Spontaneous Splenic Rupture as a Rare Initial Presentation in an Acute Lymphoblastic Leukemia Patient
Abstract
1. Introduction
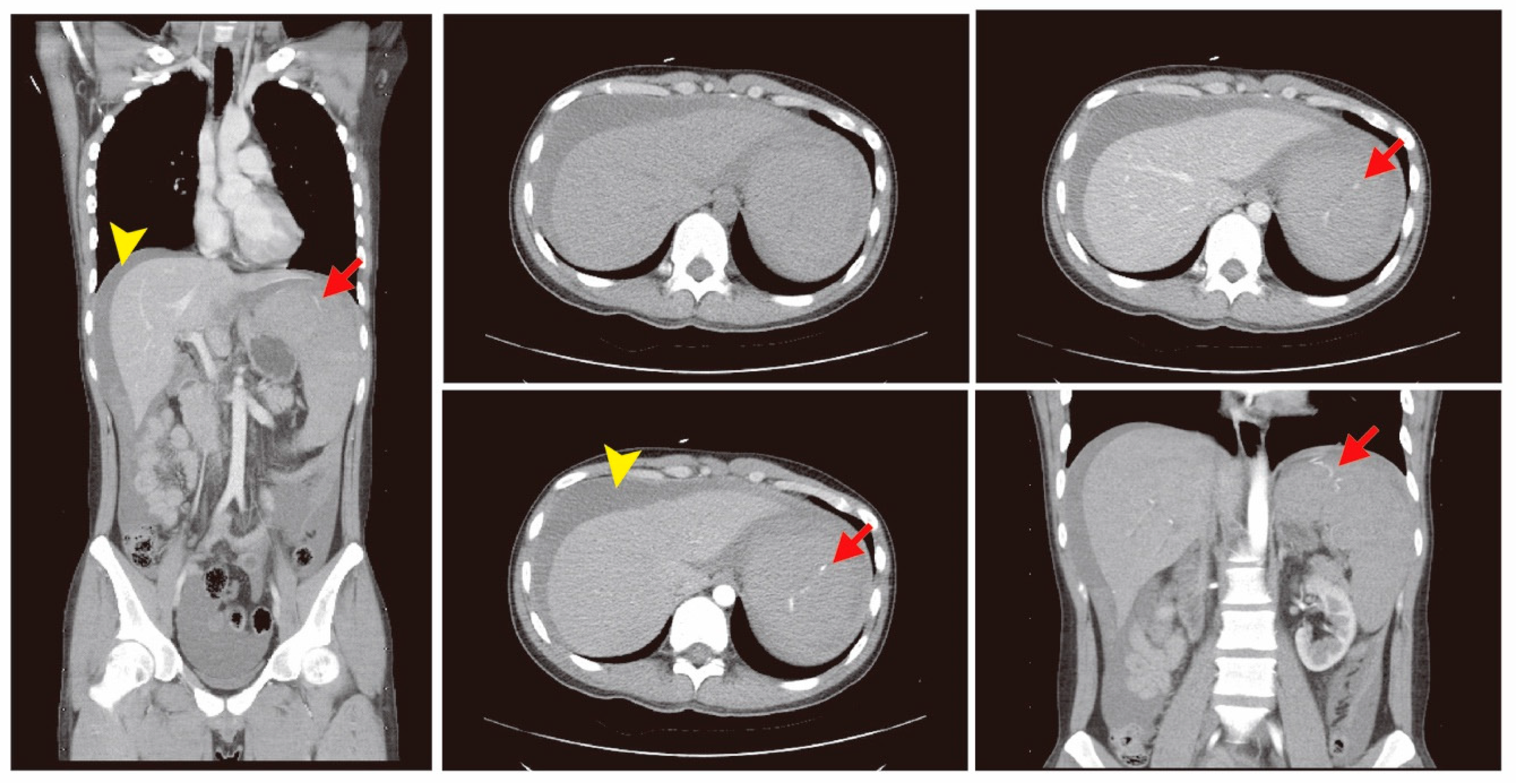
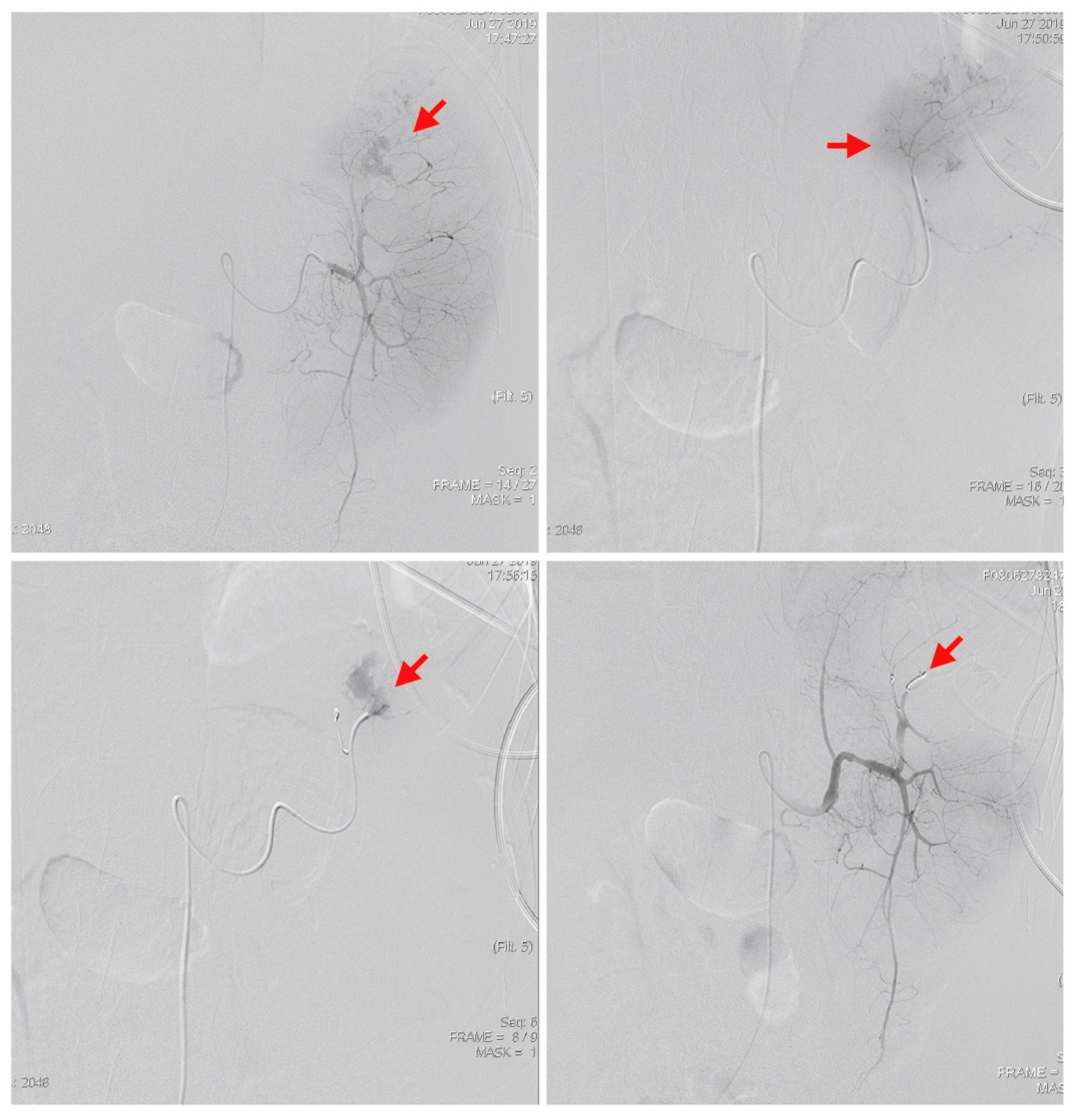
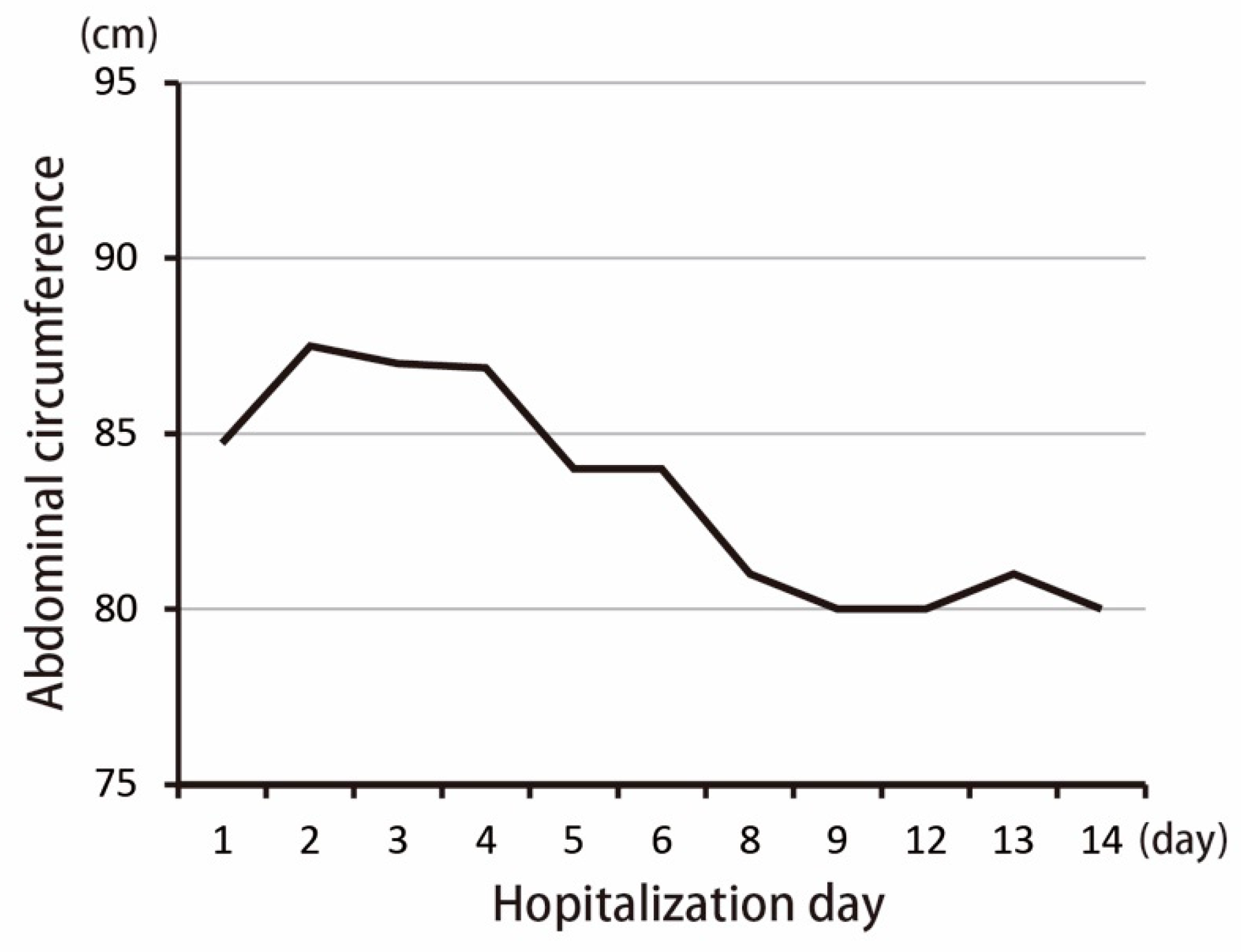
2. Case Presentation
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rhee, S.-J.; Sheena, Y.; Imber, C. Spontaneous rupture of the spleen: A rare but important differential of an acute abdomen. Am. J. Emerg. Med. 2008, 26, 733.e735–733.e736. [Google Scholar] [CrossRef]
- Karassa, F.B.; Isenberg, D.A. Spontaneous rupture of the spleen: An unusual complication of systemic lupus erythematosus. Lupus 2001, 10, 876–878. [Google Scholar] [CrossRef]
- Mujtaba, G.; Josmi, J.; Arya, M.; Anand, S. Spontaneous splenic rupture: A rare complication of acute pancreatitis in a patient with Crohn’s disease. Case Rep. Gastroenterol. 2011, 5, 179–182. [Google Scholar] [CrossRef]
- Yoshizawa, J.; Mizuno, R.; Yoshida, T.; Kanai, M.; Kurobe, M.; Yamazaki, Y. Spontaneous rupture of splenic hamartoma: A case report. J. Pediatric Surg. 1999, 34, 498–499. [Google Scholar] [CrossRef]
- Won, A.C.; Ethell, A. Spontaneous splenic rupture resulted from infectious mononucleosis. Int. J. Surg. Case Rep. 2012, 3, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Canady, M.R.; Welling, R.E.; Strobel, S.L. Splenic rupture in leukemia. J. Surg. Oncol. 1989, 41, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Shiber, J.; Fontane, E.; Prisk, D. ATraumatic Splenic Rupture: Dreaded Complication of Splenomegaly. Trop. Med. Surg. 2016, 2. [Google Scholar] [CrossRef]
- Renzulli, P.; Hostettler, A.; Schoepfer, A.M.; Gloor, B.; Candinas, D. Systematic review of atraumatic splenic rupture. Br. J. Surg. 2009, 96, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Giagounidis, A.A.; Burk, M.; Meckenstock, G.; Koch, A.J.; Schneider, W. Pathologic rupture of the spleen in hematologic malignancies: Two additional cases. Ann. Hematol. 1996, 73, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.; Mamdani, H.; Deol, A. Malignant cause of abdominal pain in leukemia: Spontaneous splenic rupture. Am. J. Med. Sci. 2015, 349, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Haskins, G.E.; Armitage, J.O. Splenic rupture in patients with hematologic malignancies. Cancer 1981, 48, 2729–2733. [Google Scholar] [CrossRef]
- Jafferbhoy, S.; Chantry, A.; Atkey, N.; Turner, D.; Wyld, L. Spontaneous splenic rupture: An unusual presentation of CML. Bmj Case Rep. 2011, 2011. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, C.; Li, D.; Li, S. Molecular and cellular bases of chronic myeloid leukemia. Protein Cell 2010, 1, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Lin, C.-H.; Hou, Y.-T.; Lin, P.-C.; Yiang, G.-T.; Tien, Y.-C.; Yeh, H.-C. Syncope as Initial Presentation in an Undifferentiated Type Acute Myeloid Leukemia Patient with Acute Intracranial Hemorrhage. Brain Sci. 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- SOORYA, D.T.; RAVINDRANATH, Y.; PHILIPPART, A.; Alpern, B.; Thomas, D. Spontaneous Splenic Rupture in Acute Lymphoblastic Leukemia: Successful Nonoperative Management. Jama Pediatrics 1980, 134, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Bernat, S.; Garcia Boyero, R.; Guinot, M.; Lopez, F.; Gozalbo, T.; Canigral, G. Pathologic rupture of the spleen as the initial manifestation in acute lymphoblastic leukemia. Haematologica 1998, 83, 760–761. [Google Scholar] [PubMed]

| Variables | Normal Range | Patient Data | Variables | Normal Range | Patient Data |
|---|---|---|---|---|---|
| White blood cells (WBC) | 3.5–11 (×109/L) | 102.04 | Blood urine nitrogen | 7–18 mg/dL | 21 |
| Segment form neutrophils | 45–70% | 6.0 | Creatinine | 0.55–1.02 mg/dL | 1.3 |
| Lymphocytes | 25–40% | 3.0 | Sodium | 136–145 mmol/L | 140 |
| Eosinophils | 1–3% | 0.0 | Potassium | 3.5–5.1 mmol/L | 4.3 |
| Monocytes | 2–8% | 1.0 | Calcium | 2.12–2.52 mmol/L | 1.91 |
| Nuclear red blood cells | 0/100WBC | 1.0 | Glucose | 70–100 mg/dL | 145 |
| Blast cell | 0% | 88.0 | Alanine aminotransferase | 16–63 U/L | 51 |
| Hemoglobin | 12–16 g/dL | 9.4 | Lipase | 73–393 IU/L | 206 |
| Platelet counts | 150–400 (×109/L) | 32 | Creatine kinase | 26–192 IU/L | 184 |
| Prothrombin time | 8.0–12.0 s | 12.7 | C-reactive protein (CRP) | 0–0.33 mg/dL | 0.66 |
| Partial thromboplastin time | 23.9–35.5 s | 30.6 | |||
| Lactic dehydrogenase (LDH) | 85–227 IU/L | 894 |
| Variables | Normal Range | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 11 | Day 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 3.5–11 (× 109/L) | 102.04 | 22.82 | 5.69 | 3.9 | 2.87 | 2.01 | 1.92 | 2.03 | 2.39 | 1.64 | 0.99 | 1.34 |
| RBC | 4.5–5.9 (× 106/uL) | 3.43 | 3.13 | 2.84 | 2.86 | 2.84 | 3.42 | 3.37 | 3.55 | 3.85 | 3.8 | 3.54 | 3.61 |
| Hb | 12–16 g/dL | 9.4 | 8.7 | 8.1 | 8.4 | 8.1 | 9.7 | 9.7 | 10.1 | 11.3 | 11 | 10.2 | 10.2 |
| PL | 150–400 (× 109/L) | 32 | 85 | 90 | 53 | 68 | 43 | 29 | 70 | 42 | 42 | 85 | 84 |
| N. band | 0–3% | 0 | 5 | 3 | 6 | 7 | 0 | 5 | 0 | 1 | 0 | 1 | 3 |
| N. seg. | 40–75% | 6 | 6 | 16 | 24 | 24 | 52 | 46 | 58.1 | 56 | 59 | 52 | 40 |
| Lym. | 20–45% | 3 | 37 | 55 | 34 | 38 | 35 | 41 | 34 | 37 | 38 | 39 | 50 |
| Mono. | 2–10% | 1 | 1 | 2 | 3 | 1 | 2 | 1 | 3 | 2 | 2 | 3 | 3 |
| Eosin. | 1–6% | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 4.9 | 0 | 1 | 3 | 4 |
| Baso. | 0–1% | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blast | 0–0% | 88 | 48 | 18 | 26 | 24 | 9 | 2 | 0 | 1 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Kao, W.-Y.; Chan, C.-Y.; Yiang, G.-T.; Liao, W.-T.; Chen, C.-S. Spontaneous Splenic Rupture as a Rare Initial Presentation in an Acute Lymphoblastic Leukemia Patient. Diagnostics 2019, 9, 152. https://doi.org/10.3390/diagnostics9040152
Wu M-Y, Kao W-Y, Chan C-Y, Yiang G-T, Liao W-T, Chen C-S. Spontaneous Splenic Rupture as a Rare Initial Presentation in an Acute Lymphoblastic Leukemia Patient. Diagnostics. 2019; 9(4):152. https://doi.org/10.3390/diagnostics9040152
Chicago/Turabian StyleWu, Meng-Yu, Woei-Yau Kao, Cheng-Yi Chan, Giou-Teng Yiang, Wan-Ting Liao, and Chien-Sheng Chen. 2019. "Spontaneous Splenic Rupture as a Rare Initial Presentation in an Acute Lymphoblastic Leukemia Patient" Diagnostics 9, no. 4: 152. https://doi.org/10.3390/diagnostics9040152
APA StyleWu, M.-Y., Kao, W.-Y., Chan, C.-Y., Yiang, G.-T., Liao, W.-T., & Chen, C.-S. (2019). Spontaneous Splenic Rupture as a Rare Initial Presentation in an Acute Lymphoblastic Leukemia Patient. Diagnostics, 9(4), 152. https://doi.org/10.3390/diagnostics9040152






