85 (95% CI 0.76–0.90) and 0.06 (95% CI 0.02–0.15) for TVS, and 0.83 (95% CI 0.76–0.88) and 0.07 (95% CI 0.03–0.14) for MRI (HSROC in Figure 2b). Heterogeneity can be evaluated by predicted ellipses in Figure 2b. Bivariate meta-regression indicated similar accuracy for TVS and MRI (p = 0.96).RVS endometriosis: Seven studies evaluated DTA of TVS and MRI for RVS endometriosis detection [21,24,25,26,27,28,30]. Pooled sensitivity and FPR were 0.47 (95% CI 0.23–0.72) and 0.05 (95% CI 0.02–0.12) for TVS, and 0.61 (95% CI 0.48–0.72) and 0.08 (95% CI 0.04–0.15) for MRI (HSROC in Figure 2c). Heterogeneity can be evaluated by predicted ellipses in Figure 2c. Bivariate meta-regression indicated similar accuracy for TVS and MRI (p = 0.47).Other localization of endometriosis (univariate approach, TVS vs. MRI):
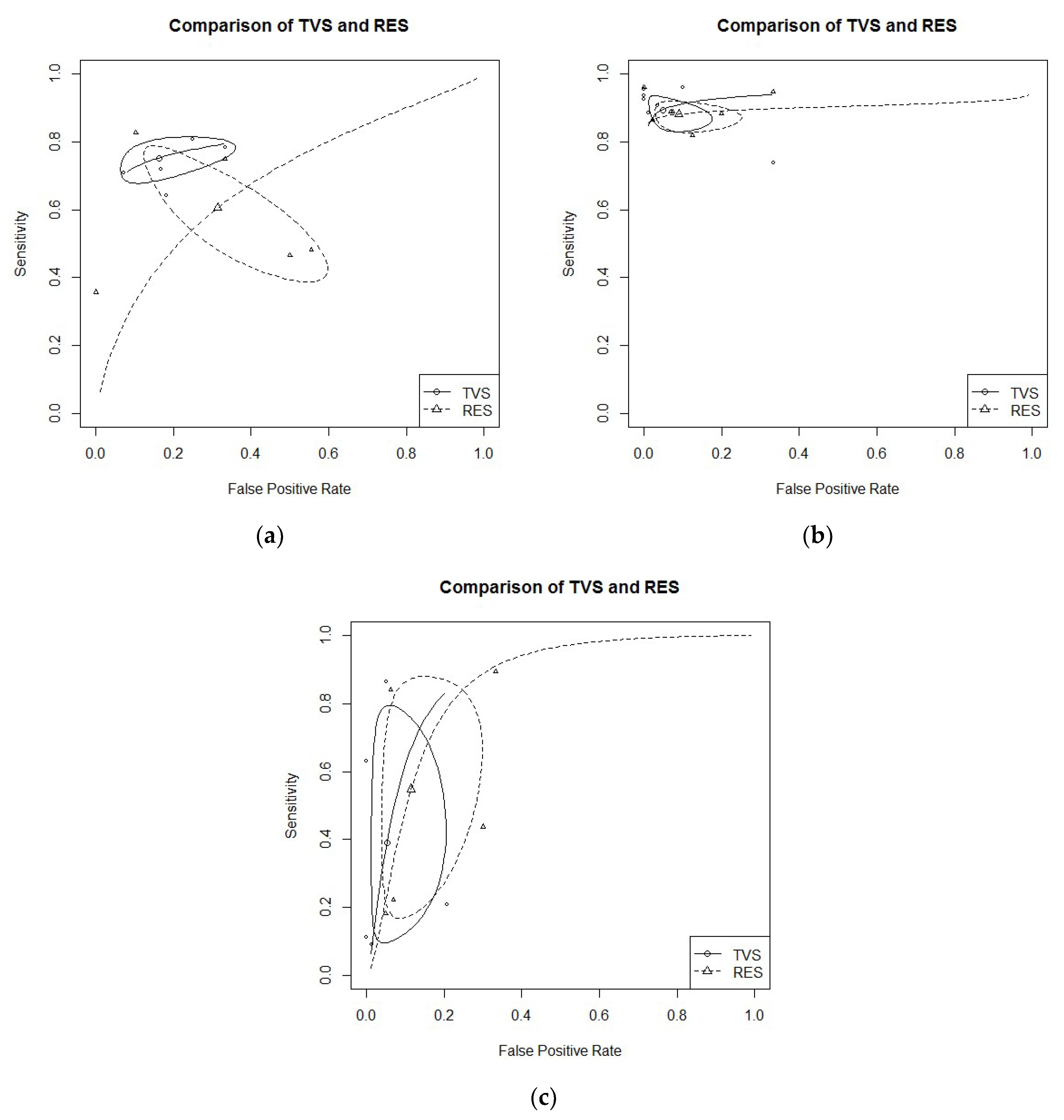
3.3. Diagnostic Performance of TVS Versus RES
- USLs endometriosis: Five studies evaluated DTA of TVS and RES for USLs endometriosis detection [18,21,22,24,30]. Pooled sensitivity and FPR were 0.75 (95% CI 0.69–0.70) and 0.16 (95% CI 0.08–0.31) for TVS, and 0.61 (95% CI 0.43–0.76) and 0.31 (95% CI 0.15–0.54) for RES (HSROC in Figure 3b). Heterogeneity can be evaluated by predicted ellipses in Figure 3a. Bivariate meta-regression indicated similar accuracy for TVS and RES (p = 0.29).
- RS endometriosis: Seven studies evaluated DTA of TVS and RES for RS endometriosis detection [15,18,20,21,22,24,30]. Pooled sensitivity and FPR were 0.89 (95% CI 0.84–0.93) and 0.05 (95% CI 0.02–0.14) for TVS, and 0.88 (95% CI 0.84–0.91) and 0.09 (95% CI 0.04–0.21) for RES (HSROC in Figure 3a). Heterogeneity can be evaluated by predicted ellipses in Figure 3b. Bivariate meta-regression indicated similar accuracy for TVS and RES (p = 0.68).RVS endometriosis: Five studies evaluated DTA of TVS and RES for RVS endometriosis detection [21,22,24,28,30]. Pooled sensitivity and FPR were 0.39 (95% CI 0.13–0.73) and 0.05 (95% CI 0.02–0.16) for TVS, and 0.55 (95% CI 0.22–0.84) and 0.11 (95% CI 0.05–0.25) for RES (HSROC in Figure 3c). Heterogeneity can be evaluated by predicted ellipses in Figure 3c. Bivariate meta-regression indicated similar accuracy for TVS and RES (p = 0.40).Other localization of endometriosis (univariate approach, TVS vs. RES):
- Only one study DTA of TVS and RES for PoD endometriosis [21]
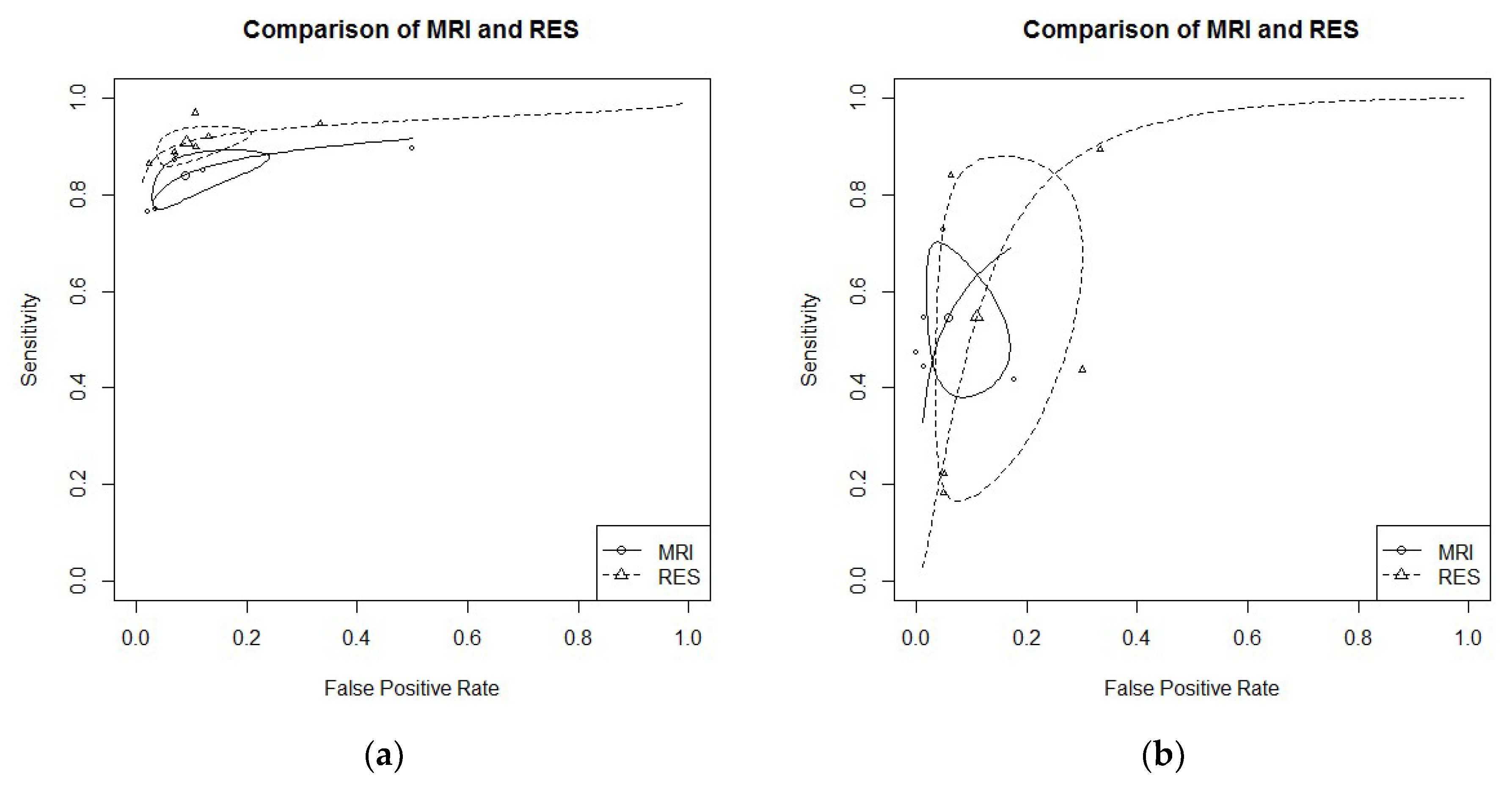
3.4. Diagnostic Performance of MRI Versus RES
- RS endometriosis: six studies evaluated DTA of MRI and RES for RS endometriosis detection [16,19,21,24,29,30]. Pooled sensitivity and FPR were 0.84 (95% CI 0.79–0.88) and 0.09 (95% CI 0.04–0.20) for MRI, and 0.91 (95% CI 0.87–0.94) and 0.13 (95% CI 0.04–0.18) for RES (HSROC in Figure 4a). Heterogeneity can be evaluated by predicted ellipses in Figure 4a. Bivariate meta-regression indicated different accuracy for MRI and RES (p = 0.03). In particular, RES offered a better sensitivity than MRI (p = 0.02), while the false positive rate was similar between the two methods (p = 0.92).
- RVS endometriosis: five studies evaluated DTA of MRI and RES for RVS endometriosis detection [19,21,24,28,30]. Pooled sensitivity and FPR were 0.55 (95% CI 0.41–0.67) and 0.06 (95% CI 0.02–0.14) for MRI, and 0.55 (95% CI 0.22–0.84) and 0.11 (95% CI 0.05–0.25) for RES (HSROC in Figure 4b). Heterogeneity can be evaluated by predicted ellipses in Figure 4b. Bivariate meta-regression indicated similar accuracy for MRI and RES (p = 0.43).Other localization of endometriosis (univariate approach, MRI vs. RES):
- No study reported data for PoD endometriosis detection.
3.5. Risk of Bias Within Studies
3.6. Applicability Concerns
4. Discussion
4.1. Synthesis of the Results
4.2. Interpretation of the Results
4.3. Comparison to Other Meta-Analysis
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Noventa, M.; Saccardi, C.; Litta, P.; Vitagliano, A.; D′Antona, D.; Abdulrahim, B.; Duncan, A.; Alexander-Sefre, F.; Aldrich, C.J.; Quaranta, M.; et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: Algorithm based on a systematic review and meta-analysis. Fertil. Steril. 2015, 104, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Diagnosis of deep endometriosis: Clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil. Steril. 2017, 108, 886–894. [Google Scholar] [CrossRef]
- Nnoaha, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. World Endometriosis Research Foundation Global Study of Women’s Health consortium. Reprint of: Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2019, 112, e137–e152. [Google Scholar] [CrossRef]
- Scioscia, M.; Bruni, F.; Ceccaroni, M.; Steinkasserer, M.; Stepniewska Minelli, L. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet. Gynecol. Scand. 2011, 90, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Noventa, M.; Saccardi, C.; Litta, P.; Quaranta, M.; D’Antona, D.; Gizzo, S. Innovative ultrasound techniques for diagnosis of deep pelvic endometriosis: More confusion or a possible solution to the dilemma? Ultrasound Obstet. Gynecol. 2015, 45, 355–356. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Minguez, J.A.; Jurado, M.; Mais, V.; Melis, G.B.; Alcazar, J.L. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 534–545. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Orozco, R.; Perniciano, M.; Jurado, M.; Melis, G.B.; Alcazar, J.L. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rectosigmoid: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 281–289. [Google Scholar] [CrossRef]
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 26, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.R.; Rosa, M.I.; Silva, B.R.; Reis, M.E.; Simon, C.S.; Dondossola, E.R.; da Cunha Filho, J.S. Accuracy of magnetic resonance in deeply infiltrating endometriosis: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2015, 291, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Sotiriadis, A.; Papatheodorou, S.I.; Martins, W.P. Synthesizing Evidence from Diagnostic Accuracy TEsts: The SEDATE guideline. Ultrasound Obstet. Gynecol. 2016, 47, 386–395. [Google Scholar] [CrossRef]
- Macaskill, P.; Gatsonis, C.; Deeks, J.J.; Harbord, R.M.; Takwoingi, Y. Chapter 10: Analysing and Presenting Results. In Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0; Deeks, J.J., Bossuyt, P.M., Gatsonis, C., Eds.; The Cochrane Collaboration: Londou, UK, 2010; Available online: http://srdta.cochrane.org/ (accessed on 1 January 2019).
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Detchev, R.; Cortez, A.; Amouyal, P.; Uzan, S.; Daraï, E. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum. Reprod. 2003, 18, 1686–1692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chapron, C.; Vieira, M.; Chopin, N.; Balleyguier, C.; Barakat, H.; Dumontier, I.; Roseau, G.; Fauconnier, A.; Foulot, H.; Dousset, B. Accuracy of rectal endoscopic ultrasonography and magnetic resonance imaging in the diagnosis of rectal involvement for patients presenting with deeply infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2004, 24, 175–179. [Google Scholar] [CrossRef]
- Abrao, M.S.; Gonçalves, M.O.; Dias JAJr Podgaec, S.; Chamie, L.P.; Blasbalg, R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum. Reprod. 2007, 22, 3092–3097. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Malzy, P.; Cortez, A.; Roseau, G.; Amouyal, P.; Daraï, E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2007, 30, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Bornier, C.; Dubernard, G.; Roseau, G.; Cortez, A.; Daraï, E. Accuracy of magnetic resonance imaging and rectal endoscopic sonography for the prediction of location of deep pelvic endometriosis. Hum. Reprod. 2007, 22, 1457–1463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piketty, M.; Chopin, N.; Dousset, B.; Millischer-Bellaische, A.E.; Roseau, G.; Leconte, M.; Borghese, B.; Chapron, C. Preoperative work-up for patients with deeply infiltrating endometriosis: Transvaginal ultrasonography must definitely be the first-line imaging examination. Hum. Reprod. 2009, 24, 602–607. [Google Scholar] [CrossRef]
- Bazot, M.; Lafont, C.; Rouzier, R.; Roseau, G.; Thomassin-Naggara, I.; Daraï, E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil. Steril. 2009, 92, 1825–1833. [Google Scholar] [CrossRef]
- Bergamini, V.; Ghezzi, F.; Scarperi, S.; Raffaelli, R.; Cromi, A.; Franchi, M. Preoperative assessment of intestinal endometriosis: A comparison of transvaginal sonography with water-contrast in the rectum, transrectal sonography, and barium enema. Abdom. Imaging 2010, 35, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Guerriero, S.; Sulcis, R.; Pilloni, M.; Ajossa, S.; Melis, G.; Mallarini, G. MRI and “tenderness guided” transvaginal ultrasonography in the diagnosis of recto-sigmoid endometriosis. J. Magn. Reson. Imaging 2012, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gauche Cazalis, C.; Koskas, M.; Martin, B.; Palazzo, L.; Madelenat, P.; Yazbeck, C. Preoperative imaging of deeply infiltrating endometriosis in: Transvaginal sonography, rectal endoscopic sonography and magnetic resonance imaging. Gynecol. Obstet. Fertil. 2012, 40, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Fiaschetti, V.; Crusco, S.; Meschini, A.; Cama, V.; Di Vito, L.; Marziali, M.; Piccione, E.; Calabria, F.; Simonetti, G. Deeply infiltrating endometriosis: Evaluation of retro-cervical space on MRI after vaginal opacification. Eur. J. Radiol. 2012, 81, 3638–3645. [Google Scholar] [CrossRef]
- Saccardi, C.; Cosmi, E.; Borghero, A.; Tregnaghi, A.; Dessole, S.; Litta, P. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2012, 40, 464–469. [Google Scholar] [CrossRef]
- Vimercati, A.; Achilarre, M.T.; Scardapane, A.; Lorusso, F.; Ceci, O.; Mangiatordi, G.; Angelelli, G.; Van Herendael, B.; Selvaggi, L.; Bettocchi, S. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2012, 40, 592–603. [Google Scholar] [CrossRef]
- Mangler, M.; Medrano, N.; Bartley, J.; Mechsner, S.; Speiser, D.; Schneider, A.; Köhler, C. Value of diagnostic procedures in rectovaginal endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2013, 53, 389–394. [Google Scholar] [CrossRef]
- Kanté, F.; Belghiti, J.; Roseau, G.; Thomassin-Naggara, I.; Bazot, M.; Daraï, E.; Ballester, M. Comparison of the accuracy of rectal endoscopic sonography and magnetic resonance imaging in the diagnosis of colorectal endometriosis. Gynecol. Obstet. Fertil. Senol. 2017, 45, 131–136. [Google Scholar]
- Alborzi, S.; Rasekhi, A.; Shomali, Z.; Madadi, G.; Alborzi, M.; Kazemi, M.; Hosseini Nohandani, A. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine 2018, 97, e9536. [Google Scholar] [CrossRef]
- Guerriero, S.; Alcázar, J.L.; Pascual, M.A.; Ajossa, S.; Perniciano, M.; Piras, A.; Mais, V.; Piras, B.; Schirru, F.; Benedetto, M.G.; et al. Deep Infiltrating Endometriosis: Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J. Ultrasound Med. 2018, 37, 1511–1521. [Google Scholar] [CrossRef]
- Doebler, P. Mada: Meta-Analysis of Diagnostic Accuracy. R Package Version 0.5.8. 2017. Available online: https://CRAN.R-project.org/package=mada (accessed on 1 January 2019).
- Team, R.C. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Reitsma, J.; Glas, A.; Rutjes, A.; Scholten, R.; Bossuyt, P.; Zwinderman, A. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kambeitz, J.; Kambeitz-Ilankovic, L.; Leucht, S.; Wood, S.; Davatzikos, C.; Malchow, B.; Falkai, P.; Koutsouleris, N. Detecting neuroimaging biomarkers for schizophrenia: A meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015, 40, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Arends, L.; Hamza, T.; Van Houwelingen, J.; Heijenbrok-Kal, M.; Hunink, M.; Stijnen, T. Bivariate Random Effects Meta-Analysis of ROC Curves. Med. Decis. Mak. 2008, 28, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Doebler, P.; Holling, H.; Boehning, D. A Mixed Model Approach to Meta-Analysis of Diagnostic Studies with Binary Test Outcome. Psychol. Methods 2012, 17, 418–436. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Piessens, S.; Healey, M.; Maher, P.; Tsaltas, J.; Rombauts, L. Can anyone screen for deep infiltrating endometriosis with transvaginal ultrasound? Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 462–468. [Google Scholar] [CrossRef]
- Reid, S.; Condous, G. Transvaginal sonographic sliding sign: Accurate prediction of pouch of Douglas obliteration. Ultrasound Obstet. Gynecol. 2013, 41, 605–607. [Google Scholar] [CrossRef]

| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Bazot et al. 2003 [15] |  |  |  |  |  |  |  |
| Chapron et al. 2004 [16] |  |  |  |  |  |  |  |
| Abrao et al. 2007 [17] |  |  |  |  |  |  |  |
| Bazot et al. 2007 a [18] |  |  |  |  |  |  |  |
| Bazot et al. 2007 b [19] |  |  |  |  |  |  |  |
| Piketty et al. 2008 [20] |  |  |  |  |  |  |  |
| Bazot et al. 2009 [21] |  |  |  |  |  |  |  |
| Bergamini et al. 2010 [22] |  |  |  |  |  |  |  |
| Saba et al. 2012 [23] |  |  |  |  |  |  |  |
| Cazalis et al. 2012 [24] |  |  |  |  |  |  |  |
| Fiaschetti et al. 2012 [25] |  |  |  |  |  |  |  |
| Saccardi et al. 2012 [26] |  |  |  |  |  |  |  |
| Vimercati et al. 2012 [27] |  |  |  |  |  |  |  |
| Mangler et al. 2013 [28] |  |  |  |  |  |  |  |
| Kanté et al. 2017 [29] |  |  |  |  |  |  |  |
| Alborzi et al. 2018 [30] |  |  |  |  |  |  |  |
| Guerriero et al. 2018 [31] |  |  |  |  |  |  |  |
 : low risk;
: low risk;  : high risk;
: high risk;  : unclear risk.
: unclear risk.| Authors and Year | Study Design | Index Test | Patients Treated (n) | Mean/Median Age | Mean BMI (kg/m2) | DPE Confirmed Localization (% (n)) | Reference Standard |
|---|---|---|---|---|---|---|---|
| Abrao et al. 2007 [17] | cross-sectional study | TVS MRI | 104 | 33.8 ± 6.1 | NS | 64.3 (63) | surgery |
| Bazot et al. 2009 [21] | retrospective study | TVS MRI RES | 92 | 31.8 (20–51) | NS | 97.8 (90) | histology |
| Saba et al. 2012 [23] | prospective study | TVS MRI | 59 | 33 (21–44) | NS | 76.3 (45) | surgery and histology |
| Cazalis et al. 2012 [24] | retrospective study | TVS MRI RES | 25 | 35.4 ± 6.1 | NS | 100 (25) | surgery and histology |
| Fiaschetti et al. 2012 [25] | prospective study | TVS MRI | 58 | 34 ± 6 | NS | 71.2 (57) | surgery |
| Saccardi et al. 2012 [26] | prospective study | TVS MRI | 54 | 32.3 ± 5.8 | 20.6 ± 2.2 | 85.2 (46) | surgery and histology |
| Vimercati et al. 2012 [27] | prospective study | TVS MRI | 90 | 34 | NS | 82.2 (74) | surgery and histology |
| Mangler et al. 2013 [28] | prospective study | TVS MRI RES | 79 | 34 (19–51) | 23 (17–35) | 61.0 (48) | surgery and histology |
| Alborzi et al. 2018 [30] | prospective study | TVS MRI RES | 317 | 31 ± 5.4 | NS | 79.5 (252) | surgery and histology |
| Guerriero et al. 2018 [31] | prospective study | TVS MRI | 159 | 33 ± 7 | nr | 66.6 (106) | surgery |
| Authors and Year | Study Design | Test Method | Patients Treated (n) | Mean/Median Age | Mean BMI (kg/m2) | DPE Confirmed Localization (% (n)) | Reference Standard |
|---|---|---|---|---|---|---|---|
| Bazot et al. 2003 [15] | prospective study | TVS RES | 30 | 32 (21 ± 50) | NS | 93.0 (28) | histology |
| Bazot et al. 2007a [18] | prospective study | TVS RES | 81 | 31.9 (20–51) | NS | 97.5 (79) | surgery and histology |
| Bazot et al. 2009 [21] | retrospective study. | TVS MRI RES | 92 | 31.8 (20–51) | NS | 97.8 (90) | histology |
| Bergamini et al. 2010 [22] | prospective study | TVS RES | 61 | 33.1 (28–37) | NS | 83.6 (51) | surgery and histology |
| Cazalis et al. 2012 [24] | retrospective study. | TVS MRI RES | 25 | 35.4 ± 6.1 | NS | 100 (25) | surgery and histology |
| Mangler et al. 2013 [28] | prospective study | TVS MRI RES | 79 | 34 (19–51) | 23 (17–35) | 61.0 (48) | surgery and histology |
| Piketty et al. 2009 [20] | prospective study | TVS RES | 134 | 32.1 ± 5.0 (22–47) | NS | 100 (134) | histology |
| Alborzi et al. 2018 [30] | prospective study | TVS MRI RES | 317 | 31 ± 5.4 | NS | 79.5 (252) | surgery and histology |
| Authors and Year | Study Design | Index Test | Patients Treated (n) | Mean/Median Age | Mean BMI (kg/m2) | DPE Confirmed Localization (% (n)) | Reference Standard |
|---|---|---|---|---|---|---|---|
| Bazot et al. 2007b [19] | prospective study | MRI RES | 88 | 32.1 (20–51) | NS | 97.7 (86) | surgery and histology |
| Bazot et al. 2009 [21] | retrospective study | TVS MRI RES | 92 | 31.8 (20–51) | NS | 97.8 (90) | surgery and histology |
| Cazalis et al. 2012 [24] | retrospective study | TVS MRI RES | 25 | 35.4 ± 61 | NS | 100 (25) | surgery and histology |
| Chapron et al. 2004 [16] | retrospective study | MRI RES | 81 | 31.9 ± 6.7 | 22.3 ± 3.3 | 100 (81) | histology |
| Kanté et al. 2017 [29] | retrospective study | MRI RES | 239 | 33 (20–53) | 22 (16–38) | NS | surgery and histology |
| Mangler et al. 2013 [28] | prospective study | TVS MRI RES | 79 | 34 (19–51) | 23 (17–35) | 61.0 (48) | surgery and histology |
| Alborzi et al. 2018 [30] | prospective study | TVS MRI RES | 317 | 31 ± 5.4 | NS | 79.5 (252) | surgery and histology |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noventa, M.; Scioscia, M.; Schincariol, M.; Cavallin, F.; Pontrelli, G.; Virgilio, B.; Vitale, S.G.; Laganà, A.S.; Dessole, F.; Cosmi, E.; et al. Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics 2019, 9, 225. https://doi.org/10.3390/diagnostics9040225
Noventa M, Scioscia M, Schincariol M, Cavallin F, Pontrelli G, Virgilio B, Vitale SG, Laganà AS, Dessole F, Cosmi E, et al. Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics. 2019; 9(4):225. https://doi.org/10.3390/diagnostics9040225
Chicago/Turabian StyleNoventa, Marco, Marco Scioscia, Michele Schincariol, Francesco Cavallin, Giovanni Pontrelli, Bruna Virgilio, Salvatore Giovanni Vitale, Antonio Simone Laganà, Francesco Dessole, Erich Cosmi, and et al. 2019. "Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis" Diagnostics 9, no. 4: 225. https://doi.org/10.3390/diagnostics9040225
APA StyleNoventa, M., Scioscia, M., Schincariol, M., Cavallin, F., Pontrelli, G., Virgilio, B., Vitale, S. G., Laganà, A. S., Dessole, F., Cosmi, E., D’Antona, D., Andrisani, A., Saccardi, C., Vitagliano, A., & Ambrosini, G. (2019). Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics, 9(4), 225. https://doi.org/10.3390/diagnostics9040225






