Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist
Abstract
1. Introduction
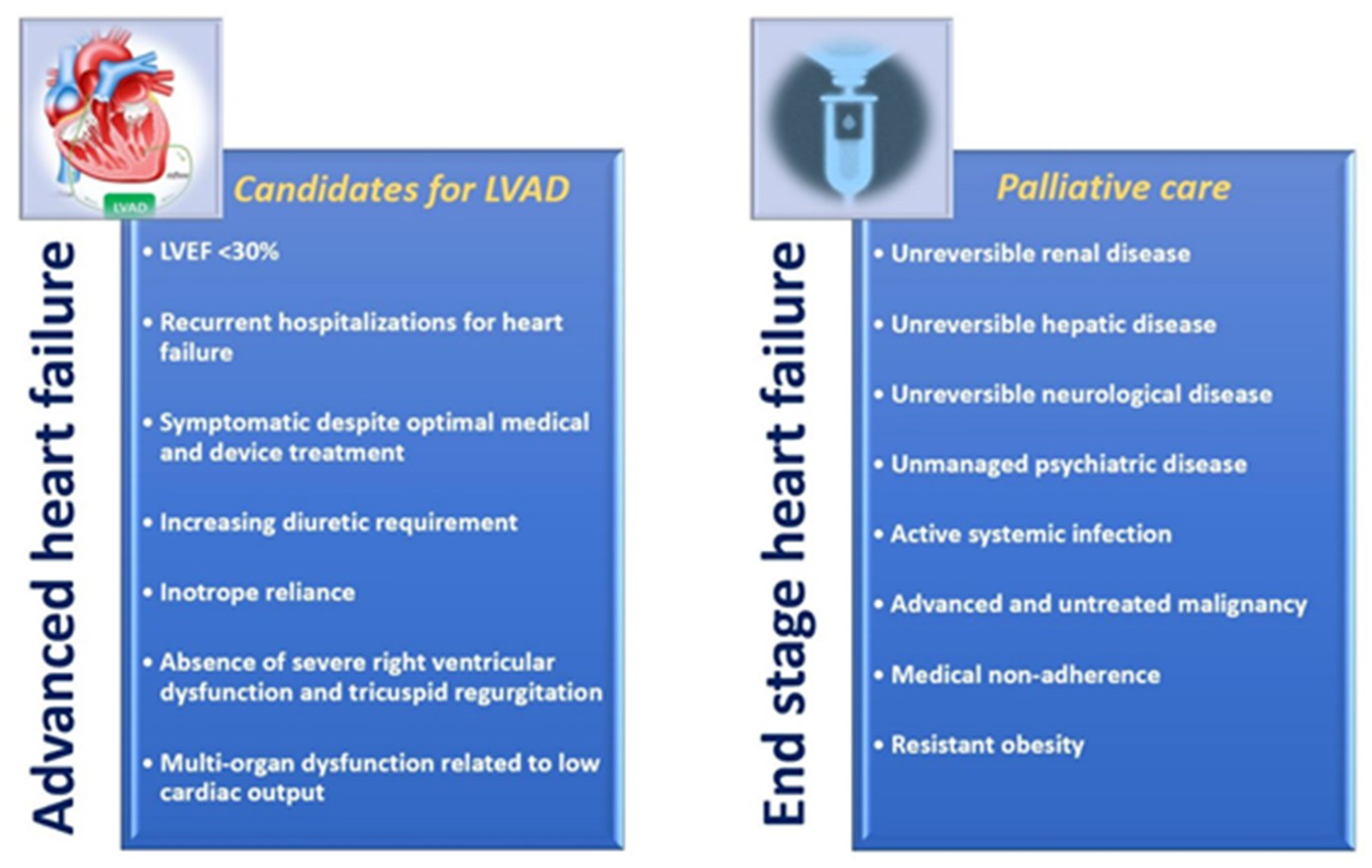
2. Advanced Heart Failure (AdHF) and End-Stage Heart Failure: Differences in Clinic, Prognosis and Therapeutic Assessment
2.1. AdHF and End-Stage HF: Difference in Clinical and Functional Assessment, and Prognosis
2.2. How AdHF and End-Stage HF Are Aligned with American College of Cardiology/American Heart Association (ACC/AHA) and New York Heart Association (NYHA) HF Classifications
2.3. Revised Criteria for AdHF Diagnostic
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Anker, S.D.; Al Habib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarson, A.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; Westergren, G.; Thimell, M.; El Allaf, D.; et al. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar]
- The Criteria Committee of the New York Heart Association. Functional Capacity and Objective Assessment. In Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed.; Dolgin, M., Ed.; Little, Brown and Company: Boston, MA, USA, 1994; pp. 253–255. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.; Adams, R.; Carnethon, M.; De Simone, G.; Ferguson, T.B.; Flegal, K.; Ford, E.; Furie, K.; Go, A.; Greenlund, K.; et al. Heart disease and stroke statistics-2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119, e21–e181. [Google Scholar]
- Metra, M.; Ponikowski, P.; Dickstein, K.; McMurray, J.J.; Gavazzi, A.; Bergh, C.H.; Fraser, A.G.; Jaarsma, T.; Pitsis, A.; Mohacsi, P.; et al. Advanced chronic heart failure: A position statement from the study group on advanced heart failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2007, 9, 684–694. [Google Scholar] [CrossRef]
- Van der Meer, P.; Gaggin, H.K.; Dec, G.W. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 2756–2768. [Google Scholar] [CrossRef]
- Stewart, G.C.; Stevenson, L.W. Keeping left ventricular assist device acceleration on track. Circulation 2011, 123, 1559–1568. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Wilson, S.R.; Mudge, G.H.; Stewart, G.C.; Givertz, M.M. Evaluation for a ventricular assist device: Selecting the appropriate candidate. Circulation 2009, 119, 2225–2232. [Google Scholar] [CrossRef]
- Fedele, F.; Severino, P.; Bruno, N.; Stio, R.; Caira, C.; D’Ambrosi, A.; Brasolin, B.; Ohanyan, V.; Mancone, M. Role of ion channels in coronary microcirculation: A review of the literature. Future Cardiol. 2013, 9, 897–905. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Chilian, W.M.; Severino, P.; Canali, E.; Logan, S.; De Marchis, M.L.; Volterrani, M.; Palmirotta, R.; Guadagni, F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res. Cardiol. 2013, 108, 387. [Google Scholar] [CrossRef] [PubMed]
- Baumwol, J. I Need Help—A mnemonic to aid timely referral in advanced heart failure. J. Heart Lung Transplant. 2017, 36, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Stevenson, L.W.; Grady, K.L.; Goldstein, N.E.; Matlock, D.D.; Arnold, R.M.; Cook, N.R.; Felker, G.M.; Francis, G.S.; Hauptman, P.J. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation 2012, 125, 1928–1952. [Google Scholar] [CrossRef] [PubMed]
- Flint, K.M.; Matlock, D.D.; Lindenfeld, J.; Allen, L.A. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ. Heart Fail. 2012, 5, 286–293. [Google Scholar] [CrossRef]
- Severino, P.; Netti, L.; Mariani, M.V.; Maraone, A.; D’Amato, A.; Scarpati, R.; Infusino, F.; Pucci, M.; Lavalle, C.; Maestrini, V.; et al. Prevention of Cardiovascular Disease: Screening for Magnesium Deficiency. Cardiol. Res. Pract. 2019, 4874921. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Mariani, M.V.; Netti, L.; Infusino, F.; Mancone, M.; Fedele, F. Myocardial Ischemia in Women When Genetic Susceptibility Matters. J. Mol. Genet. Med. 2019, 13, 409. [Google Scholar]
- Miller, R.J.H.; Teuteberg, J.J.; Hunt, S.A. Innovations in Ventricular Assist Devices for End-Stage Heart Failure. Annu. Rev. Med. 2019, 27, 33–44. [Google Scholar] [CrossRef]
- Miller, L.W.; Rogers, J.G. Evolution of Left Ventricular Assist Device Therapy for Advanced Heart Failure: A Review. JAMA Cardiol. 2018, 3, 650–658. [Google Scholar] [CrossRef]
- De Freitas Campos Guimarães, L.; Urena, M.; Wijeysundera, H.C.; Munoz-Garcia, A.; Serra, V.; Benitez, L.M.; Auffret, V.; Cheema, A.N.; Amat-Santos, I.J.; Fisher, Q.; et al. Long-term outcomes after transcatheter aortic valve-in-valve replacement. Circ. Cardiovasc. Interv. 2018, 11, e007038. [Google Scholar] [CrossRef]
- Meier, B. His master’s art, Andreas Grüntzig’s approach to performing and teaching coronary angioplasty. EuroIntervention 2017, 13, 15–27. [Google Scholar] [CrossRef]
- Basoli, A.; Cametti, C.; Satriani, F.G.; Mariani, P.; Severino, P. Hemocompatibility of stent materials: Alterations in electrical parameters of erythrocyte membranes. Vasc. Health Risk Manag. 2012, 8, 197–204. [Google Scholar] [PubMed]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; De Marchis, M.; Palmirotta, R.; Volterrani, M.; Mancone, M.; Fedele, F. Diabetes mellitus and ischemic heart disease: The role of ion channels. Int. J. Mol. Sci. 2018, 19, 802. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Baughman, K.L.; Dec, W.G.; Elkayam, U.; Forker, A.D.; Gheorghiade, M.; Hermann, D.; Konstam, M.A.; Liu, P.; Massie, B.M.; et al. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction-pharmacological approaches. Pharmacotherapy 2000, 20, 495–522. [Google Scholar] [CrossRef]
- Ho, K.K.; Pinsky, J.L.; Kannel, W.B.; Levy, D. The epidemiology of heart failure: The Framingham Study. J. Am. Coll. Cardiol. 1993, 22, 6A–13A. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2010: A report from the American Heart Association. Circulation 2010, 121, e46–e215. [Google Scholar]
- Levine, A.; Gupta, C.A.; Gass, A. Advanced Heart Failure Management and Transplantation. Cardiol. Clin. 2019, 37, 105–111. [Google Scholar] [CrossRef]
- Miller, L.; Birks, E.; Guglin, M.; Lamba, H.; Frazier, O.H. Use of Ventricular Assist Devices and Heart Transplantation for Advanced Heart Failure. Circ. Res. 2019, 124, 1658–1678. [Google Scholar] [CrossRef]
- Everly, M.J. Cardiac transplantation in the United States: An analysis of the UNOS registry. Clin. Transpl. 2008, 35–43. [Google Scholar]
- Lowey, S.E. Palliative Care in the Management of Patients with Advanced Heart Failure. Adv. Exp. Med. Biol. 2018, 1067, 295–311. [Google Scholar]
- Martens, P.; Vercammen, J.; Ceyssens, W.; Jacobs, L.; Luwel, E.; Van Aerde, H.; Potargent, P.; Renaers, M.; Dupont, M.; Mullens, W. Effects of intravenous home dobutamine in palliative end-stage heart failure on quality of life, heart failure hospitalization, and cost expenditure. ESC Heart Fail. 2018, 5, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J. Diabetes Res. 2019, 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Mariani, M.V.; Maraone, A.; Piro, A.; Ceccacci, A.; Tarsitani, L.; Maestrini, V.; Mancone, M.; Lavalle, C.; Pasquini, M.; et al. Triggers for Atrial Fibrillation: The Role of Anxiety. Cardiol. Res. Pract. 2019, 2019, 1208505. [Google Scholar] [CrossRef]
- Severino, P.; Maestrini, V.; Mariani, M.V.; Birtolo, L.I.; Scarpati, R.; Mancone, M.; Fedele, F. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail. Rev. 2019. [Google Scholar] [CrossRef]
- Wood, P.W.; Choy, J.B.; Nanda, N.C.; Becher, H. Left ventricular ejection fraction and volumes: It depends on the imaging method. Echocardiography 2014, 31, 87–100. [Google Scholar] [CrossRef]
- Gaasch, W.H.; Meyer, T.E. Left ventricular response to mitral regurgitation: Implications for management. Circulation 2008, 118, 2298–2303. [Google Scholar] [CrossRef]
- Berko, B.; Gaasch, W.H.; Tanigawa, N.; Smith, D.; Craige, E. Disparity between ejection and end-systolic indexes of left ventricular contractility in mitral regurgitation. Circulation 1987, 75, 1310–1319. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Adamo, F.; Severino, P. Heart failure with preserved, mid-range, and reduced ejection fraction: The misleading definition of the new guidelines. Cardiol. Rev. 2017, 25, 4–5. [Google Scholar] [CrossRef]
- Fedele, F.; Gatto, M.C.; D’Ambrosi, A.; Mancone, M. TNM-like classification: A new proposed method for heart failure staging. Sci. World, J. 2013, 2013, 175925. [Google Scholar] [CrossRef] [PubMed]
- Fedele, F.; Severino, P.; Calcagno, S.; Mancone, M. Heart failure: TNM-like classification. J. Am. Coll. Cardiol. 2014, 63, 1959–1960. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, V.; Birtolo, L.I.; Cimino, S.; Severino, P.; Mancone, M.; Francone, M.; Banypersad, S.M.; Ventriglia, F.; Tritapepe, L.; Miraldi, F.; et al. Giant right atrium and subvalvular pulmonary stenosis: A case report of an interesting combination. Echocardiography 2019, 36, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Buerke, M.; Parissis, J.; Ben-Gal, T.; Pollesello, P.; Kivikko, M.; Karavidas, A.; Severino, P.; Comín-Colet, J.; Wikström, G.; et al. Pharmaco-economics of levosimendan in cardiology: A European perspective. Int. J. Cardiol. 2015, 199, 337–341. [Google Scholar] [CrossRef]
- Gold, M.R.; Padhiar, A.; Mealing, S.; Sidhu, M.K.; Tsintzos, S.I.; Abraham, W.T. Economic value and cost-effectiveness of cardiac resynchronization therapy among patients with mild heart failure: Projections from the REVERSE Long-Term Follow-Up. J. Am. Coll. Cardiol. HF 2017, 5, 204–212. [Google Scholar]
- Sandhu, A.T.; Ollendorf, D.A.; Chapman, R.H.; Pearson, S.D.; Heidenreich, P.A. Cost-effectiveness of Sacubitril-Valsartan in patients with heart failure with reduced ejection fraction. Ann. Intern. Med. 2016, 165, 681–689. [Google Scholar] [CrossRef]
- Ollendorf, D.; Sandhu, A.T.; Pearson, S.D. Sacubitril-Valsartan for the treatment of heart failure effectiveness and value. JAMA Intern. Med. 2016, 176, 249–250. [Google Scholar] [CrossRef]
- Severino, P.; Scarpati, R.; Pucci, M.; Alfarano, M.; Infusino, F.; Cimino, S.; Calcagno, S.; Alunni Fegatelli, D.; Maestrini, V.; Vestri, A.; et al. Prognostic role of TNM-like classification for heart failure at 12 months of follow-up: Comparison with others nosologies. J. Am. Coll. CardioL. 2019, 73, 929. [Google Scholar] [CrossRef]
- Severino, P.; Mariani, M.V.; Fedele, F. Futility in cardiology: The need for a change in perspectives. Eur. J. Heart Fail. 2019, 29. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severino, P.; Mather, P.J.; Pucci, M.; D’Amato, A.; Mariani, M.V.; Infusino, F.; Birtolo, L.I.; Maestrini, V.; Mancone, M.; Fedele, F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics 2019, 9, 170. https://doi.org/10.3390/diagnostics9040170
Severino P, Mather PJ, Pucci M, D’Amato A, Mariani MV, Infusino F, Birtolo LI, Maestrini V, Mancone M, Fedele F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics. 2019; 9(4):170. https://doi.org/10.3390/diagnostics9040170
Chicago/Turabian StyleSeverino, Paolo, Paul J. Mather, Mariateresa Pucci, Andrea D’Amato, Marco Valerio Mariani, Fabio Infusino, Lucia Ilaria Birtolo, Viviana Maestrini, Massimo Mancone, and Francesco Fedele. 2019. "Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist" Diagnostics 9, no. 4: 170. https://doi.org/10.3390/diagnostics9040170
APA StyleSeverino, P., Mather, P. J., Pucci, M., D’Amato, A., Mariani, M. V., Infusino, F., Birtolo, L. I., Maestrini, V., Mancone, M., & Fedele, F. (2019). Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics, 9(4), 170. https://doi.org/10.3390/diagnostics9040170





