Neonatal Mass Urine Screening Approach for Early Detection of Mucopolysaccharidoses by UPLC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Urine Specimen Collection on Filter Paper
2.3. Reagents
2.3.1. Analyte Stock Solutions
2.3.2. Internal Standard Stock Solutions
2.3.3. Standard Curves and Quality Control Working Solutions
2.3.4. Resuspension Solution
2.4. Sample Processing
2.5. Instrumentation and Parameters
2.5.1. UPLC Parameters
2.5.2. MS/MS Parameters
2.5.3. Quantification Parameters
2.6. Method Validation
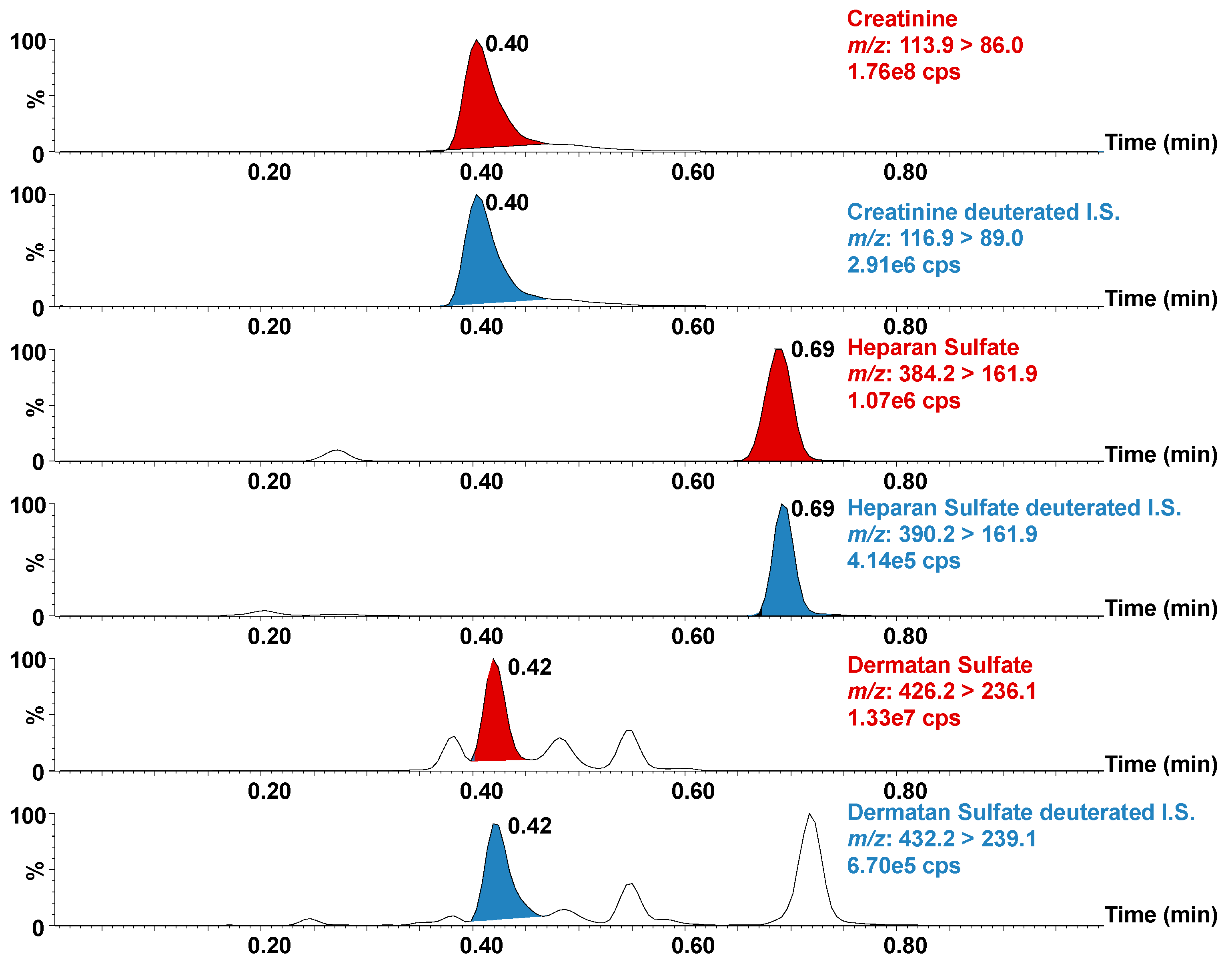
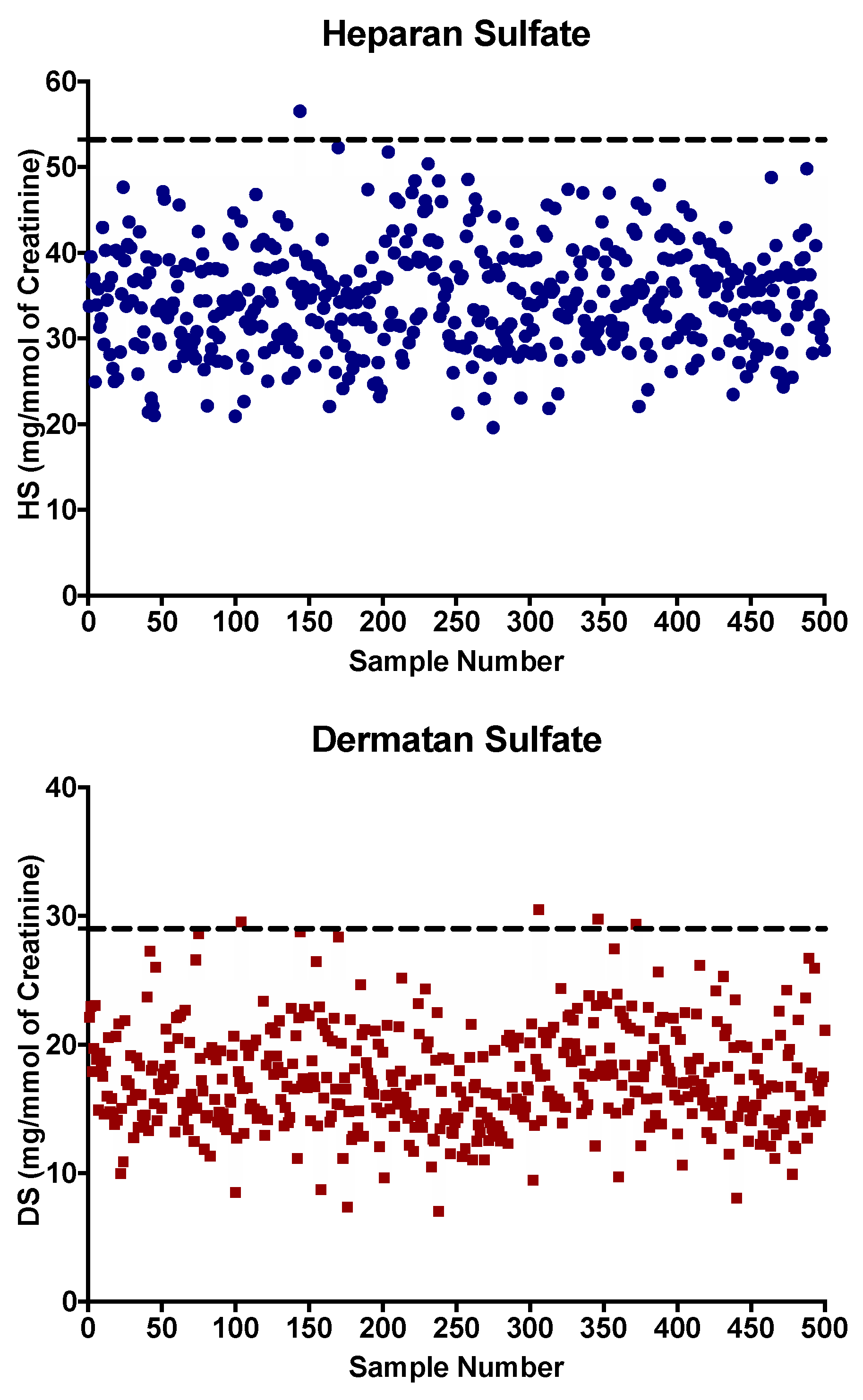
3. Results and Discussion
3.1. Method Development and Validation
3.2. Reference Values
3.3. Urine Samples from Confirmed MPS Patients
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palmieri, C.; Giger, U.; Wang, P.; Pizarro, M.; Shivaprasad, H.L. Pathological and Biochemical Studies of Mucopolysaccharidosis Type IIIB (Sanfilippo Syndrome Type B) in Juvenile Emus (Dromaius novaehollandiae). Vet. Pathol. 2015, 52, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Muenzer, J. Overview of the mucopolysaccharidoses. Rheumatology 2011, 50, v4–v12. [Google Scholar] [CrossRef]
- Lawrence, R.; Brown, J.R.; Lorey, F.; Dickson, P.I.; Crawford, B.E.; Esko, J.D. Glycan-based biomarkers for mucopolysaccharidoses. Mol. Genet. Metab. 2014, 111, 73–83. [Google Scholar] [CrossRef]
- Khan, S.A.; Peracha, H.; Ballhausen, D.; Wiesbauer, A.; Rohrbach, M.; Gautschi, M.; Mason, R.W.; Giugliani, R.; Suzuki, Y.; Orii, K.E.; et al. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab. 2017, 121, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Lavoie, P.; Tomatsu, S.; Valayannopoulos, V.; Mitchell, J.J.; Raiman, J.; Beaudoin, M.; Maranda, B.; Clarke, J.T. UPLC-MS/MS detection of disaccharides derived from glycosaminoglycans as biomarkers of mucopolysaccharidoses. Anal. Chim. Acta 2016, 936, 139–148. [Google Scholar] [CrossRef]
- Mitchell, J.; Berger, K.I.; Borgo, A.; Braunlin, E.A.; Burton, B.K.; Ghotme, K.A.G.; Kircher, S.G.; Molter, D.; Orchard, P.J.; Palmer, J.; et al. Unique medical issues in adult patients with mucopolysaccharidoses. Eur. J. Intern. Med. 2016, 34, 2–10. [Google Scholar] [CrossRef]
- Gaffke, L.; Pierzynowska, K.; Piotrowska, E.; Węgrzyn, G. How close are we to therapies for Sanfilippo disease? Metab. Brain Dis. 2017, 33, 1–10. [Google Scholar] [CrossRef]
- Giugliani, R.; Federhen, A.; Rojas, M.V.; Vieira, T.; Artigalás, O.; Pinto, L.L.; Azevedo, A.C.; Acosta, A.; Bonfim, C.; Lourenço, C.M.; et al. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010, 33, 589–604. [Google Scholar] [CrossRef]
- Kubaski, F.; Mason, R.W.; Nakatomi, A.; Shintaku, H.; Xie, L.; Van Vlies, N.N.; Church, H.; Giugliani, R.; Kobayashi, H.; Yamaguchi, S.; et al. Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J. Inherit. Metab. Dis. 2016, 40, 151–158. [Google Scholar] [CrossRef]
- Muenzer, J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014, 111, 63–72. [Google Scholar] [CrossRef]
- Gabrielli, O.; Clarke, L.A.; Bruni, S.; Coppa, G.V. Enzyme-Replacement Therapy in a 5-Month-Old Boy With Attenuated Presymptomatic MPS I: 5-Year Follow-up. Pediatrics 2010, 125, e183–e187. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.A.; Atherton, A.M.; Burton, B.K.; Day-Salvatore, D.L.; Kaplan, P.; Leslie, N.D.; Scott, C.R.; Stockton, D.W.; Thomas, J.A.; Muenzer, J. Mucopolysaccharidosis Type I Newborn Screening: Best Practices for Diagnosis and Management. J. Pediatr. 2017, 182, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Lam, W.K.; Wiggins, L.D.; Kemper, A.R. Cognitive outcomes and age of detection of severe mucopolysaccharidosis type 1. Genet. Med. 2017, 19, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Shone, S.M. Newborn Screening Policy Decisions. North Carol. Med. J. 2019, 80, 42–44. [Google Scholar] [CrossRef]
- Laraway, S.; Breen, C.; Mercer, J.; Jones, S.; Wraith, J.E. Does early use of enzyme replacement therapy alter the natural history of mucopolysaccharidosis I? Experience in three siblings. Mol. Genet. Metab. 2013, 109, 315–316. [Google Scholar] [CrossRef]
- Chan, M.-J.; Liao, H.-C.; Gelb, M.H.; Chuang, C.-K.; Liu, M.-Y.; Chen, H.-J.; Kao, S.-M.; Lin, H.-Y.; Huang, Y.-H.; Kumar, A.B.; et al. Taiwan National Newborn Screening Program by Tandem Mass Spectrometry for Mucopolysaccharidoses Types I, II, and VI. J. Pediatr. 2019, 205, 176–182. [Google Scholar] [CrossRef]
- Hopkins, P.V.; Campbell, C.; Klug, T.; Rogers, S.; Raburn-Miller, J.; Kiesling, J. Lysosomal Storage Disorder Screening Implementation: Findings from the First Six Months of Full Population Pilot Testing in Missouri. J. Pediatr. 2015, 166, 172–177. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Cyr, D.; Drouin, R. Quebec neonatal mass urinary screening programme: From micromolecules to macromolecules. J. Inherit. Metab. Dis. 2007, 30, 515–521. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Giguère, R.; Lemieux, B. Newborn urine screening programme in the province of Quebec: an update of 30 years’ experience. J. Inherit. Metab. Dis. 2003, 26, 393–402. [Google Scholar] [CrossRef]
- Auray-Blais, C.; Lavoie, P.; Zhang, H.; Gagnon, R.; Clarke, J.T.; Maranda, B.; Young, S.P.; An, Y.; Millington, D.S. An improved method for glycosaminoglycan analysis by LC–MS/MS of urine samples collected on filter paper. Clin. Chim. Acta 2012, 413, 771–778. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, G.; Zhang, R.; He, J.; Zhang, Y.; Xu, J.; Yang, W.; Chen, X.; Song, Y.; Abliz, Z. Combination of Injection Volume Calibration by Creatinine and MS Signals’ Normalization to Overcome Urine Variability in LC-MS-Based Metabolomics Studies. Anal. Chem. 2013, 85, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Young, S.P.; Millington, D.S. Quantification of Glycosaminoglycans in Urine by Isotope-Dilution Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Curr. Protoc. Hum. Genet. 2013, 76, 17.12. 1–17.12. 14. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Ellaway, C.; Foster, H.E.; Giugliani, R.; Goizet, C.; Goring, S.; Hawley, S.; Jurecki, E.; Khan, Z.; Lampe, C.; et al. Understanding the Early Presentation of Mucopolysaccharidoses Disorders. J. Inborn. Errors Metab. Screen 2018, 6, 232640981880034. [Google Scholar] [CrossRef]
- Kubaski, F.; Brusius-Facchin, A.C.; Mason, R.W.; Patel, P.; Burin, M.G.; Michelin-Tirelli, K.; Kessler, R.G.; Bender, F.; Leistner-Segal, S.; Moreno, C.A.; et al. Elevation of glycosaminoglycans in the amniotic fluid of a fetus with mucopolysaccharidosis VII. Prenat. Diagn. 2017, 37, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mason, R.W.; Giugliani, R.; Orii, K.; Fukao, T.; Suzuki, Y.; Yamaguchi, S.; Kobayashi, H.; Orii, T.; Tomatsu, S. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018, 125, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Menkovic, I.; Lavoie, P.; Boutin, M.; Auray-Blais, C. Distribution of heparan sulfate and dermatan sulfate in mucopolysaccharidosis type II mouse tissues pre- and post-enzyme-replacement therapy determined by UPLC-MS/MS. Bioanalusis 2019, 11, 727–740. [Google Scholar] [CrossRef]
- Colón, C.; Alvarez, J.V.; Castaño, C.; Gutierrez-Solana, L.G.; Marquez, A.M.; O’Callaghan, M.; Sánchez-Valverde, F.; Yeste, C.; Couce, M.L. A selective screening program for the early detection of mucopolysaccharidosis. Medicine 2017, 96. [Google Scholar] [CrossRef]
| UPLC Parameters | |
|---|---|
| Column | BEH Amide |
| ID × Length | 2.1 × 50 mm |
| Particle size | 1.7 μm |
| Column temperature | 40 °C |
| Weak Wash solvent | 95:5 ACN:H2O + 0.2% FA |
| Strong Wash solvent | 50:50 MeOH:H2O |
| Injection volume | 4.0 μL |
| Injector | Flow-through needle |
| Autosampler temperature | 10 °C |
| Mobile phase A | 95:5 ACN:H2O + 0.2% FA + 10 mM CH3COONH4 |
| Mobile phase B | 10:90 ACN:H2O + 0.2% FA + 10 mM CH3COONH4 |
| Flow rate | 0.8 mL/min |
| Gradient (% mobile phase B) | 0.0 → 0.1 min: 10% 0.1 → 0.5 min: 10 →60% (Linear gradient) 0.5 → 1.0 min: 10% |
| MS/MS Parameters | |||
| Ionization mode | ESI | ||
| Polarity | Positive | ||
| Acquisition mode | MRM | ||
| Capillary voltage | 3.44 kV | ||
| Desolvation temperature | 500 °C | ||
| Desolvation gas flow | 700 L/h | ||
| Cone gas flow | 150 L/h | ||
| Source temperature Dwell time | 120 °C 0.05 s | ||
| Span | 0 Da | ||
| Transitions | |||
| Compound | Transitions (m/z) | Cone Voltage (V) | Collision Energy (V) |
| DS | 426.2 > 236.1 | 36 | 12 |
| DS-D6 IS | 432.2 > 239.1 | 36 | 12 |
| HS | 384.2 > 161.9 | 18 | 18 |
| HS-D6 IS | 390.2 > 161.9 | 18 | 18 |
| Creatinine | 113.9 > 86.0 | 34 | 10 |
| Creatinine-D3 | 116.9 > 89.0 | 34 | 10 |
| Patient | MPS Type | Gender | Age (Years) | ERT | HS * | DS * | Control HS * | Control DS * |
|---|---|---|---|---|---|---|---|---|
| 1 | I (H) | M | 1.7 | U | 86.4 | 42.6 | 8.4 | 1.7 |
| 2 | I (H/S) | M | 8.0 | U | 68.7 | 36.4 | 9.7 | 1.4 |
| 3 | I (H/S) | F | 10 | U | 48.1 | 24.2 | 4.0 | 1.4 |
| 4 | II | M | 3.0 | U | 150.3 | 72.6 | 10.6 | 1.3 |
| 5 | II | M | 5.0 | U | 45.4 | 15.1 | 9.6 | 1.7 |
| 6 | II | M | 5.0 | U | 47.5 | 14.9 | 9.4 | 0.8 |
| 7 | II | M | 5.0 | T | 13.4 | 3.8 | 7.3 | 2.3 |
| 8 | II | M | 6.0 | T | 16.3 | 4.9 | 5.8 | 0.8 |
| 9 | II | M | 7.0 | T | 12.9 | 5.2 | 4.8 | 1.2 |
| 10 | IIIA | M | 3.0 | U | 142.1 | 4.0 | 8.4 | 1.8 |
| 11 | IIIB | F | 1.8 | U | 56.5 | 1.6 | 10.2 | 1.6 |
| 12 | VI | F | 10 | U | 65.4 | 46.4 | 7.7 | 2.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menkovic, I.; Marchand, A.-S.; Boutin, M.; Auray-Blais, C. Neonatal Mass Urine Screening Approach for Early Detection of Mucopolysaccharidoses by UPLC-MS/MS. Diagnostics 2019, 9, 195. https://doi.org/10.3390/diagnostics9040195
Menkovic I, Marchand A-S, Boutin M, Auray-Blais C. Neonatal Mass Urine Screening Approach for Early Detection of Mucopolysaccharidoses by UPLC-MS/MS. Diagnostics. 2019; 9(4):195. https://doi.org/10.3390/diagnostics9040195
Chicago/Turabian StyleMenkovic, Iskren, Anne-Sophie Marchand, Michel Boutin, and Christiane Auray-Blais. 2019. "Neonatal Mass Urine Screening Approach for Early Detection of Mucopolysaccharidoses by UPLC-MS/MS" Diagnostics 9, no. 4: 195. https://doi.org/10.3390/diagnostics9040195
APA StyleMenkovic, I., Marchand, A.-S., Boutin, M., & Auray-Blais, C. (2019). Neonatal Mass Urine Screening Approach for Early Detection of Mucopolysaccharidoses by UPLC-MS/MS. Diagnostics, 9(4), 195. https://doi.org/10.3390/diagnostics9040195





