Otolaryngologists and the Early Diagnosis of Mucopolysaccharidoses: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
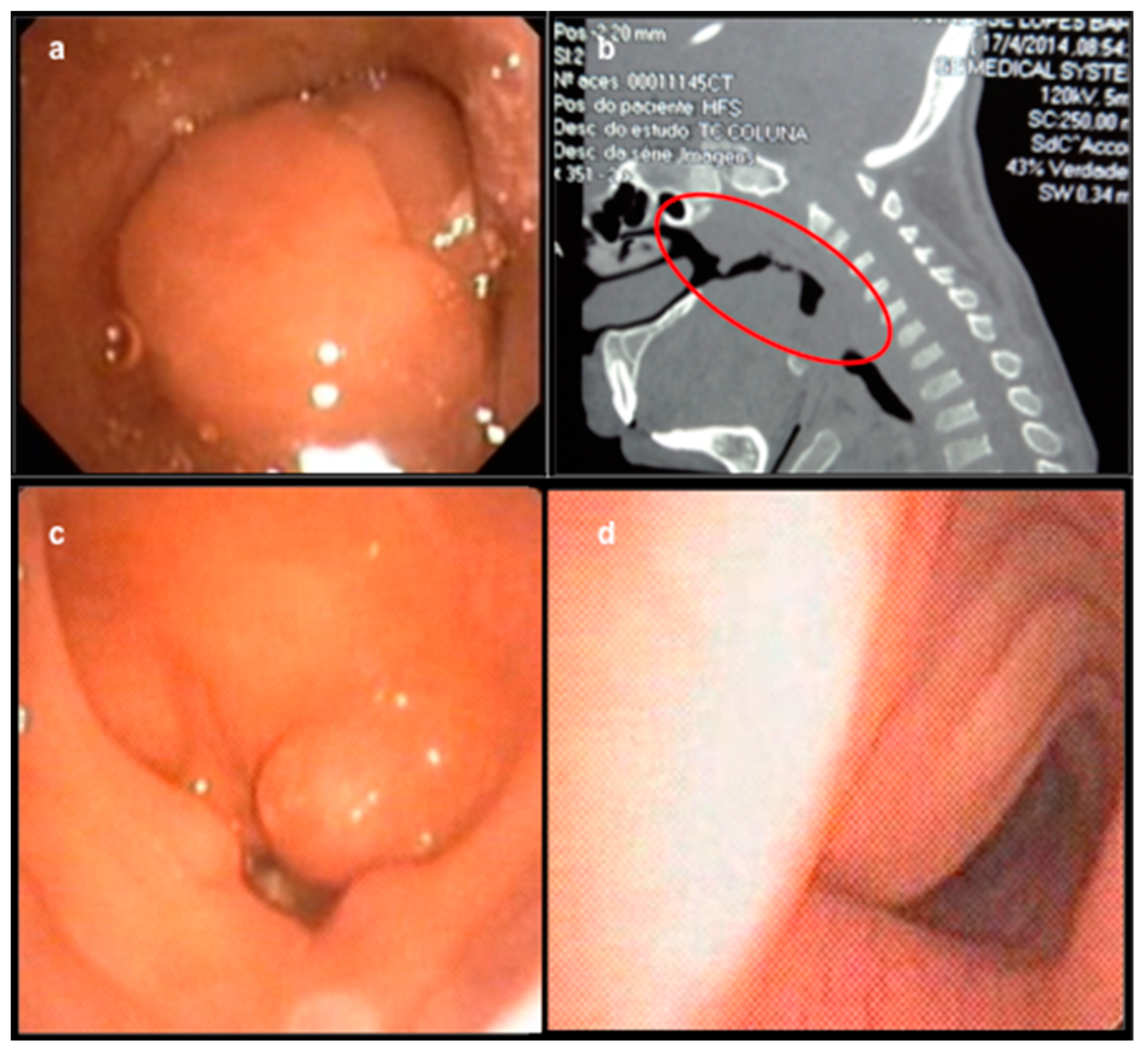
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MPS | Mucopolysaccharidoses |
| ENT | Ear, nose and throat |
| ERT | Enzyme replacement therapy |
References
- Nelson, J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum. Genet. 1997, 101, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Poorthuis, B.J.; Wevers, R.A.; Kleijer, W.J.; Groener, J.E.; de Jong, J.G.; van Weely, S.; Niezen-Koning, K.E.; van Diggelen, O.P. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999, 105, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Applegarth, D.A.; Toone, J.R.; Lowry, R.B. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics 2000, 105, e10. [Google Scholar] [CrossRef]
- Nelson, J.; Crowhurst, J.; Carey, B.; Greed, L. Incidence of the mucopolysaccharidoses in Western Australia. Am. J. Med. Genet. A 2003, 123A, 310–313. [Google Scholar] [CrossRef]
- Baehner, F.; Schmiedeskamp, C.; Krummenauer, F.; Miebach, E.; Bajbouj, M.; Whybra, C.; Kohlschutter, A.; Kampmann, C.; Beck, M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J. Inherit. Metab. Dis. 2005, 28, 1011–1017. [Google Scholar] [CrossRef]
- Malm, G.; Lund, A.M.; Mansson, J.E.; Heiberg, A. Mucopolysaccharidoses in the Scandinavian countries: Incidence and prevalence. Acta Paediatr. 2008, 97, 1577–1581. [Google Scholar] [CrossRef]
- Ben Turkia, H.; Tebib, N.; Azzouz, H.; Abdelmoula, M.S.; Ben Chehida, A.; Chemli, J.; Monastiri, K.; Chaabouni, M.; Sanhagi, H.; Zouari, B.; et al. Incidence of mucopolysaccharidoses in Tunisia. Tunis Med. 2009, 87, 782–785. [Google Scholar]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Niu, D.M.; Chen, M.R.; Tsai, F.J.; Chao, M.C.; Chiu, P.C.; Lin, S.J.; Tsai, L.P.; et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A. 2009, 149A, 960–964. [Google Scholar] [CrossRef]
- Tomatsu, S.; Fujii, T.; Fukushi, M.; Oguma, T.; Shimada, T.; Maeda, M.; Kida, K.; Shibata, Y.; Futatsumori, H.; Montano, A.M.; et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013, 110, 42–53. [Google Scholar] [CrossRef]
- Wraith, J.E. Mucopolysaccharidoses and mucolipidoses. Handb Clin. Neurol 2013, 113, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Keilmann, A.; Nakarat, T.; Bruce, I.A.; Molter, D.; Malm, G.; Investigators, H.O.S. Hearing loss in patients with mucopolysaccharidosis II: Data from HOS - the Hunter Outcome Survey. J. Inherit. Metab Dis. 2012, 35, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Keilmann, A.; Lassig, A.K.; Pollak-Hainz, A.; Mann, W.J.; Beck, M.; Hainz, M. Adenoids of patients with mucopolysaccharidoses demonstrate typical alterations. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.M.; Derkay, C.S.; Darrow, D.H.; Proud, V. Role of the pediatric otolaryngologist in diagnosis and management of children with mucopolysaccharidoses. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chuang, C.K.; Lee, K.S.; Lin, S.P. Awareness of Mucopolysaccharidosis in an Otorhinolaryngologic Clinic. Pediatr Neonatol 2017, 58, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Federhen, A.; Munoz Rojas, M.V.; Vieira, T.A.; Artigalas, O.; Pinto, L.L.; Azevedo, A.C.; Acosta, A.X.; Bomfim, C.; Lourenco, C.M.; et al. [Enzyme replacement therapy for mucopolysaccharidoses I, II and VI: Recommendations from a group of Brazilian F experts]. Rev. Assoc. Med. Bras. (1992) 2010, 56, 271–277. [Google Scholar] [CrossRef]
- Muenzer, J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014, 111, 63–72. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Valayannopoulos, V.; Wijburg, F.A. Therapy for the mucopolysaccharidoses. Rheumatology 2011, 50 (Suppl. 5), v49–v59. [Google Scholar] [CrossRef]
- Simmons, M.A.; Bruce, I.A.; Penney, S.; Wraith, E.; Rothera, M.P. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 589–595. [Google Scholar] [CrossRef]
- Berger, K.I.; Fagondes, S.C.; Giugliani, R.; Hardy, K.A.; Lee, K.S.; McArdle, C.; Scarpa, M.; Tobin, M.J.; Ward, S.A.; Rapoport, D.M. Respiratory and sleep disorders in mucopolysaccharidosis. J. Inherit. Metab. Dis. 2013, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Petty, R.E. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology 2011, 50 (Suppl. 5), v19–v25. [Google Scholar] [CrossRef]
- Oussoren, E.; Brands, M.M.; Ruijter, G.J.; der Ploeg, A.T.; Reuser, A.J. Bone, joint and tooth development in mucopolysaccharidoses: Relevance to therapeutic options. Biochim. Biophys Acta 2011, 1812, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Nichani, J.; Rothera, M.P.; Wraith, J.E.; Jones, S.A.; Walker, R.; Bruce, I.A. Tracheostomy in mucopolysaccharidosis type II (Hunter’s Syndrome). Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Gonuldas, B.; Yilmaz, T.; Sivri, H.S.; Gucer, K.S.; Kilinc, K.; Genc, G.A.; Kilic, M.; Coskun, T. Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, J.K.; Smith, M.E.; Dudley, J.P.; Williams, J.C.; Crumley, R.L.; Crockett, D.M. Otolaryngologic manifestations of the mucopolysaccharidoses. Ann. Otol. Rhinol. Laryngol. 1992, 101, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, N.J.; Harmatz, P.; Bodamer, O.; Burton, B.K.; Giugliani, R.; Jones, S.A.; Lampe, C.; Malm, G.; Steiner, R.D.; Parini, R.; et al. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): Data from the Hunter Outcome Survey. Genet. Med. 2010, 12, 816–822. [Google Scholar] [CrossRef]
- Walker, R.W.; Allen, D.L.; Rothera, M.R. A fibreoptic intubation technique for children with mucopolysaccharidoses using the laryngeal mask airway. Paediatr. Anaesth. 1997, 7, 421–426. [Google Scholar] [CrossRef]
- Arn, P.; Bruce, I.A.; Wraith, J.E.; Travers, H.; Fallet, S. Airway-related symptoms and surgeries in patients with mucopolysaccharidosis I. Ann. Otol. Rhinol. Laryngol. 2015, 124, 198–205. [Google Scholar] [CrossRef]
| ENT Problems | MPS Patients (n = 23) |
|---|---|
| Otalgia, n (%) | 10 (43) |
| Airway disorders, n (%) | 13 (57) |
| Snoring, respiratory distress at night and/or sleep apnea, n (%) | 15 (65) |
| Speech delay, n (%) | 12 (52) |
| Suspected hearing loss, n (%) | 9 (39) |
| At least one of the above, n (%) | 20 (87) |
| MPS Type | Surgical Procedure | Age at the Surgical Procedure (yrs.) | Age at the Diagnosis of MPS (yrs.) | Interval from Surgery to Diagnosis (mo.) |
|---|---|---|---|---|
| I | umbilical herniorrhaphy | 3.0 | 7.5 | 54.0 |
| II | umbilical herniorrhaphy | 6.0 | 17.4 | 136.8 |
| II | umbilical herniorrhaphy | 30.0 | 33.0 | 36.0 |
| II | adenotonsillectomy with paracentesis of tympanic membranes | 2.9 | 3.9 | 12.0 |
| VI | umbilical herniorrhaphy | 0.8 | 2.5 | 20.4 |
| VI | cardiac valvuloplasty | 1.1 | 1.6 | 6.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, D.d.A.; Barth, A.L.; Valente, M.P.d.M.; Mello, P.P.d.; Horovitz, D.D.G. Otolaryngologists and the Early Diagnosis of Mucopolysaccharidoses: A Cross-Sectional Study. Diagnostics 2019, 9, 187. https://doi.org/10.3390/diagnostics9040187
Torres DdA, Barth AL, Valente MPdM, Mello PPd, Horovitz DDG. Otolaryngologists and the Early Diagnosis of Mucopolysaccharidoses: A Cross-Sectional Study. Diagnostics. 2019; 9(4):187. https://doi.org/10.3390/diagnostics9040187
Chicago/Turabian StyleTorres, Danielle de Araujo, Anneliese Lopes Barth, Mariana Pires de Mello Valente, Paulo Pires de Mello, and Dafne Dain Gandelman Horovitz. 2019. "Otolaryngologists and the Early Diagnosis of Mucopolysaccharidoses: A Cross-Sectional Study" Diagnostics 9, no. 4: 187. https://doi.org/10.3390/diagnostics9040187
APA StyleTorres, D. d. A., Barth, A. L., Valente, M. P. d. M., Mello, P. P. d., & Horovitz, D. D. G. (2019). Otolaryngologists and the Early Diagnosis of Mucopolysaccharidoses: A Cross-Sectional Study. Diagnostics, 9(4), 187. https://doi.org/10.3390/diagnostics9040187





