Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Exome Sequencing and Data Analysis
2.2. AED Lists
2.3. Disease Causative Genes
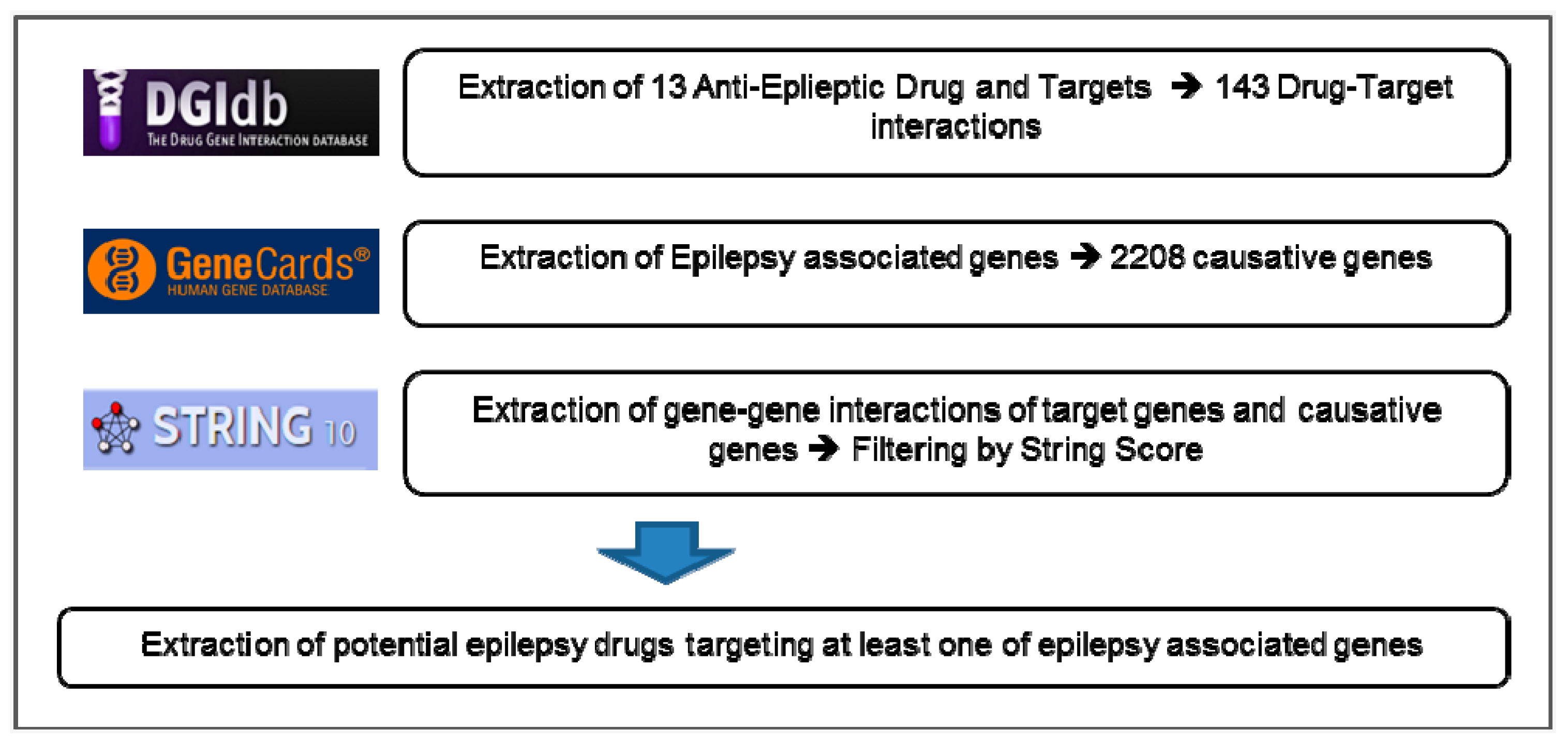
2.4. Drug-Target Interactions (DGIs)
2.5. Network Database
2.6. Construction of EDT-DRUG Network
3. Results
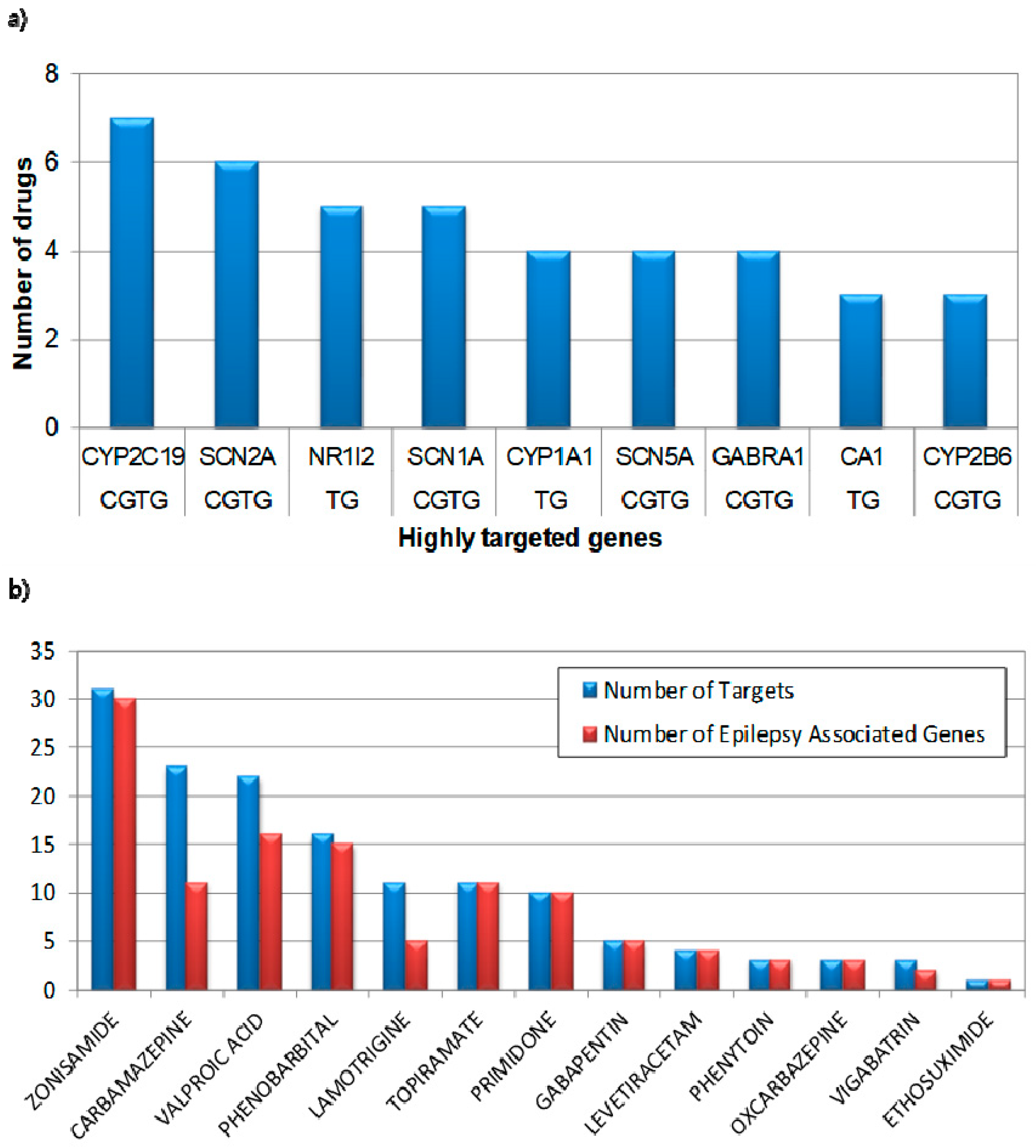
3.1. Characteristics of Clinically Used Anti-Epileptic Drugs
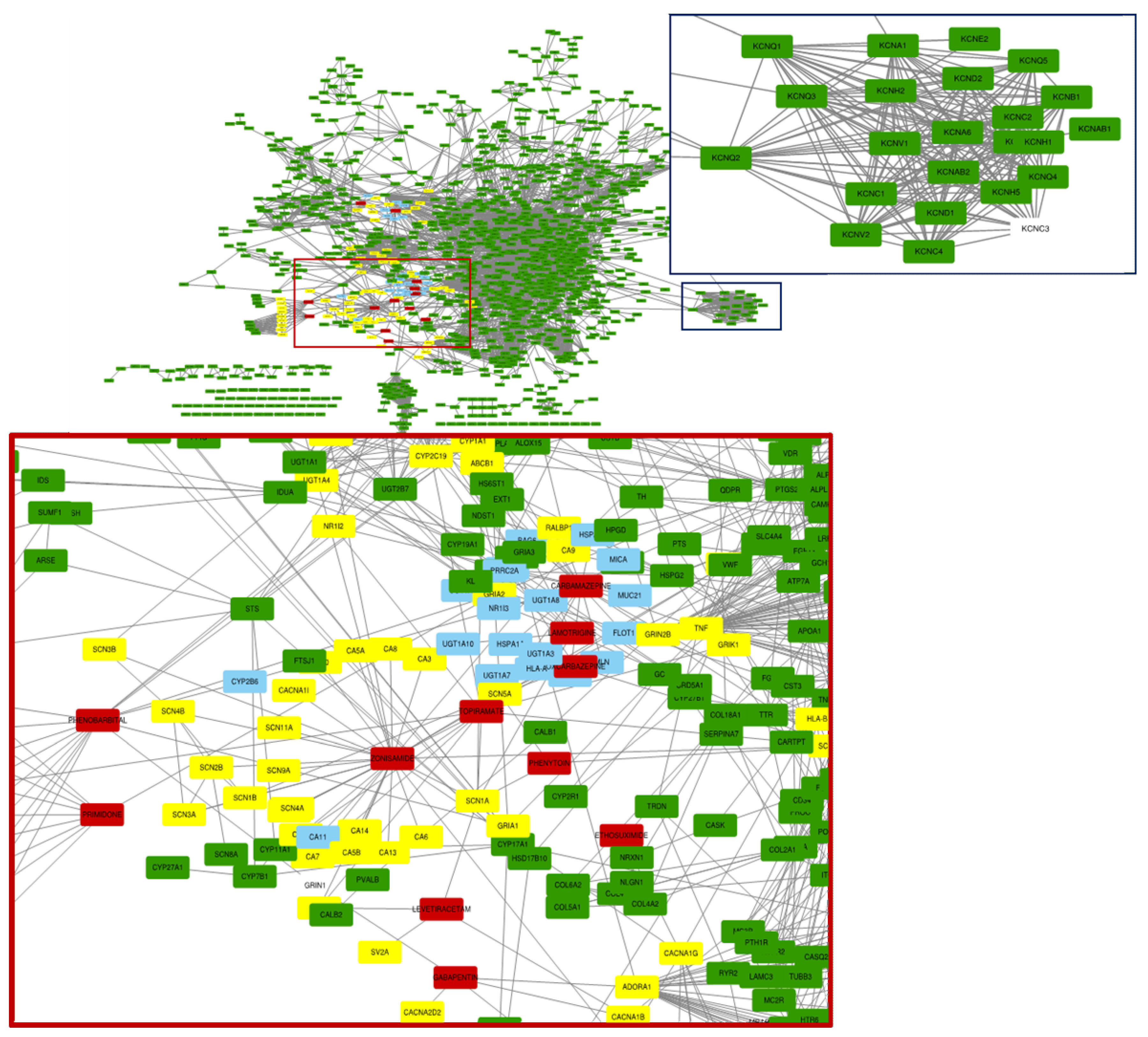
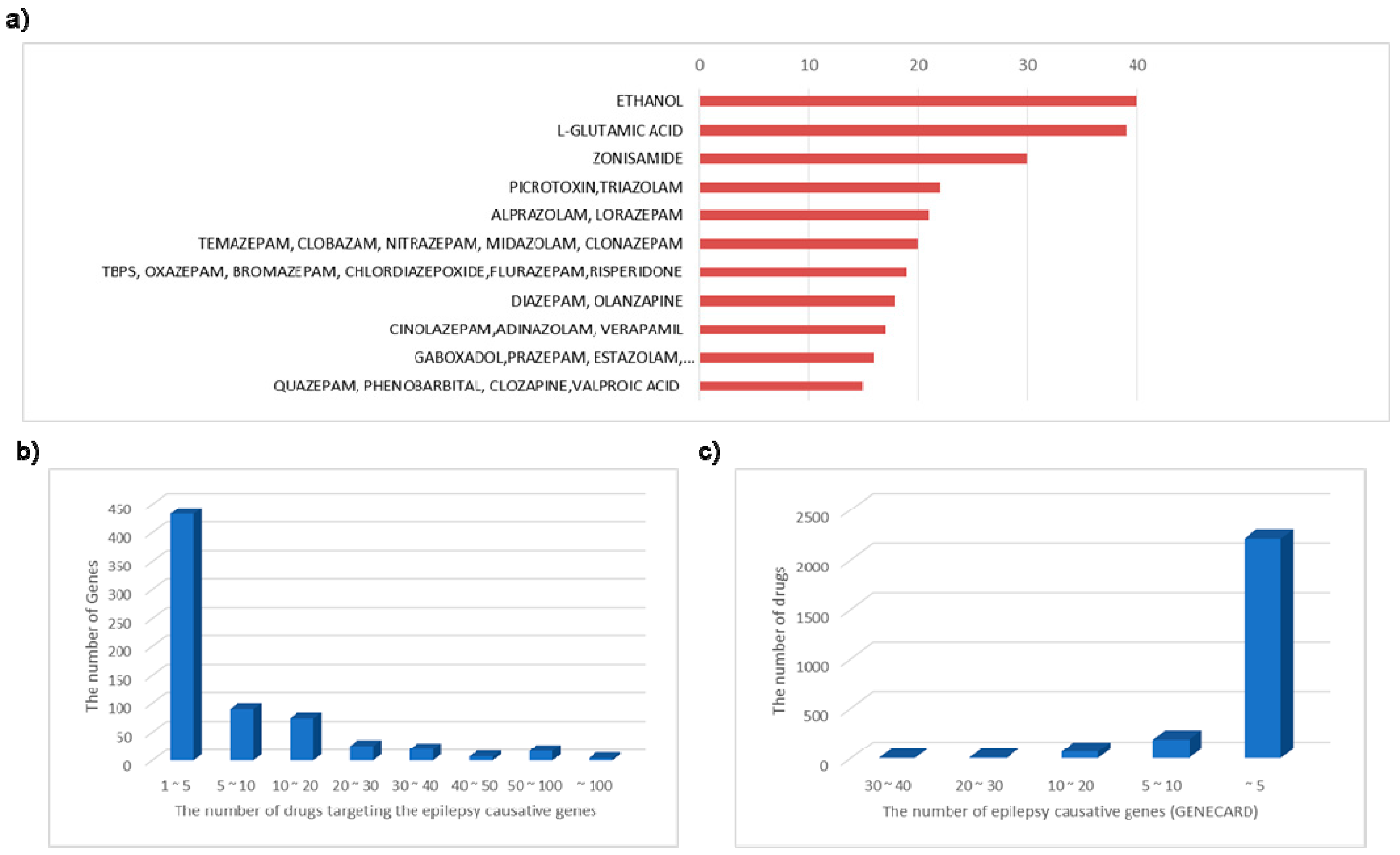
3.2. Epilepsy Associated Genes and Active Targeting Drug
3.3. Identification of Causative Genes from Intractable Epilepsy Patients
3.4. Accurate Diagnosis for Epilepsy Patients Through NGS
3.5. NGS for Epilepsy Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beghi, E. Treating epilepsy across its different stages. Ther. Adv. Neurol. Disord. 2010, 3, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A. Difficulties in Treatment and Management of Epilepsy and Challenges in New Drug Development. Pharmaceuticals (Basel) 2010, 3, 2090–2110. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415. [Google Scholar]
- Shorvon, S.D. Drug treatment of epilepsy in the century of the ILAE: The second 50 years, 1959–2009. Epilepsia 2009, 50 (Suppl. S3), 93–130. [Google Scholar] [CrossRef]
- Brodie, M.J.; Sills, G.J. Combining antiepileptic drugs--rational polytherapy? Seizure 2011, 20, 369–375. [Google Scholar] [CrossRef]
- Lee, J.W.; Dworetzky, B. Rational Polytherapy with Antiepileptic Drugs. Pharmaceuticals (Basel) 2010, 3, 2362–2379. [Google Scholar] [CrossRef]
- Poolos, N.P.; Castagna, C.E.; Williams, S.; Miller, A.B.; Story, T.J. Association between antiepileptic drug dose and long-term response in patients with refractory epilepsy. Epilepsy Behav. 2017, 69, 59–68. [Google Scholar] [CrossRef]
- Baranano, K.W.; Hartman, A.L. The ketogenic diet: Uses in epilepsy and other neurologic illnesses. Curr. Treat. Options Neurol. 2008, 10, 410–419. [Google Scholar] [CrossRef]
- D’Andrea Meira, I.; Romao, T.T.; Pires do Prado, H.J.; Kruger, L.T.; Pires, M.E.P.; da Conceicao, P.O. Ketogenic Diet and Epilepsy: What We Know So Far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef]
- Lefevre, F.; Aronson, N. Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics 2000, 105, E46. [Google Scholar] [CrossRef]
- Madaan, P.; Jauhari, P.; Chakrabarty, B.; Gulati, S. Ketogenic Diet in Epilepsy of Infancy With Migrating Focal Seizures. Pediatr. Neurol. 2019, 95, 92. [Google Scholar] [CrossRef] [PubMed]
- Rogovik, A.L.; Goldman, R.D. Ketogenic diet for treatment of epilepsy. Can. Fam. Physician 2010, 56, 540–542. [Google Scholar] [PubMed]
- Engel, J., Jr. Approaches to refractory epilepsy. Ann. Indian Acad. Neurol. 2014, 17, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Cascino, G.D. Intractable partial epilepsy: Evaluation and treatment. Mayo Clin. Proc. 1990, 65, 1578–1586. [Google Scholar] [CrossRef]
- Barrese, V.; Miceli, F.; Soldovieri, M.V.; Ambrosino, P.; Iannotti, F.A.; Cilio, M.R.; Taglialatela, M. Neuronal potassium channel openers in the management of epilepsy: Role and potential of retigabine. Clin. Pharmacol. 2010, 2, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S.; Rogawski, M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007, 4, 18–61. [Google Scholar] [CrossRef]
- Rogawski, M.A. Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res. 2006, 68, 22–28. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Loscher, W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat. Med. 2004, 10, 685–692. [Google Scholar] [CrossRef]
- Woodbury, D.M. Convulsant drugs: Mechanisms of action. Adv. Neurol. 1980, 27, 249–303. [Google Scholar]
- Brewer, C.T.; Chen, T. PXR variants: The impact on drug metabolism and therapeutic responses. Acta Pharm. Sin. B 2016, 6, 441–449. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, T.M.B.; De Melo, J.M.A.J.; Lopes, L.B.; Bessa, M.C.; Santos, J.G.; Vasconcelos, L.C.; Vieira Neto, A.E.; Borges, L.T.N.; Fonteles, M.M.F.; Chaves Filho, A.J.M.; et al. Ivabradine possesses anticonvulsant and neuroprotective action in mice. Biomed. Pharmacother. 2019, 109, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Huang, C.C.; Wu, S.N. The opening effect of pregabalin on ATP-sensitive potassium channels in differentiated hippocampal neuron-derived H19-7 cells. Epilepsia 2006, 47, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Wu, S.N.; Cheng, J.T.; Tsai, J.J.; Huang, C.C. Diazoxide reduces status epilepticus neuron damage in diabetes. Neurotox. Res. 2010, 17, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Lin, K.M.; Hung, T.Y.; Chuang, Y.C.; Wu, S.N. Multiple Actions of Rotenone, an Inhibitor of Mitochondrial Respiratory Chain, on Ionic Currents and Miniature End-Plate Potential in Mouse Hippocampal (mHippoE-14) Neurons. Cell. Physiol. Biochem. 2018, 47, 330–343. [Google Scholar] [CrossRef]
- Huang, C.W.; Huang, C.C.; Wu, S.N. Activation by zonisamide, a newer antiepileptic drug, of large-conductance calcium-activated potassium channel in differentiated hippocampal neuron-derived H19-7 cells. J. Pharmacol. Exp. Ther. 2007, 321, 98–106. [Google Scholar] [CrossRef]
- Ebrahimi, H.A.; Ebrahimi, S. Evaluation of the Effects of Charged Amino Acids on Uncontrolled Seizures. Neurol. Res. Int. 2015, 2015, 124507. [Google Scholar] [CrossRef]
- Hennecke, H.; Wiechert, P. Seizures and the dose of L-glutamic acid in rats. Epilepsia 1970, 11, 327–331. [Google Scholar] [CrossRef]
- Singh, S.; Hota, D.; Prakash, A.; Khanduja, K.L.; Arora, S.K.; Chakrabarti, A. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin-induced seizure model in mice. Pharmacol. Biochem. Behav. 2010, 94, 416–422. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Large, C.H.; Sankar, R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 2012, 53, 412–424. [Google Scholar] [CrossRef]
- Clark, S.; Antell, A.; Kaufman, K. New antiepileptic medication linked to blue discoloration of the skin and eyes. Ther. Adv. Drug Saf. 2015, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
| Anti-Epileptic Drug Name | Type | Usage/Effects | Serious Side Effects |
|---|---|---|---|
| ZONISAMIDE | classical anti-epileptic drug/broad-spectrum AED | used as adjunctive therapy, indicated for partial seizures in adults | Ataxis |
| CARBAMAZEPINE | classical anti-epileptic drug/narrow-spectrum AED | decreasing nerve impulses that cause seizure and pain, indicated for temporal love epilepsy, grand mal and mixed type seizures | Dermatologic reactions, bone marrow suppression |
| VALPROIC ACID | classical anti-epileptic drug/broad-spectrum AED | preventing of absence seizures, partial seizures, and generalized seizures | Hepatotoxicity, pancreatitis, congenital malformations |
| PHENOBARBITAL | classical anti-epileptic drug/narrow-spectrum AED | slows the activity of the brain and nervous system, used for tonic clonic seizure | CNS depression |
| LAMOTRIGINE | new anti-epileptic drug/broad-spectrum AED | used for focal seizures, tonic-clonic seizures, Lenox–Gastaut syndrome | Stevens–Johnson syndrome |
| TOPIRAMATE | new anti-epileptic drug/broad-spectrum AED | used for generalized tonic-clonic seizure and developmental delay | Neuropsychiatric reactions, visual field defect |
| PRIMIDONE | classical anti-epileptic drug | used for grand mal or focal seizure and neuropathic pain | Ataxia, vertigo |
| GABAPENTIN | new anti-epileptic drug/narrow-spectrum AED | used for partial seizures or neuralgia, affects chemicals and nerves in the body | Hypersensitivity |
| LEVETIRACETAM | new anti-epileptic drug/broad-spectrum AED | used for partial-onset or tonic-clonic seizures, myoclonic seizures | Psychiatric symptoms, anaphylaxis |
| PHENYTOIN | classical anti-epileptic drug/narrow-spectrum AED | used for status epilepticus, slow down impulses in the brain causing seizures | Hypotension, arrhythmias |
| OXCARBAZEPINE | new anti-epileptic drug/narrow-spectrum AED | used for partial seizure, decrease nerve impulses | Hyponatremia, hypersensitivity |
| VIGABATRIN | new anti-epileptic drug/narrow-spectrum AED | used in combination with other medicines, treats for complex partial seizures | Vision loss |
| ETHOSUXIMIDE | classical anti-epileptic drug | treat absence seizures | Allergic reactions |
| Patient ID | Symptom | Identified Causative Genes from Whole Exome Sequencing |
|---|---|---|
| 1 | Seizure, Rett Syndrome | MECP2 |
| 2 | Epilepsy, West syndrome | CDKL5 |
| 3 | Early infantile epileptic encephalopathy, West syndrome, mental retardation, microcephaly | KCNQ2 |
| 4 | Epilepsy, metabolic disorder, cerebral palsy, leukodystrophy, mitochondrial myopathy, delayed development | PRODH |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.; Lee, C.; Lee, Y.; Lee, J.-S. Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs. Diagnostics 2019, 9, 208. https://doi.org/10.3390/diagnostics9040208
Ko Y, Lee C, Lee Y, Lee J-S. Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs. Diagnostics. 2019; 9(4):208. https://doi.org/10.3390/diagnostics9040208
Chicago/Turabian StyleKo, Younhee, Chulho Lee, Youngmok Lee, and Jin-Sung Lee. 2019. "Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs" Diagnostics 9, no. 4: 208. https://doi.org/10.3390/diagnostics9040208
APA StyleKo, Y., Lee, C., Lee, Y., & Lee, J.-S. (2019). Systematic Approach for Drug Repositioning of Anti-Epileptic Drugs. Diagnostics, 9(4), 208. https://doi.org/10.3390/diagnostics9040208





