The Newly Normed SKT Reveals Differences in Neuropsychological Profiles of Patients with MCI, Mild Dementia and Depression
Abstract
1. Introduction
2. Methods
2.1. Samples
2.2. Measures
2.3. Statistics
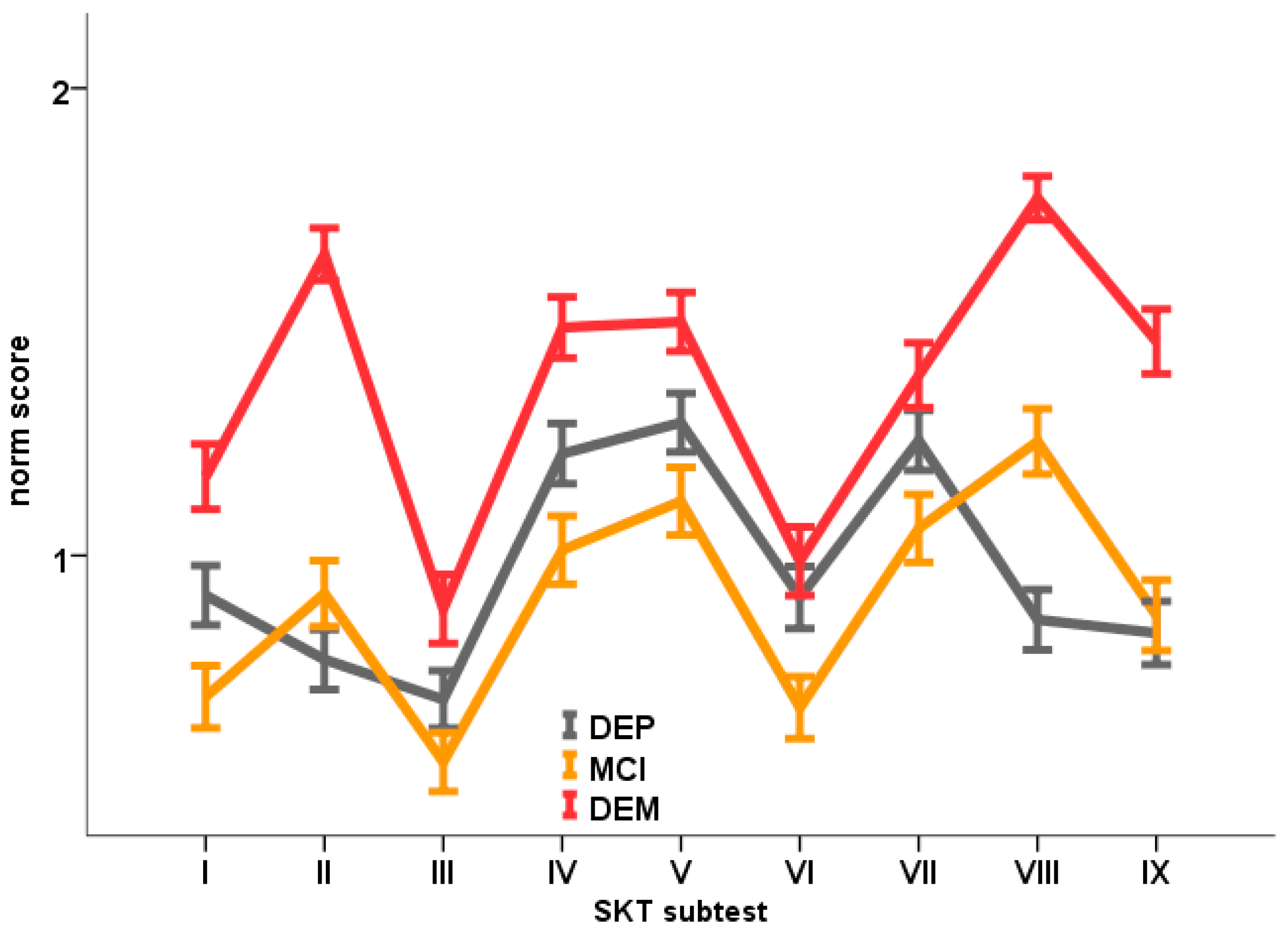
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wernicke, T.F.; Linden, M.; Gilberg, R.; Helmchen, H. Ranges of psychiatric morbidity in the old and the very old–results from the Berlin Aging Study (BASE). Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Mohsen, N.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Barnes, D.; Yaffe, K.; Byers, A.L.; McCormick, M.; Schaefer, C.; Whitmer, R. Midlife vs Late-Life Depressive Symptoms and Risk of Dementia Differential Effects for Alzheimer Disease and Vascular Dementia. Arch. Gen. Psychiatry 2012, 69, 493–498. [Google Scholar] [PubMed]
- Singh-Manoux, A.; Dugravot, A.; Fournier, A.; Abell, J.; Ebmeier, K.; Kivimäki, M.; Sabia, S. Trajectories of Depressive Symptoms Before Diagnosis of Dementia: A 28-Year Follow-up Study. JAMA Psychiatry 2017. [Google Scholar] [CrossRef] [PubMed]
- Amjad, H.; Roth, D.L.; Sheehan, O.C.; Lyketsos, C.G.; Wolff, J.L.; Samus, Q.M. Underdiagnosis of Dementia: An Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. J. Gen. Intern. Med. 2018, 33, 1131–1138. [Google Scholar] [CrossRef]
- Depression: Fact sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 July 2019).
- Dybedal, G.S.; Tanum, L.; Sundet, K.; Gaarden, T.L.; Bjølseth, T.M. Neuropsychological functioning in late-life depression. Front. Psychol. 2013, 4, 381. [Google Scholar] [CrossRef]
- Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996, 46, 130–135. [Google Scholar] [CrossRef]
- Zhao, Q.-F.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Dilling, H.; Mombour, W.; Schmidt, M.H.; Schulte-Markwort, M. Weltgesundheitsorganisation: Internationale Klassifikation psychischer Störungen: ICD-10 Kapitel V (F). Diagnostische Kriterien für Forschung und Praxis, 3rd ed.; Verlag Hans Huber: Toronto, ON, Canada, 1999; pp. 181–183. [Google Scholar]
- APA American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Lee, R.S.; Hermens, D.F.; Porter, M.A.; Redoblado-Hodge, M.A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 2012, 140, 113–124. [Google Scholar] [CrossRef]
- Beblo, T.; Sinnamon, G.; Baune, B.T. Specifying the neuropsychology of affective disorders: Clinical, demographic and neurobiological factors. Neuropsychol. Rev. 2011, 21, 337–359. [Google Scholar] [CrossRef]
- Butters, M.A.; Whyte, E.M.; Nebes, R.D.; Begley, A.E.; Dew, M.A.; Mulsant, B.H.; Zmuda, M.D.; Bhalla, R.; Meltzer, C.C.; Pollock, B.G.; et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch. Gen. Psychiatry. 2004, 61, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, C.T.; Johnson, L.G.; Benedict, K.B. Neurocognition in depression: Patients on and off medication versus healthy comparison subjects. J. Neuropsychiatry Clin. Neurosci. 2016, 18, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Künig, G.; Jäger, M.; Stief, V.; Kaldune, A.; Urbaniok, F.; Endrass, J. The impact of the CERAD-NP on diagnosis of cognitive deficiencies in late onset depression and Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2006, 21, 911–916. [Google Scholar] [CrossRef]
- Barth, S.; Schönknecht, P.; Pantel, J.; Schröder, J. Neuropsychologische Profile in der Demenzdiagnostik: Eine Untersuchung mit der CERAD-NP-Testbatterie. [Mild Cognitive Impairment and Alzheimer’s Disease: An Investigation of the CERAD−NP Test Battery. Fortschr. Der. Neurol. Und. Der. Psychiatr 2005, 73, 568–576. [Google Scholar] [CrossRef]
- Dierckx, E.; Engelborghs, S.; Raedt, R.D.; Deyn, P.P.D.; Ponjaert-Kristoffersen, I. Differentiation between mild cognitive impairment, Alzheimer’s disease and depression by means of cued recall. Psychol. Med. 2007, 37, 747–755. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dis. Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Ismail, Z.; Elbayoumi, H.; Fischer, C.E.; Hogan, D.B.; Millikin, C.P.; Schweizer, T.; Mortby, M.E.; Smith, E.E.; Patten, S.B.; Fiest, K.M. Prevalence of depression in patients with mild cognitive impairment: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 58–67. [Google Scholar] [CrossRef]
- Zihl, J.; Reppermund, S.; Thum, S.; Unger, K. Neuropsychological profiles in MCI and in depression: Differential cognitive dysfunction patterns or similar final common pathway disorder? J. Psychiatr. Res. 2010, 44, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, M.; Lehfeld, H.; Horn, R. SKT Manual Edition 2015; Geromed GmbH: Spardorf, Bavaria, Germany, 2015. [Google Scholar]
- Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Cha, R.H.; Pankratz, V.S.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Rocca, W.A.; Petersen, R.C. The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology 2012, 78, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [PubMed]
- Erzigkeit, H. Manual for the Syndrom-Kurztest; Geromed GmbH: Spardorf, Germany, 1997. [Google Scholar]
- Monsch, A. CERAD—Neuropsychological Test Battery; Memory Clinic: Basel, Switzerland, 1997. [Google Scholar]
- Hautzinger, M.; Bailer, M. ADS, General Depression Scale; Hogrefe Publishing GmbH: Göttingen, Germany, 1993. [Google Scholar]
- Collegium Internationale Psychiatriae Scalarum (Hrsg.). International Scales for Psychiatry, 5th ed; Hogrefe Publishing GmbH: Göttingen, Germany, 2005. [Google Scholar]
- Hindmarch, I.; Lehfeld, H.; de Jongh, P.; Erzigkeit, H. The Bayer Activities of Daily Living Scale (B-ADL Scale). Dement. Geriatr. Cogn. Disord. 1998, 9, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Stroessenreuther, N.; Lehfeld, H.; Niklewski, N. PANDA—A screening tool Parkinson neuropsychometric dementia assessment. In Proceedings of the DGPPN Congress, Berlin, Germany, 24–27 November 2010. [Google Scholar]
- Stemmler, M.; Lehfeld, H.; Erzigkeit, A. The English Validation of the SKT according to Erzigkeit. In SKT Manual Edition 2019; Geromed GmbH: Spardorf, Bavaria, Germany, 2019. [Google Scholar]
- Erzigkeit, H. Manual for the SKT forms A-E, 4th ed.; Beltz: Weinheim, Germany, 1989. [Google Scholar]
- Lehfeld, H.; Erzigkeit, H. The SKT—A short cognitive performance test for assessing deficits of memory and attention. Int. Psychogeriatr. 1997, 9, 115–121. [Google Scholar] [CrossRef]
- Hessler, J.B.; Stemmler, M.; Bickel, H. Cross-Validation of the newly-normed SKT for the detection of MCI and dementia. GeroPsych. 2017, 30, 19–25. [Google Scholar] [CrossRef]
- Stemmler, M.; Hessler, J.B.; Bickel, H. Predicting cognitive decline and dementia with the newly-normed SKT Short Cognitive Performance Test. Dement. Geriatr. Cogn. Disord. Extra 2019, 9, 184–193. [Google Scholar] [CrossRef]
- Butters, M.A.; Young, J.B.; Lopez, O.; Aizenstein, H.J.; Mulsant, B.H.; Reynolds III, C.F.; DeKosky, S.T.; Becker, J.T. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 2008, 10, 345–357. [Google Scholar]
- Wang, L.; Potter, G.G.; Krishnan, R.K.; Dolcos, F.; Smith, G.S.; Steffens, D.C. Neural correlates associated with cognitive decline in late-life depression. Am. J. Geriatr Psychiatry 2012, 20, 653–663. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Baskys, A.; Boldrini, M.; Butters, M.A.; Diniz, B.S.; Jaiswal, M.K.; Jellinger, K.A.; Kruglov, L.S.; Meshandin, I.A.; Mijajlovic, M.D.; et al. Vascular depression consensus report—A critical update. Bmc Med. 2016, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, C.; Lee, D.; Ng, K.P.; Chander, R.; Ng, D.; Ji, F.; Shim, H.Y.; Hilal, S.; Venketasubramanian, N.; Chen, C.; et al. Mechanisms Linking White Matter Lesions, Tract Integrity and Depression in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nibbelink, D.W.; Overall, J.E. Factor structure and scoring of the SKT test battery. J. Clin. Psychol. 1993, 49, 61–71. [Google Scholar] [CrossRef]
- Lehfeld, H.; Rudinger, G.; Rietz, C.; Heinrich, C.; Wied, V.; Fornazzari, L.; Pittas, J.; Hindmarch, I.; Erzigkeit, H. Evidence of the cross-cultural stability of the factor structure of the SKT short test for assessing deficits of memory and attention. Int. Psychogeriatr. 1997, 9, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, M.; Pittas, J. The use of SKT for the assessment of dementia in Greece. Encephalos 1995, 32, 336–345. [Google Scholar]
- Flaks, M.K.; Forlenza, O.V.; Pereira, F.S.; Viola, L.F.; Yassuda, M.S. Short cognitive performance test: Diagnostic accuracy and education bias in older Brazilian adults. Arch. Clin. Neuropsychol. 2009, 24, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Fornazzari, L.; Cumsille, F.; Quevedo, F.; Quiroga, P.; Rioseco, P.; Klaasen, G.; Martinez, C.G.; Rhode, G.; Sacks, C.; Rivera, E.; et al. Spanish validation of the Syndrom Kurztest (SKT). Alzheimer Dis. Assoc. Disord. 2001, 15, 211–215. [Google Scholar] [CrossRef]
- Ostrosky-Solís, F.; Davila, G.; Ortiz, X.; Vega, F.; Garcia Ramos, G.; de Celis, M.; Dávila, L.; Gómez, C.; Jiménez, S.; Juárez, S.; et al. Determination of normative criteria and validation of the SKT for use in Spanish-speaking populations. Int. Psychogeriatr. 1999, 11, 171–180. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, B.H.; Hahm, D.S.; Jeong, J.H.; Ha, C.K.; Han, S.H.; Erzigkeit, H.; Na, D.L. Validation of the Korean version of the Syndrom Kurztest (SKT): A short test for the assessment of memory and attention. Hum. Psychopharmacol. 2004, 19, 495–501. [Google Scholar] [CrossRef]
- Stemmler, M.; Lehfeld, H. Validation of the SKT short cognitive performance test for the detection of early cognitive decline in English-speaking countries. In Proceedings of the CTAD Conference, San Diego CA, USA, 4–7 December 2019. [Google Scholar]

| Name of Subtest | Content of Subtest | Domain |
|---|---|---|
| I Naming Objects | twelve objects have to be named and memorized at the same time | attention/speed |
| II Immediate Recall | recall of objects shown in subtest I | memory |
| III Naming Numerals | two digit numbers written on magnetic blocks placed on a board have to be read out loud | attention/speed |
| IV Arranging Blocks | the magnetic blocks have to be arranged in ascending order of the numbers | attention/speed |
| V Replacing Blocks | the blocks have to be replaced in their original positions | attention/speed |
| VI Counting Symbols | symbols printed on a tableau have to be counted | attention/speed |
| VII Reversal Naming | two rows composed of two letters in random order are to be read by naming each letter with the name of the other | attention/speed |
| VIII Delayed Recall | recall of objects shown in subtest I | memory |
| IX Recognition Memory | identification of objects shown in subtest I from a table containing 48 objects | memory |
| MCI M (SD) | DEM M (SD) | DEP M (SD) | p | Group Comparisons | |
|---|---|---|---|---|---|
| sample size | 172 | 166 | 211 | ||
| age | 73.9 (7.3) | 76.6 (7.6) | 72.5 (7.5) | 0.000 | DEP < MCI < DEM |
| gender (f/m) | 90/82 | 102/64 | 134/77 | 0.045 | |
| education * | 12,0 (2,8) | 11,3 (2,9) | 11,4 (2,8) | n.s. | |
| MMSE ** | 26.5 (2.2) | 22.6 (3.1) | 26.2 (3.0) | 0.000 | DEM < MCI = DEP |
| depression (z-scores) *** | −0.42 (0.77) | −0.27 (0.81) | 0.55 (1.04) | 0.000 | DEM = MCI, MCI < DEP, DEM < DEP |
| SKT I | 0.70 (0.87) | 1.17 (0.90) | 0.91 (0.93) | 0.000 | MCI < (tend.) DEP < DEM |
| SKT II | 0.92 (0.93) | 1.64 (0,72) | 0.78 (0.93) | 0.000 | MCI = DEP; DEP < DEM; MCI < DEM |
| SKT III | 0.56 (0.83) | 0.89 (0.95) | 0.69 (0.90) | 0.003 | MCI = DEP; DEP = DEM; MCI < DEM |
| SKT IV | 1.01 (0.96) | 1.49 (0.84) | 1.22 (0.94) | 0.000 | MCI < (tend.) DEP < DEM |
| SKT V | 1.12 (0.95) | 1.50 (0.81) | 1.28 (0.91) | 0.000 | MCI = DEP; DEP < (tend.) DEM; MCI < DEM |
| SKT VI | 0.67 (0.87) | 0.99 (0.95) | 0.91 (0.96) | 0.009 | MCI < DEP; DEP = DEM; MCI < DEM |
| SKT VII | 1.06 (0.95) | 1.39 (0.89) | 1.25 (0.93) | 0.005 | MCI = DEP; DEP = DEM; MCI < DEM |
| SKT VIII | 1.24 (0.92) | 1.77 (0.60) | 0.86 (0.94) | 0.000 | DEP < MCI < DEM |
| SKT IX | 0.87 (0.99) | 1.46 (0.89) | 0.83 (0.99) | 0.000 | DEP = MCI; DEP < DEM; MCI < DEM |
| SKT memory | 3.03 (1.85) | 4.87 (1.44) | 2.47 (2.06) | 0.000 | DEP < MCI < DEM |
| SKT attention | 5.12 (3.58) | 7.42 (3.37) | 6.27 (3.95) | 0.000 | MCI < DEP < DEM |
| SKT sum score | 8.15 (3.97) | 12.28 (3.55) | 8.74 (4.78) | 0.000 | MCI= DEP; DEP < DEM; MCI < DEM |
| MCI | DEM | DEP | |
|---|---|---|---|
| SKT sum score | 0.83 | 0.96 | 0.81 |
| SKT memory subscore | 0.77 | 0.93 | 0.68 |
| SKT attention subscore | 0.74 | 0.88 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehfeld, H.; Stemmler, M. The Newly Normed SKT Reveals Differences in Neuropsychological Profiles of Patients with MCI, Mild Dementia and Depression. Diagnostics 2019, 9, 163. https://doi.org/10.3390/diagnostics9040163
Lehfeld H, Stemmler M. The Newly Normed SKT Reveals Differences in Neuropsychological Profiles of Patients with MCI, Mild Dementia and Depression. Diagnostics. 2019; 9(4):163. https://doi.org/10.3390/diagnostics9040163
Chicago/Turabian StyleLehfeld, Hartmut, and Mark Stemmler. 2019. "The Newly Normed SKT Reveals Differences in Neuropsychological Profiles of Patients with MCI, Mild Dementia and Depression" Diagnostics 9, no. 4: 163. https://doi.org/10.3390/diagnostics9040163
APA StyleLehfeld, H., & Stemmler, M. (2019). The Newly Normed SKT Reveals Differences in Neuropsychological Profiles of Patients with MCI, Mild Dementia and Depression. Diagnostics, 9(4), 163. https://doi.org/10.3390/diagnostics9040163






