Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Echocardiogram
2.2. Measurement of Oxidative Stress Biomarkers
2.3. Statistical Analysis
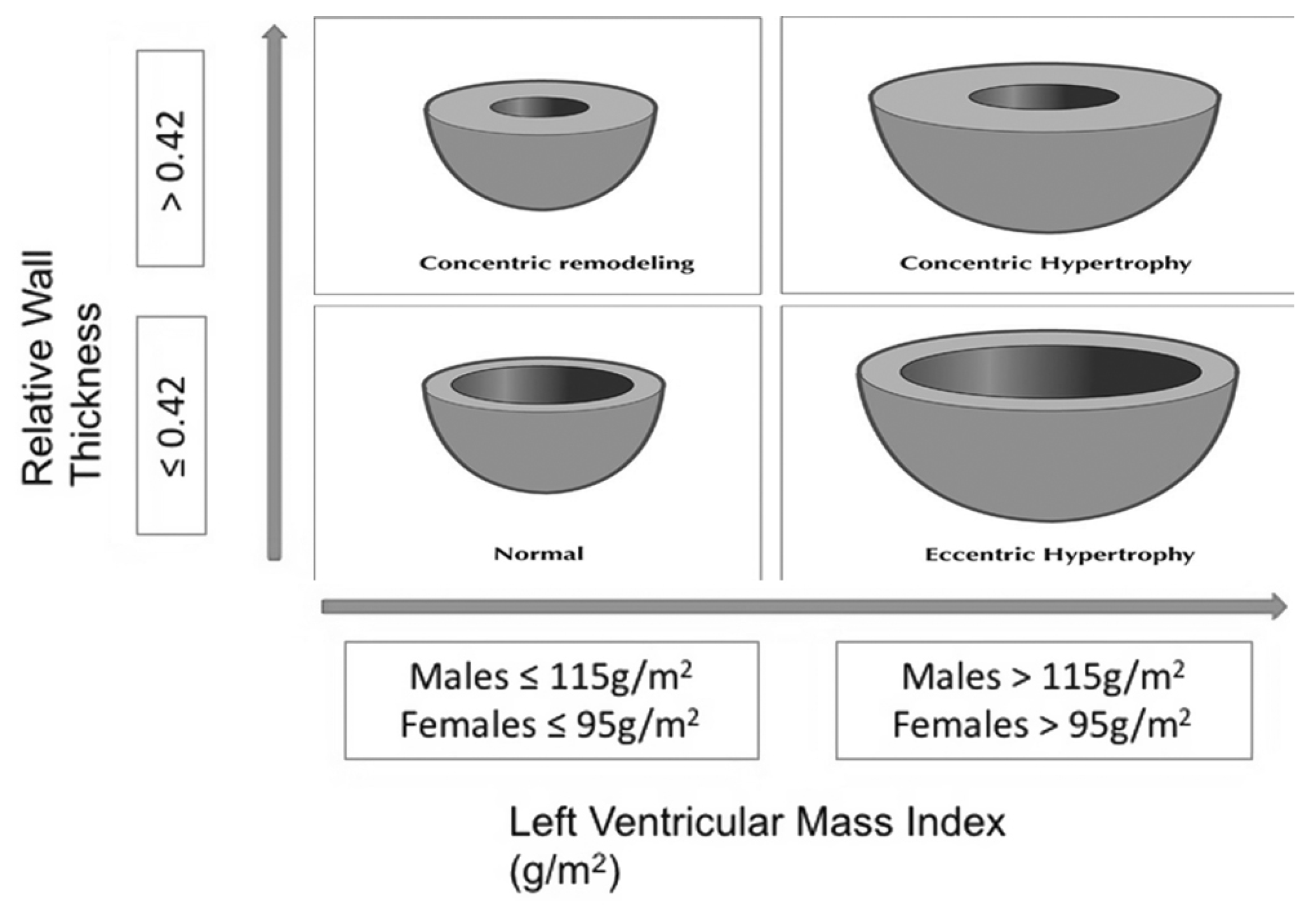
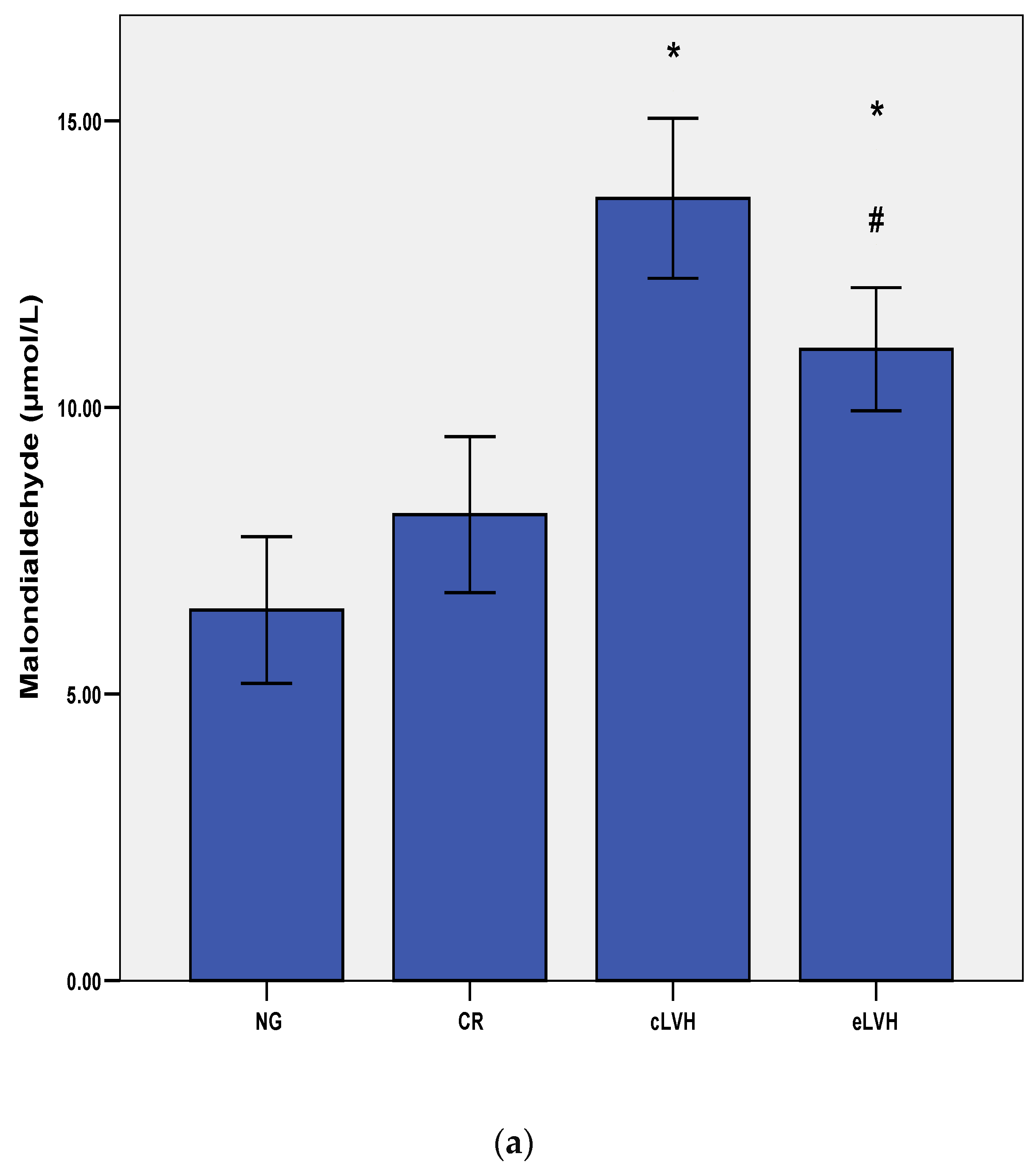
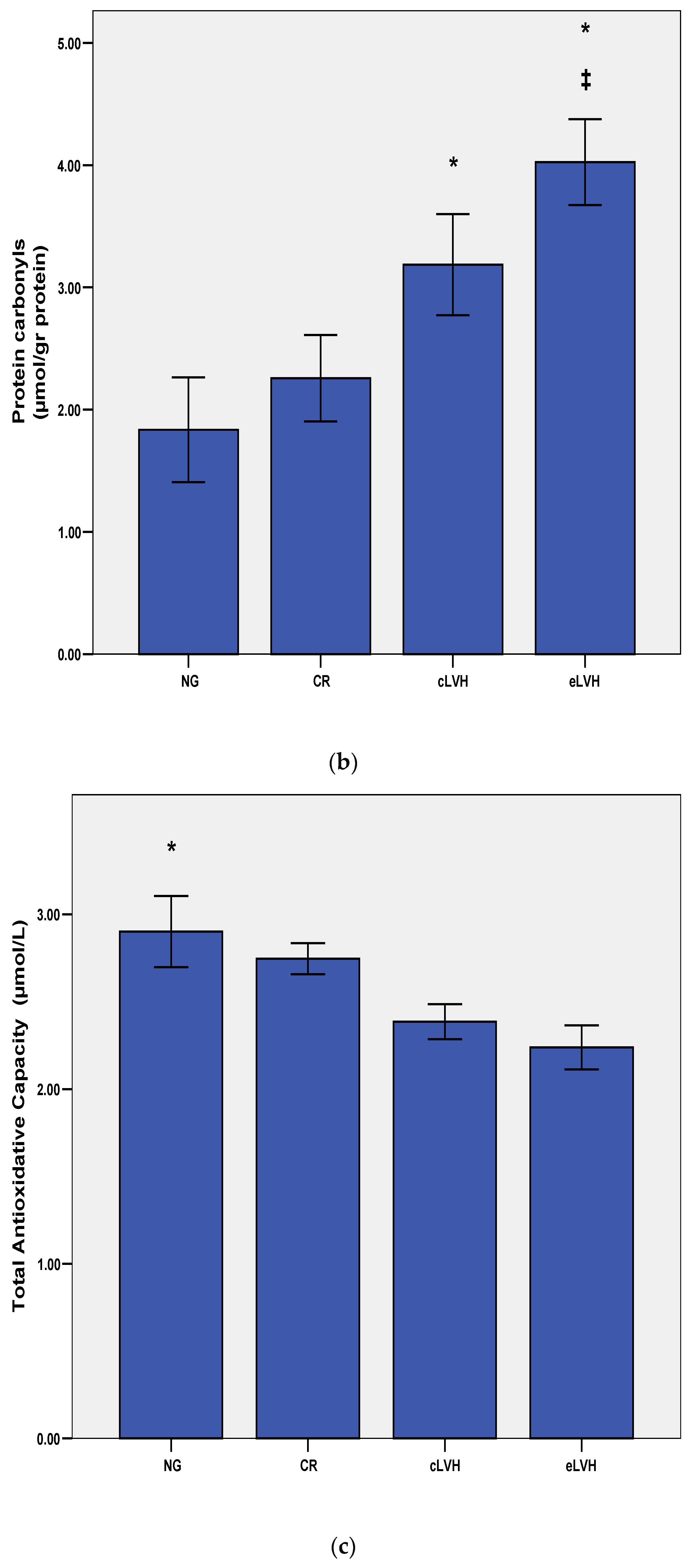
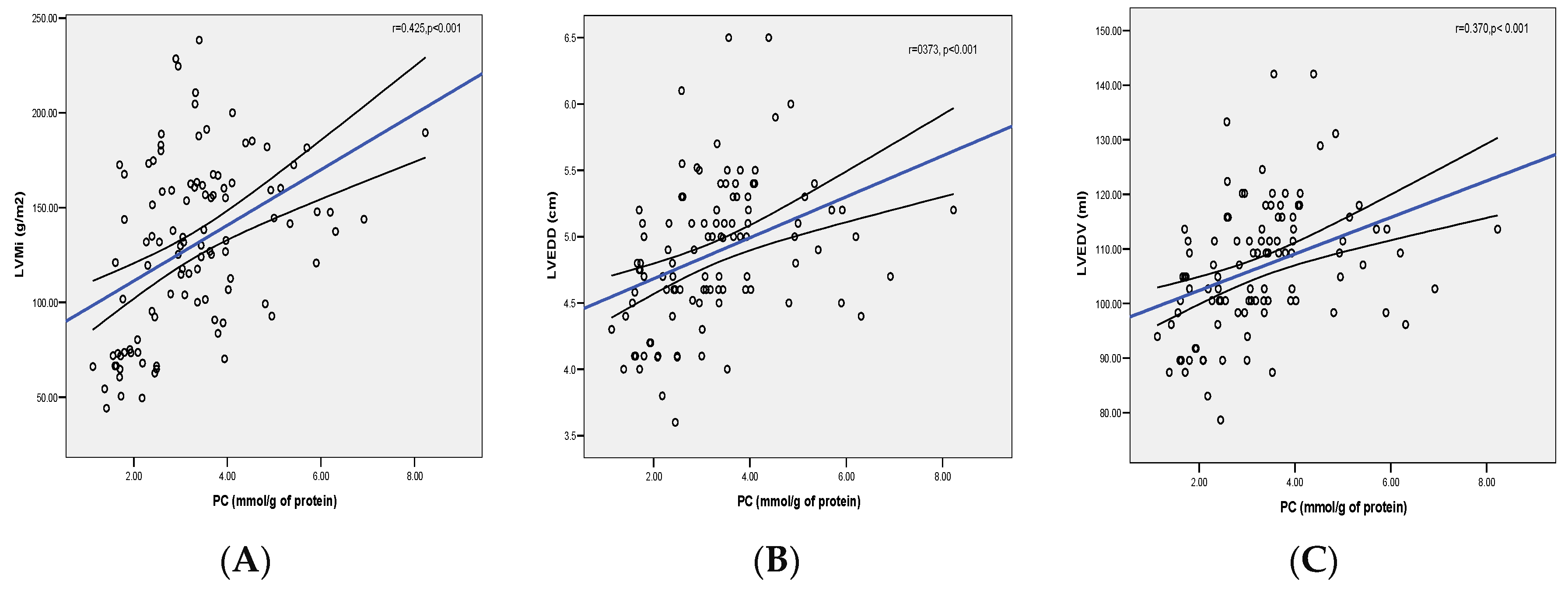
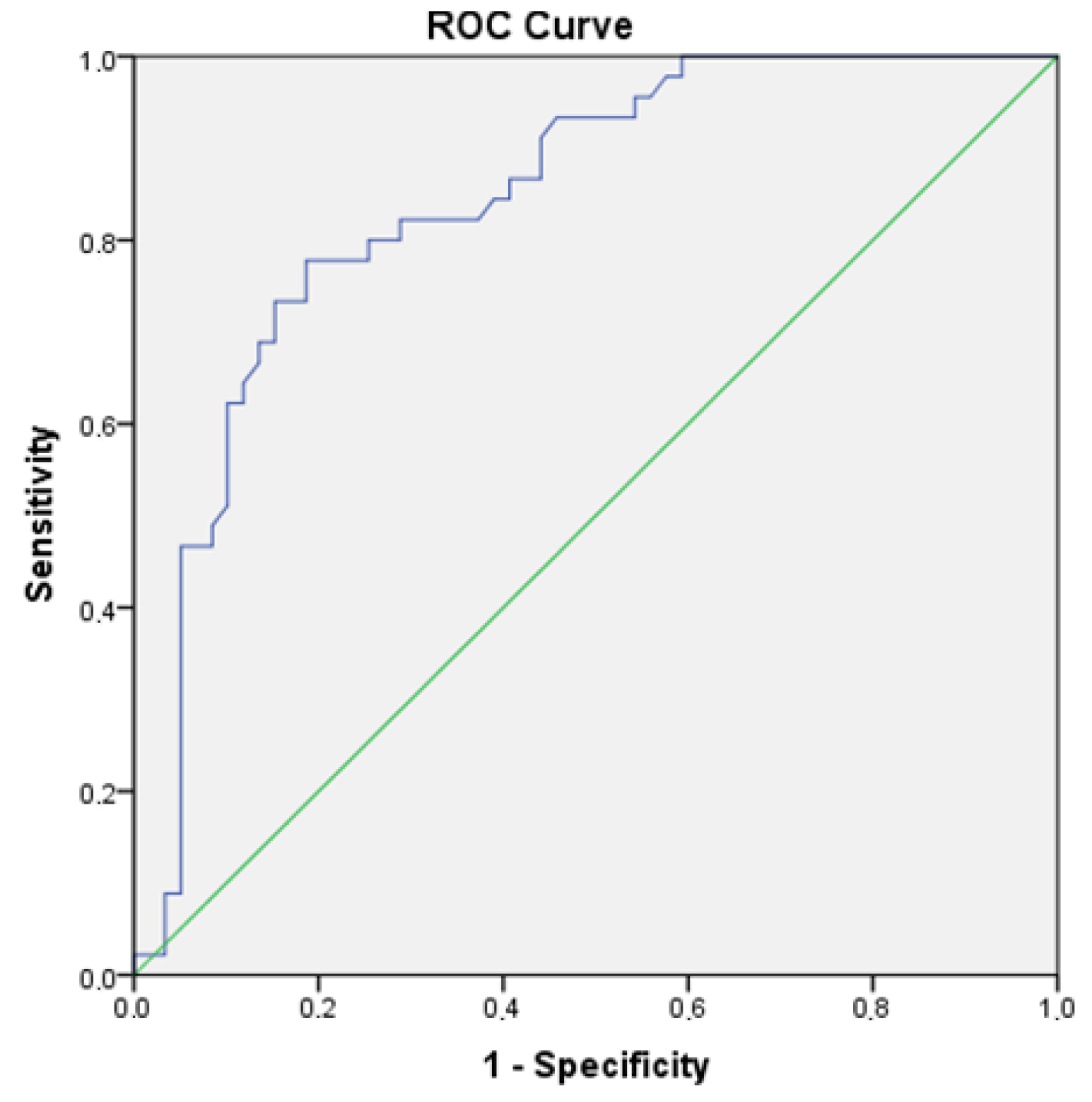
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paoletti, E.; Bellino, D.; Cassottana, P.; Rolla, D.; Cannella, G. LVH in nondiabetic predialysis CKD. Am. J. Kidney Dis. 2005, 46, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Pecoits-Filho, R.; Barberato, S.H. Left ventricular mass in chronic kidney disease and ESRD. Clin. J. Am. Soc. Nephrol. 2009, 4, 79–91. [Google Scholar] [CrossRef]
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Larson, M.; Levy, D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J. Am. Coll. Cardiol. 1995, 25, 879–884. [Google Scholar] [CrossRef]
- Shigematsu, Y. Clinical evidence for association between left ventricular geometric adaptation and extracardiac target organ damage in essential hypertension. J. Hypertens. 1995, 13, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Battistelli, M.; Barroccini, C.; Santucci, A.; Santucci, C.; Reboldi, G.; Porcellati, C. Adverse prognostic significance of concentric remodelling of the left ventricle in hypertensive patients with normal left ventricular mass. J. Am. Coll. Cardiol. 1995, 25, 871–878. [Google Scholar] [CrossRef]
- Parfrey, P.S. Cardiac disease in dialysis patients: Diagnosis, burden of disease, prognosis, risk factors and management. Nephrol. Dial. Transplant. 2000, 15, 58–68. [Google Scholar] [CrossRef]
- Naito, Y.; Tsujino, T.; Matsumoto, M.; Sakoda, T.; Ohyanagi, M.; Masuyama, T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H585–H593. [Google Scholar] [CrossRef]
- Martin, L.C.; Franco, R.J.; Gavras, I.; Matsubara, B.B.; Garcia, S.; Caramori, J.T.; Barretti, B.B.; Balbi, A.L.; Barsanti, R.; Padovani, C.; et al. Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am. J. Hypertens. 2004, 17, 1163–1169. [Google Scholar] [CrossRef]
- MacRae, J.M.; Levin, A.; Belenkie, I. The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: A cause for concern. Semin. Dial. 2006, 19, 349–352. [Google Scholar] [CrossRef]
- Fujii, H.; Kim, J.I.; Abe, T.; Umezu, M.; Fukagawa, M. Relationship between parathyroid hormone and cardiac abnormalities in chronic dialysis patients. Intern. Med. 2007, 46, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.L.; Ritz, E. Hypertrophy and fibrosis in the cardiomyopathy of uremia-beyond coronary heart disease. Semin. Dial. 2008, 21, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Kurokawa, K.; Van Ypersele De Strihou, C. Relevance of oxidative and carbonyl stress to long-term uremic complications. Kidney Int. Suppl. 2000, 70, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Witko-Sarsat, V. Importance of oxidatively modified proteins in chronic renal failure. Kidney Int. 2001, 59, 108–113. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. AGEs and carbonyl stress: Potential pathogenetic factors of long–term uremic complications. Nephrol. Dial. Transpl. 2000, 15, 7–11. [Google Scholar] [CrossRef]
- Kaya, Y.; Ari, E.; Demir, H.; Soylemez, N.; Cebi, A.; Alp, H.; Bakan, E.; Gecit, I.; Asicioglu, E.; Beytur, A. Accelerated atherosclerosis in haemodialysis patients; correlation of endothelial function with oxidative DNA damage. Nephrol. Dial. Transplant. 2012, 27, 1164–1169. [Google Scholar] [CrossRef]
- Alvarez, M.C.; Caldiz, C.; Fantinelli, J.C.; Garciarena, C.D.; Console, G.M.; Chiappe de Cingolani, G.E.; Mosca, S.M. Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress? Hypertens. Res. 2008, 31, 1465–1476. [Google Scholar] [CrossRef]
- Sag, C.M.; Santos, C.X.; Shah, A.M. Redox regulation of cardiac hypertrophy. J. Mol. Cell Cardiol. 2014, 73, 103–111. [Google Scholar] [CrossRef]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L. Wishful Thinking: The Surprisingly Sparse Evidence for a Relationship between Oxidative Stress and Cardiovascular Disease in Hemodialysis Patients. Semin. Dial. 2015, 28, 224–230. [Google Scholar] [CrossRef]
- Himmelfarb, J. Oxidative stress in hemodialysis. Contrib. Nephrol. 2008, 161, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, C.; Li, X.H.; Deng, B.Q. The prognostic value of oxidative stress and inflammation in Chinese hemodialysis patients. Ren. Fail. 2017, 39, 54–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimitrijevic, Z.M.; Cvetkovic, T.P.; Djordjevic, V.M.; Pavlovic, D.D.; Stefanovic, N.Z.; Stojanovic, I.R.; Paunovic, G.J.; Velickovic-Radovanovic, R.M. How the duration period of erythropoietin treatment influences the oxidative status of hemodialysis patients. Int. J. Med. Sci. 2012, 9, 808–815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sangeetha, L.B.; Harini, D.N.; Suchitra, M.M.; Srinivasa Rao, P.V.L.N.; Siva, K.V. Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren. Fail. 2018, 40, 534–540. [Google Scholar] [CrossRef]
- Ding, Y.F.; Brower, G.L.; Zhong, Q.; Murray, D.; Holland, M.; Janicki, J.S.; Zhong, J. Defective intracellular Ca2+ homeostasis contributes to myocyte dysfunction during ventricular remodelling induced by chronic volume overload in rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 827–835. [Google Scholar] [CrossRef]
- Qin, F.; Lennon-Edwards, S.; Lancel, S.; Biolo, A.; Siwik, D.A.; Pimentel, D.R.; Dorn, G.W.; Kang, Y.J.; Colucci, W.S. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circ. Heart Fail. 2010, 3, 306–313. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Andreeva, I.L.; Kozemjakin, A.L.; Kiskun, A.A. Modifikacija metoda opredelenia perekisej lipidov v test s tiobarbiturovoj kislotoj. Lab Delo. 1988, 11, 41–43. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–561. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Curtis, B.M.; Randell, E.W.; Parfrey, P.S. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 805–813. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Guerin, A.P.; Blacher, J.; Marchais, S.J.; Darne, B.; Metivier, F.; Adda, H.; Safar, M.E. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J. Am. Soc. Nephrol. 2001, 12, 2759–2767. [Google Scholar] [PubMed]
- Tian, J.P.; Wang, T.; Wang, H.; Cheng, L.T.; Tian, X.K.; Lindholm, B.; Axelsson, J.; Du, F.H. The prevalence of left ventricular hypertrophy in Chinese hemodialysis patients is higher than that in peritoneal dialysis patients. Ren. Fail. 2008, 30, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; De Nicola, L.; Gabbai, F.B.; Chiodini, P.; Ravera, M.; Pieracci, L.; Marre, S.; Cassottana, P.; Lucà, S.; Vettoretti, S.; et al. Associations of Left Ventricular Hypertrophy and Geometry with Adverse Outcomes in Patients with CKD and Hypertension. Clin. J. Am. Soc. Nephrol. 2016, 11, 271–279. [Google Scholar] [CrossRef]
- Bansal, N.; Keane, M.; Delafontaine, P.; Dries, D.; Foster, E.; Gadegbeku, C.A.; Go, A.S.; Hamm, L.L.; Kusek, J.W.; Ojo, A.O.; et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2013, 8, 355–362. [Google Scholar] [CrossRef]
- Park, M.; Hsu, C.Y.; Li, Y.; Mishra, R.K.; Keane, M.; Rosas, S.E.; Dries, D.; Xie, D.; Chen, J.; He, J.; et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J. Am. Soc. Nephrol. 2012, 23, 1725–1734. [Google Scholar] [CrossRef]
- Cai, Q.Z.; Lu, X.Z.; Lu, Y.; Wang, A.Y. Longitudinal changes of cardiac structure and function in CKD (CASCADE study). J. Am. Soc. Nephrol. 2014, 25, 1599–1608. [Google Scholar] [CrossRef]
- De Roij van Zuijdewijn, C.L.; Hansildaar, R.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Grooteman, M.P.; Kamp, O.; ter Wee, P.M.; Nubé, M.J. Eccentric Left Ventricular Hypertrophy and Sudden Death in Patients with End-Stage Kidney Disease. Am. J. Nephrol. 2015, 42, 1226–1233. [Google Scholar] [CrossRef]
- Dimitrijevic, Z.; Cvetkovic, T.; Stojanovic, M.; Paunovic, K.; Djordjevic, V. Prevalence and risk factors of myocardial remodeling in hemodialysis patients. Ren. Fail. 2009, 31, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin. Dial. 2009, 22, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxid. Med Cell Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Cucchiari, D.; Astori, E.; Garavaglia, M.L.; Portinaro, N.M.; Saino, N.; Finazzi, S.; Milzani, A.; Badalamenti, S.; et al. Plasma Protein Carbonylation in Haemodialysed Patients: Focus on Diabetes and Gender. Oxid. Med. Cell Longev. 2018, 2018, 4149681. [Google Scholar] [CrossRef]
- Mimić-Oka, J.; Simić, T.; Djukanović, L.; Reljić, Z.; Davicević, Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin. Nephrol. 1999, 51, 233–241. [Google Scholar]
- Valentini, J.; Grotto, D.; Paniz, C.; Roehrs, M.; Burg, G.; Garcia, S.C. The influence of the hemodialysis treatment time under oxidative stress biomarkers in chronic renal failure patients. Biomed. Pharmacother. 2008, 62, 378–382. [Google Scholar] [CrossRef]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.; Green, M.; Fainaru, M.; Smetana, S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999, 56, 1078–1083. [Google Scholar] [CrossRef]
- Miller, M.A.; Cappuccio, F.P. Cellular adhesion molecules and their relationship with measures of obesity and metabolic syndrome in a multiethnic population. Int. J. Obes. 2006, 30, 1176–1182. [Google Scholar] [CrossRef]
- Katunga, L.A.; Anderson, E.J. Carbonyl Stress as a Therapeutic Target for Cardiac Remodeling in Obesity/Diabetes. Austin J. Pharmacol. Ther. 2014, 2, 1047. [Google Scholar]
- Ramasamy, R.; Schmidt, A.M. Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Curr. Heart Fail. Rep. 2012, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Creagh-Brown, B.C.; Quinlan, G.J.; Evans, T.W.; Burke-Gaffney, A. The RAGE axis in systemic inflammation, acute lung injury and myocardial dysfunction: An important therapeutic target? Intensive Care Med. 2010, 36, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Yamazaki, K.G.; Gonzalez, E.; Zambon, A.C. Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: Role of the cardiac fibroblast. J. Cardiovasc. Transl. Res. 2012, 5, 805–813. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Manso, P.H.; Carmona, F.; Dal-Pizzol, F.; Petronilho, F.; Cardoso, F.; Castro, M.; Carlotti, A.P. Oxidative stress markers are not associated with outcomes after pediatric heart surgery. Paediatr. Anaesth. 2013, 23, 188–194. [Google Scholar] [CrossRef]
- Cournot, M.; Burillo, E.; Saulnier, P.J.; Planesse, C.; Gand, E.; Rehman, M.; Ragot, S.; Rondeau, P.; Catan, A.; Gonthier, M.P.; et al. Circulating Concentrations of Redox Biomarkers Do Not Improve the Prediction of Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2015, 7, e007397. [Google Scholar] [CrossRef]
- Casoinic, F.; Sampelean, D.; Buzoianu, A.D.; Hancu, N.; Baston, D. Serum Levels of Oxidative Stress Markers in Patients with Type 2 Diabetes Mellitus and Non-alcoholic Steatohepatitis. Rom. J. Intern. Med. 2016, 54, 228–236. [Google Scholar] [CrossRef]
- Radovanovic, S.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Djukic, T.; Suvakov, S.; Krotin, M.; Simic, D.V.; Matic, M.; Radojicic, Z.; Pekmezovic, T.; et al. Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure. J. Card Fail. 2012, 18, 493–501. [Google Scholar] [CrossRef] [PubMed]
| Parameters | NG n = 12 | CR n = 14 | cLVH n = 33 | eLVH n = 45 |
|---|---|---|---|---|
| Age (years) | 51.3 ± 7.4 | 56.7 ± 24.2 | 61.1 ± 15.3 | 60.0 ± 12.7 |
| Gender (f/m) | 4/8 | 4/10 | 15/18 | 17/28 |
| HD vintage (months) | 52.7 ± 47.3 | 56.7 ± 55.2 | 53.5 ± 45.6 | 57.1 ± 43.8 |
| Body mass index (kg/m2) | 22.7 ± 1.6 | 22.1 ± 3.1 | 23.5 ± 3.6 | 23.7 ± 3.5 |
| Kt/V | 1.35 ± 0.6 | 1.39 ± 0.8 | 1.33 ± 0.7 | 1.36 ± 0.7 |
| Vascular access (AV fistula) | 10 (83%) | 12 (75%) | 25 (78%) | 38 (84%) |
| IDWG (kg) | 2.3 ± 1.1 | 2.8 ± 1.0 | 2.6 ± 0.9 | 3.1 ± 0.8 a |
| sBP (mmHg) | 126.7 ± 21.5 | 135.0 ± 14.0 | 150.5 ± 15.6 a,c | 141.8 ± 20.0 d |
| dBP (mmHg) | 63.6 ± 13.2 | 65.6 ± 10.0 | 73.2 ± 7.8 d | 72.0 ± 9.5 d |
| Hemoglobin (g/dL) | 11.7 ± 2.0 | 11.0 ± 1.4 | 10.3 ± 1.5 a,e | 11.2 ± 1.0 |
| Serum albumin (g/dL) | 37.3 ± 0.6 | 34.7 ± 5.1 | 31.8 ± 5.5 | 31.7 ± 6.0 |
| CRP (mg/L) | 3.3 ± 2.4 | 3.9 ± 0.7 | 4.9 ± 0.5 | 4.1 ± 1.3 |
| Cholesterol (mmol/L) | 4.4 ± 0.9 | 4.16 ± 0.9 | 4.6 ± 1.3 | 4.8 ± 1.2 |
| LDL–cholesterol (mmol/L) | 1.7 ± 0.2 | 2.4 ± 0.8 | 2.7 ± 1.1 a | 3.3 ± 0.9 a |
| HDL–cholesterol (mmol/L) | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.1 ± 0.1 | 1.1 ± 0.4 |
| Triglycerides (mmol/L) | 2.6 ± 0.9 | 2.1 ± 1.7 | 2.0 ± 1.2 | 2.1 ± 1.3 |
| LVEDD (cm) | 4.54 ± 0.36 | 4.24 ± 0.40 | 4.86 ± 0.41 b,c | 5.17 ± 0.49 c,e |
| IVST (cm) | 0.85 ± 0.17 | 0.96 ± 0.11 | 1.34 ± 0.14 b,c,e | 1.25 ± 0.13 b,c |
| PWT (cm) | 0.80 ± 0.11 | 0.92 ± 0.09 | 1.41 ± 0.16 b,c,f | 1.15 ± 0.24.c |
| LVWT (cm) | 1.75 ± 0.39 | 2.12 ± 0.27 b | 2.83 ± 0.22 b,c,f | 2.46 ± 0.41 b,c |
| RWT (cm) | 0.30 (0.25–0.37) | 0.44 (0.43–0.46) b | 0.44 (0.43–0.52) b,c,f | 0.36 (0.28–0.41) b,c |
| LVM (g) | 121.62 (75.84–188.02) | 129.38 (85.96–200.78) b | 276.74 (204.79–373.13) b,c,f | 249.96 (153.27–340.78) b,c |
| LVMI (g/m2) | 67.73 (44.17–101.74) | 72.70 (49.60–104.46) b | 167.47 (138.50–238.37) b,c | 154.07 (130.24–200.07) b,c |
| LVEDV (mL) | 99.42 ± 8.16 | 92.86 ± 8.85 | 106.34 ± 8.99 f | 113.04 ± 10.84 b,c |
| LVESV (mL) | 37.33 ± 11.06 | 26.34 ± 5.02 a | 43.26 ± 11.01 a,b | 37.32 ± 10.84 a,b,c |
| LVEF (%) | 63 (57–70) | 57 (51–60) b | 60 (55–68) b | 61 (57–69)b |
| β | p | |
|---|---|---|
| Dependent variable: LVMI a R2 = 0.57; p < 0.001 | ||
| Independent variable | ||
| MDA | 0.266 | 0.011 |
| PC | 0.328 | <0.001 |
| TAC | −0.177 | 0.043 |
| HD vintage | 0.231 | 0.010 |
| Hemoglobin | −0.337 | <0.001 |
| Dependent variable: LVWT b R2 = 0.61; p < 0.001 | ||
| Independent variable | ||
| PC | 0.288 | 0.013 |
| TAC | 0.266 | 0.011 |
| MDA | 0.038 | 0.22 |
| Hemoglobin | −0.31 | 0.002 |
| Dependent variable: RWT c R2 = 0.53; p < 0.001 | ||
| Independent variable | ||
| IDWG | −0.22 | 0.002 |
| MDA | 0.04 | 0.072 |
| PC | 0.19 | 0.003 |
| Hgb | −0.33 | 0.002 |
| sBP | 0.27 | 0.001 |
| Dependent variable: LVEDD d R2 = 0.50; p < 0.001 | ||
| Independent variable | ||
| PC | 0.32 | <0.001 |
| MDA | 0.16 | 0.06 |
| TAC | 0.18 | 0.008 |
| IDWG | −0.17 | 0.01 |
| Dependent variable: LVEDV e R2 = 0.48; p < 0.001 | ||
| Independent variable | ||
| PC | 0.28 | <0.001 |
| TAC | 0.12 | 0.04 |
| IDWG | −0.10 | 0.02 |
| Variables | Eccentric LVH | Concentric LVH | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| PC (Per 1 SD increase) | 1.344 (1.203–1.503 a | 1.256 (0.998–1.514) a | 1.321(1.285–1.408) a | 1.094 (0.875–2.181) c |
| Tertile of PC | ||||
| T1 (≤3.49) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| T2 (3.50–5.85) | 1.446 (1.277–1.615) a | 1.366 (1.218–1.533) a | 1.421 (1.277–1603) a | 1.248 (1.180–1.390) c |
| T3 (>5.85) | 1.766 (1.510–2.029) a | 1.517 (1.287–1.747) a | 1688 (1.492–1884) a | 1.344 (1.211–1.507) c |
| p for trend | <0.001 | <0.001 | <0.05 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrijevic, Z.M.; Salinger Martinovic, S.S.; Nikolic, V.N.; Cvetkovic, T.P. Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients. Diagnostics 2019, 9, 202. https://doi.org/10.3390/diagnostics9040202
Dimitrijevic ZM, Salinger Martinovic SS, Nikolic VN, Cvetkovic TP. Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients. Diagnostics. 2019; 9(4):202. https://doi.org/10.3390/diagnostics9040202
Chicago/Turabian StyleDimitrijevic, Zorica M., Sonja S. Salinger Martinovic, Valentina N. Nikolic, and Tatjana P. Cvetkovic. 2019. "Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients" Diagnostics 9, no. 4: 202. https://doi.org/10.3390/diagnostics9040202
APA StyleDimitrijevic, Z. M., Salinger Martinovic, S. S., Nikolic, V. N., & Cvetkovic, T. P. (2019). Protein Carbonyl Content Is a Predictive Biomarker of Eccentric Left Ventricular Hypertrophy in Hemodialysis Patients. Diagnostics, 9(4), 202. https://doi.org/10.3390/diagnostics9040202





