Comparing the Diagnostic Accuracy of Two Cognitive Screening Instruments in Different Dementia Subtypes and Clinical Depression
Abstract
1. Introduction
2. Methods
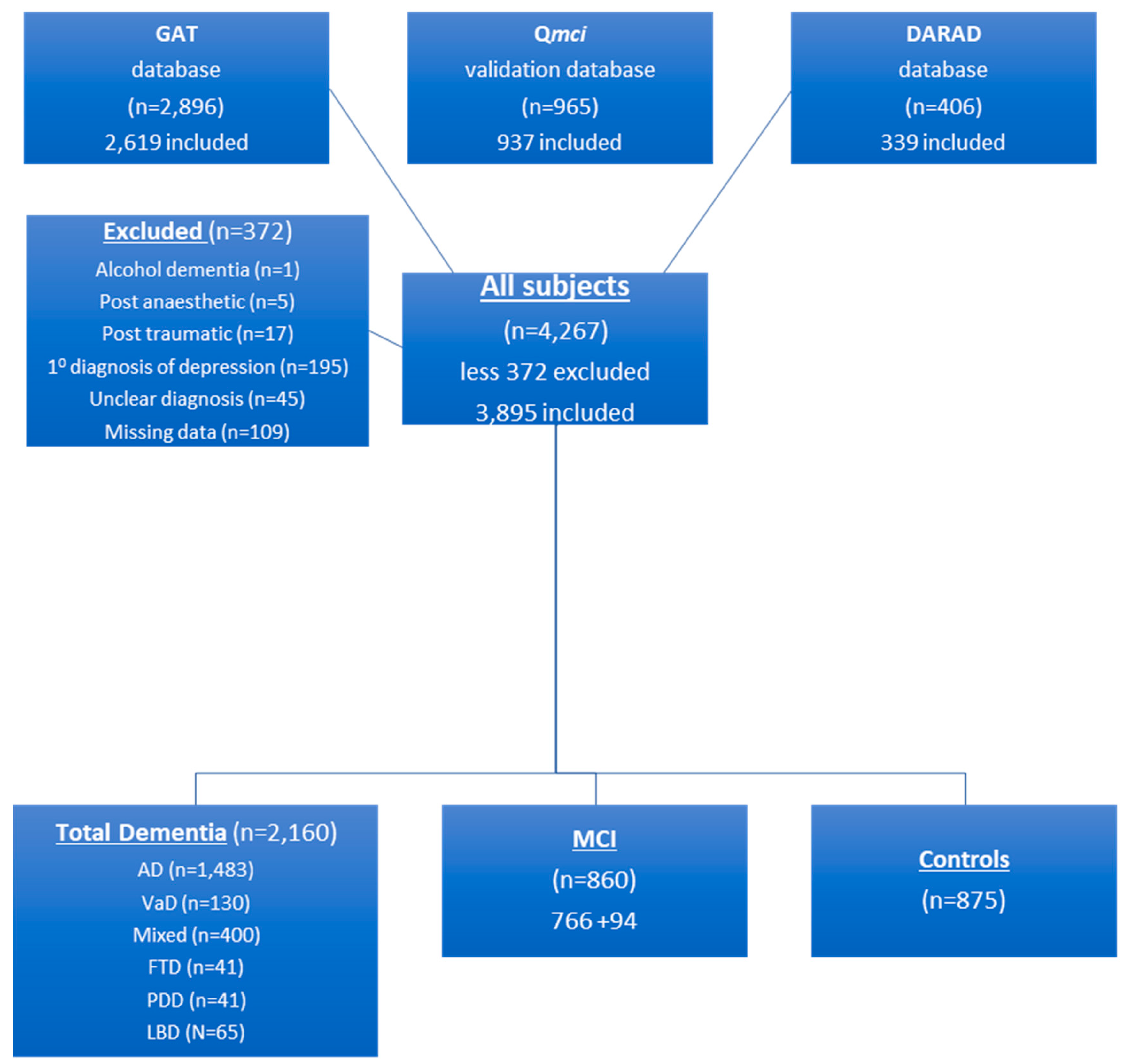
2.1. Data Collection
2.2. Participants
2.3. Outcome Measures
2.4. Analysis
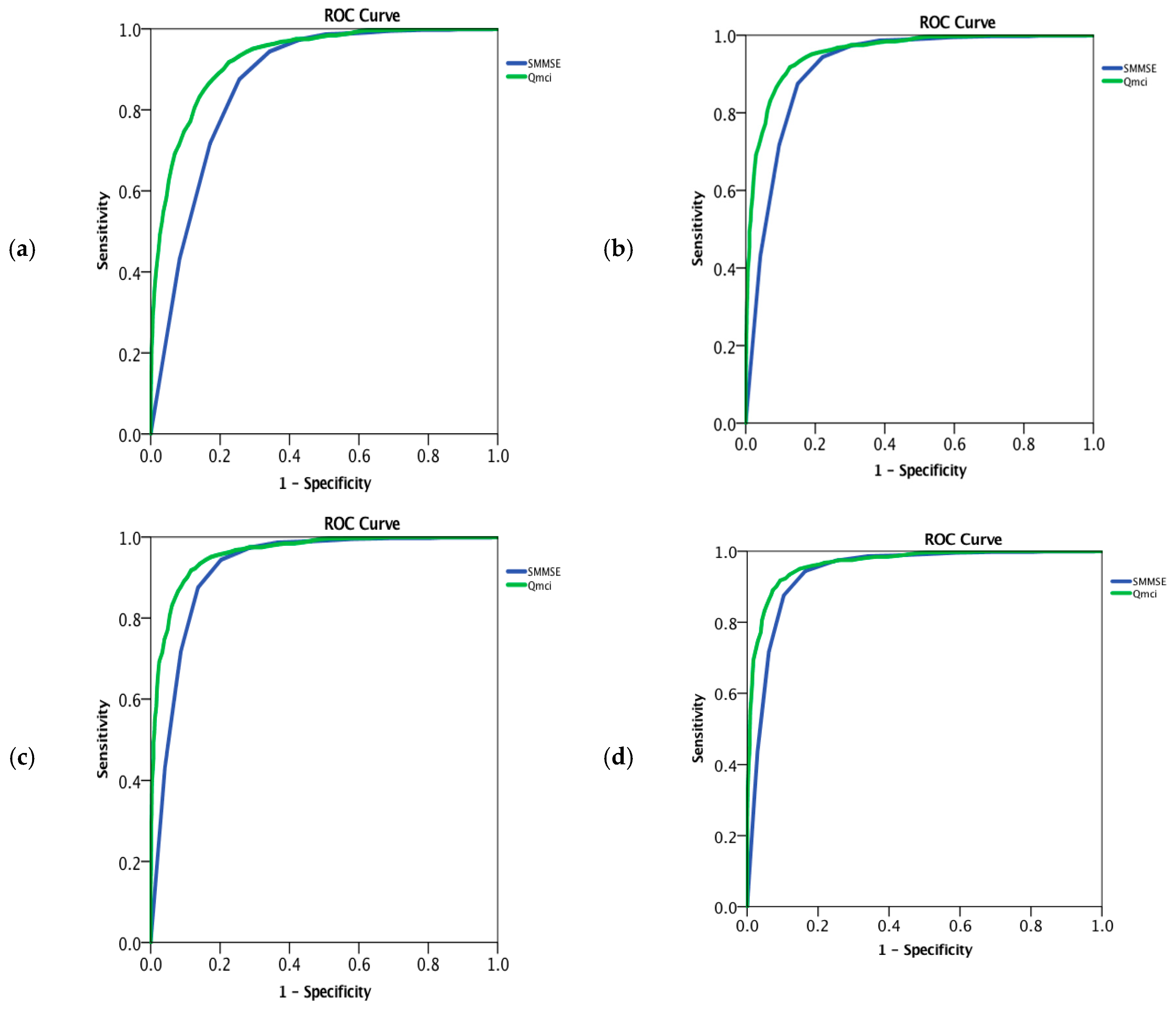
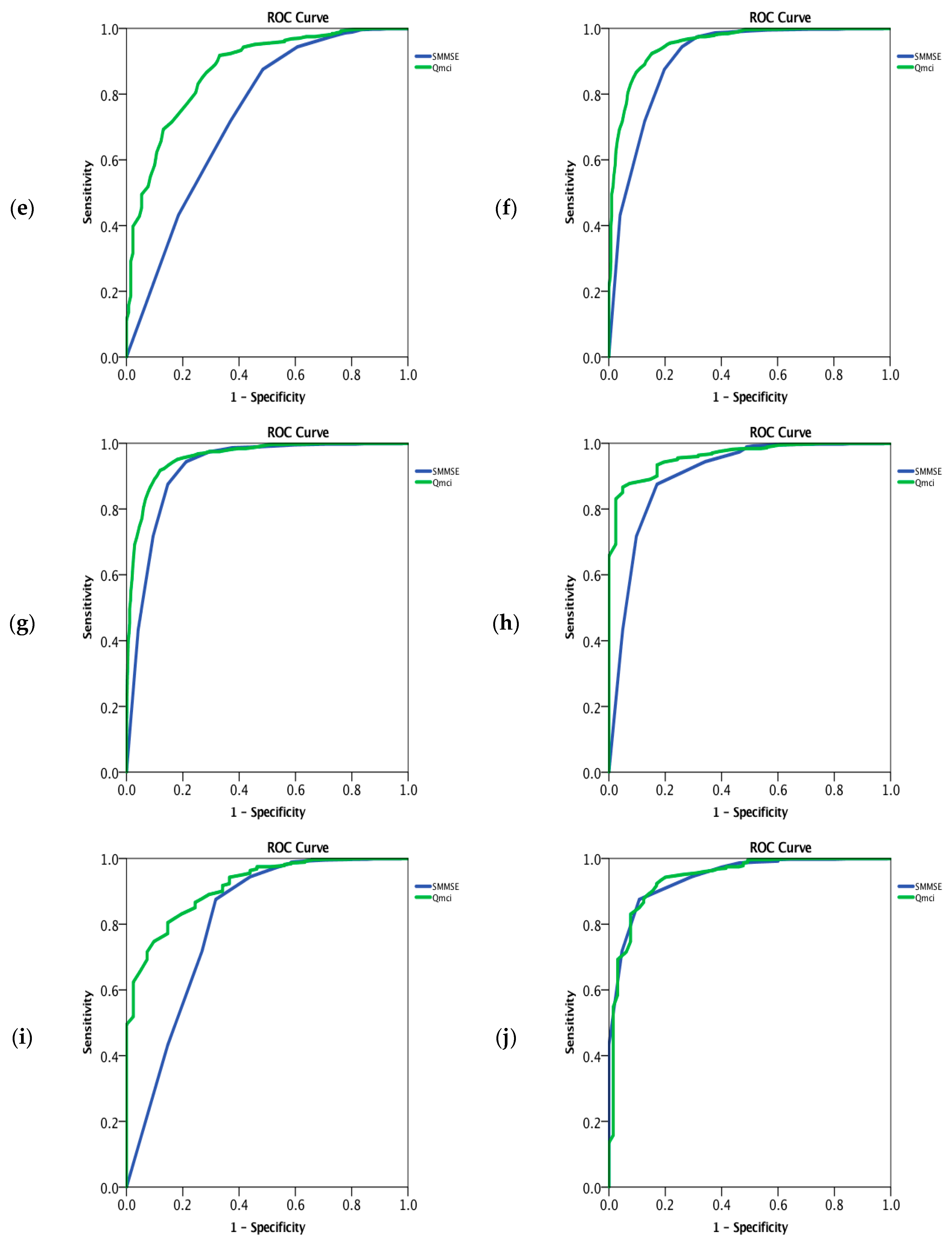
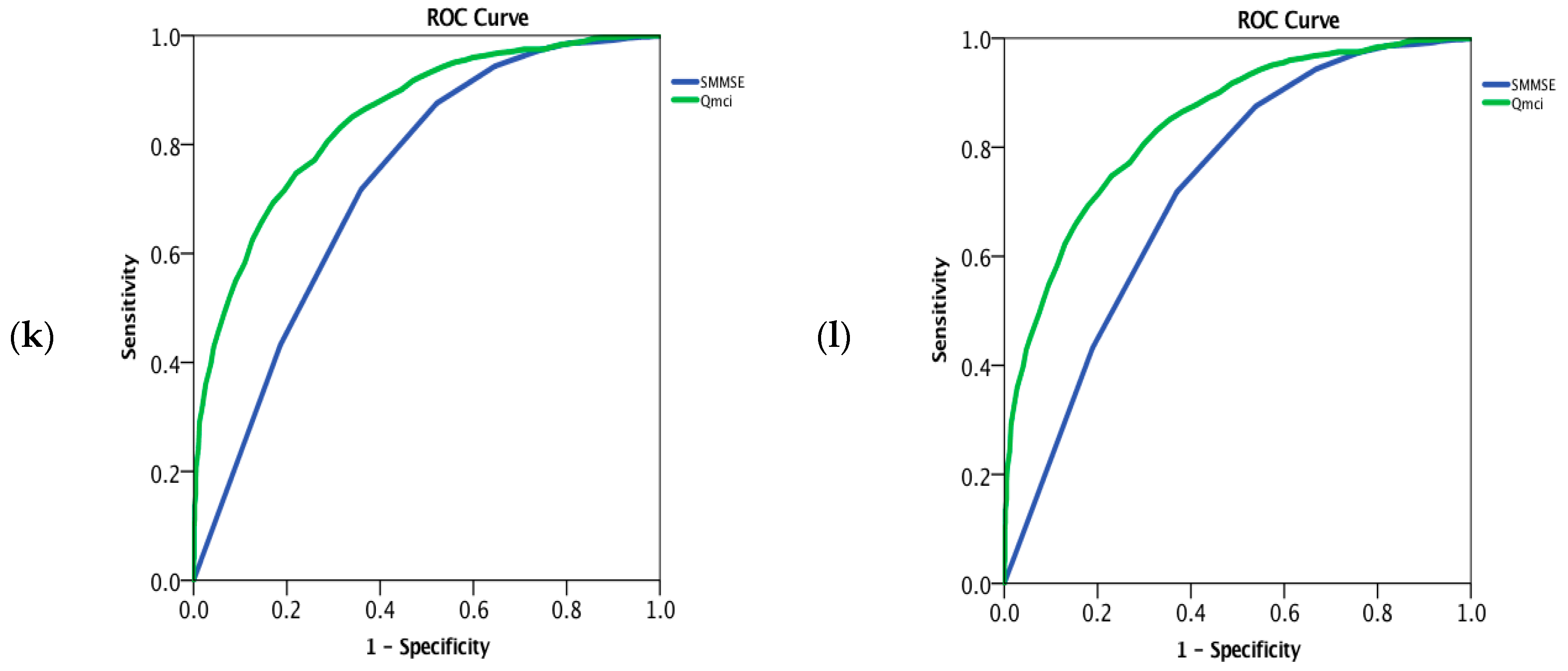
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatry Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Molloy, D.W.; Alemayehu, E.; Roberts, R. Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am. J. Psychiatry 1991, 148, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Molloy, D.W.; Standish, T.I.M. A guide to the Standardized Mini-Mental State Examination. Int. Psychogeriatry 1997, 9, 87–94. [Google Scholar] [CrossRef]
- Woodford, H.J.; George, J. Cognitive assessment in the elderly: A review of clinical methods. QJM Int. J. Med. 2007, 100, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Breton, A.; Casey, D.; Arnaoutoglou, N.A. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int. J. Geriatr. Psychiatry 2019, 34, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Snyder, P.J. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Rami, L.; Molinuevo, J.L.; Sanchez-Valle, R.; Bosch, B.; Villar, A. Screening for amnestic mild cognitive impairment and early Alzheimer’s disease with M@T (Memory Alteration Test) in the primary care population. Int. J. Geriatr. Psychiatry 2007, 22, 294–304. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; McGlade, C.; Healy, L.; Gallagher, P.; Timmons, S.; Molloy, D.W. Comparison of the Quick Mild Cognitive Impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 2012, 41, 624–629. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Mitchell, A.J. A Meta-Analysis of the Accuracy of the Mini-Mental State Examination in the Detection of Dementia and Mild Cognitive Impairment. J. Psychiatry Res. 2009, 43, 411–431. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatry Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. J. Am. Med. Assoc. 1993, 269, 2386–2391. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Svendovski, A.; Gallagher, P.; Eustace, J.; Molloy, D.W. Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. J. Alzheimers Dis. 2017, 57, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, L.; Ryu, S.H.; Raczek, M.; Philpot, M.; Lindesay, J.; Critchfield, M.; Livingston, G. Review of brief cognitive tests for patients with suspected dementia. Int. Psychogeriatry 2014, 26, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Nazem, S.; Siderowf, A.D.; Duda, J.E.; Have, T.T.; Colcher, A.; Horn, S.S.; Moberg, P.J.; Wilkinson, J.R.; Hurtig, H.I.; Stern, M.B.; et al. Montreal Cognitive Assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J. Am. Geriatr. Soc. 2009, 57, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Osher, J.E.; Wicklund, A.H.; Rademaker, A.; Johnson, N.; Weintraub, S. The Mini-Mental State Examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am. J. Alzheimer’s Dis. 2008, 22, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, M.Z.A.A.; Miptah, H.N.; O’Caoimh, R. Cognitive screening instruments to identify vascular cognitive impairment: A systematic review. Int. J. Geriatr. Psychiatry 2019, 34, 1114–1127. [Google Scholar] [CrossRef]
- Bak, T.H.; Rogers, T.T.; Crawford, L.M.; Hearn, V.C.; Mathuranath, P.S.; Hodges, J.R. Cognitive bedside assessment in atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2005, 76, 420–422. [Google Scholar] [CrossRef]
- Russo, M.; Mahon, K.; Burdick, K. Measuring cognitive functions in MDD: Emerging assessment tools. Depress. Anxiety 2015, 32, 262–269. [Google Scholar] [CrossRef]
- Richardson, L.; Adams, S. Cognitive deficits in patients with depression. J. Nur. Pract. 2018, 14, 437–443. [Google Scholar] [CrossRef]
- Van der Mussele, S.; Fransen, E.; Struyfs, H.; Luyckx, J.; Mariën, P.; Saerens, J.; Somers, N.; Goeman, J.; De Deyn, PP.; Engelborghs, S. Depression in Mild Cognitive Impairment is associated with Progression to Alzheimer’s Disease: A Longitudinal Study. J. Alzheimers Dis. 2014, 42, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Sachdev, P.S.; Brodaty, H.; Trollor, J.N.; Andrews, G. Effects of sociodemographic and health variables on Mini-Mental State Exam scores in older Australians. Am. J. Ger. Psychiatry 2007, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; O’Caoimh, R.; Healy, L.; Kerins, D.M.; Eustace, J.; Guyatt, G.; Sammon, D.; Molloy, D.W. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open 2013, 3, e002881. [Google Scholar] [CrossRef] [PubMed]
- Molloy, D.W.; Standish, T.I.; Zhou, Q.; Guyatt, G. DARAD Study Group. A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer’s disease: The DARAD trial. Int. J. Geriatr. Psychiatry 2013, 28, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization an outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- O’Caoimh, R.; Gao, Y.; Gallagher, P.F.; Eustace, J.; McGlade, C.; Molloy, D.W. Which part of the Quick Mild Cognitive Impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 2013, 42, 324–330. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Screening for Mild Cognitive Impairment: Comparison of “MCI Specific” Screening Instruments. J. Alzheimer’s Dis. 2016, 51, 619–629. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Svendrovski, A.; Johnston, B.C.; Gao, Y.; McGlade, C.; Eustace, J.; Timmons, S.; Guyatt, G.; Molloy, D.W. The Quick Mild Cognitive Impairment screen correlated with the Standardized Alzheimer’s Disease Assessment Scale–cognitive section in clinical trials. J. Clin. Epidemiol. 2014, 67, 87–92. [Google Scholar] [CrossRef]
- Bunt, S.; O’Caoimh, R.; Krijnen, W.P.; Molloy, D.W.; Goodijk, G.P.; Van der Schans, C.P.; Hobbelen, H.J.S.M. Validation of the Dutch version of the quick mild cognitive impairment screen (Qmci-D). BMC Geriatr. 2015, 15, 115. [Google Scholar] [CrossRef]
- Yavuz, B.B.; Varan, H.D.; O’Caoimh, R.; Kizilarslanoglu, M.C.; Kilic, M.K.; Molloy, D.W.; Dogrul, R.T.; Karabulut, E.; Svendrovski, A.; Sagir, A.; et al. Validation of the Turkish Version of the Quick Mild Cognitive Impairment Screen. Am. J. Alzheimer’s Dis. 2017, 32, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, A.; Carpinelli Mazzi, M.; Russo, G.; D’Anna, F.; Peluso, S.; Mazzeo, P.; De Luca, V.; De Michele, G.; Iaccarino, G.; Abete, P.; et al. The Italian version of the Quick Mild Cognitive Impairment (Qmci-I) screen: Normative study on 307 healthy subjects. Aging Clin. Exp. Res. 2018, 31, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; O’Caoimh, R.; Murayama, H.; Molloy, D.W.; Inoue, S.; Shobugawa, Y.; Fujiwara, T. Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen. Int. J. Environ. Res. Public Health 2019, 16, 917. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.E. Basic principles of ROC curve analysis. Sem. Nucl. Med. 1979, 8, 283–298. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kvitting, A.S.; Fällman, K.; Wressle, E.; Marcusson, J. Age-Normative MMSE Data for Older Persons Aged 85 to 93 in a Longitudinal Swedish Cohort. J. Am. Geriatr. Soc. 2019, 67, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Biundo, R.; Weis, L.; Bostantjopoulou, S.; Stefanova, E.; Falup-Pecurariu, C.; Kramberger, M.G.; Geurtsen, G.J.; Antonini, A.; Weintraub, D.; Aarsland, D. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: A multicenter 1-year follow-up study. J. Neural Transm. 2016, 123, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gnanalingham, K.K.; Byrne, E.J.; Thornton, A.; Sambrook, M.A.; Bannister, P. Motor and cognitive function in Lewy body dementia: Comparison with Alzheimer’s and Parkinson’s diseases. J. Neur. Neurosurg. Psychiatry 1997, 62, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kida, J.; Nemoto, K.; Ikejima, C.; Bun, S.; Kakuma, T.; Mizukami, K.; Asada, T. Impact of depressive symptoms on conversion from mild cognitive impairment subtypes to Alzheimer’s disease: A community-based longitudinal study. J. Alzheimer’s Dis. 2016, 51, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. MACE versus MoCA: Equivalence or superiority? Pragmatic diagnostic test accuracy study. Int. Psychogeriatr. 2017, 29, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Speed versus accuracy in cognitive assessment when using CSIs. Prog. Neurol. Psychiatry 2015, 19, 21–24. [Google Scholar] [CrossRef]
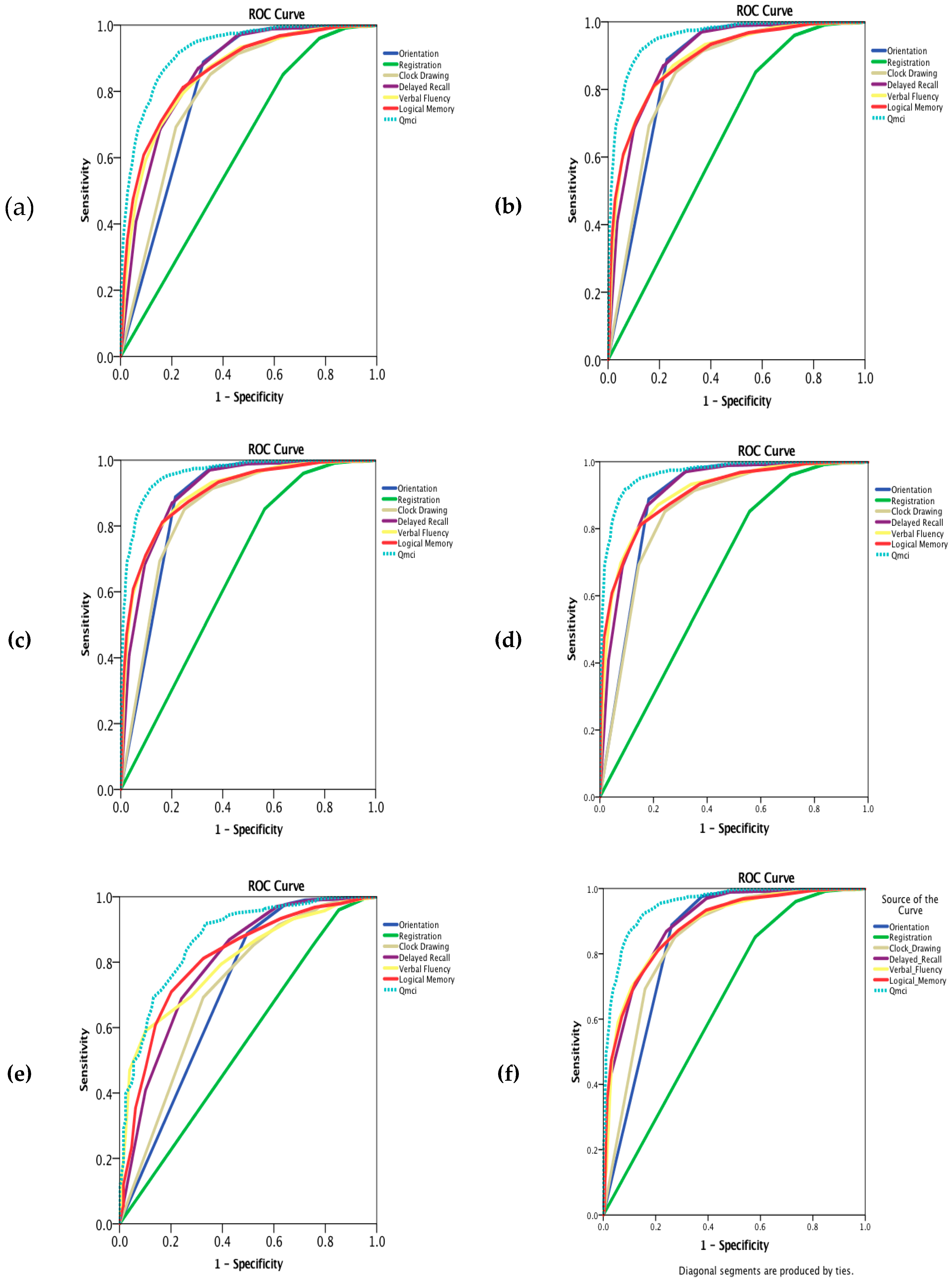
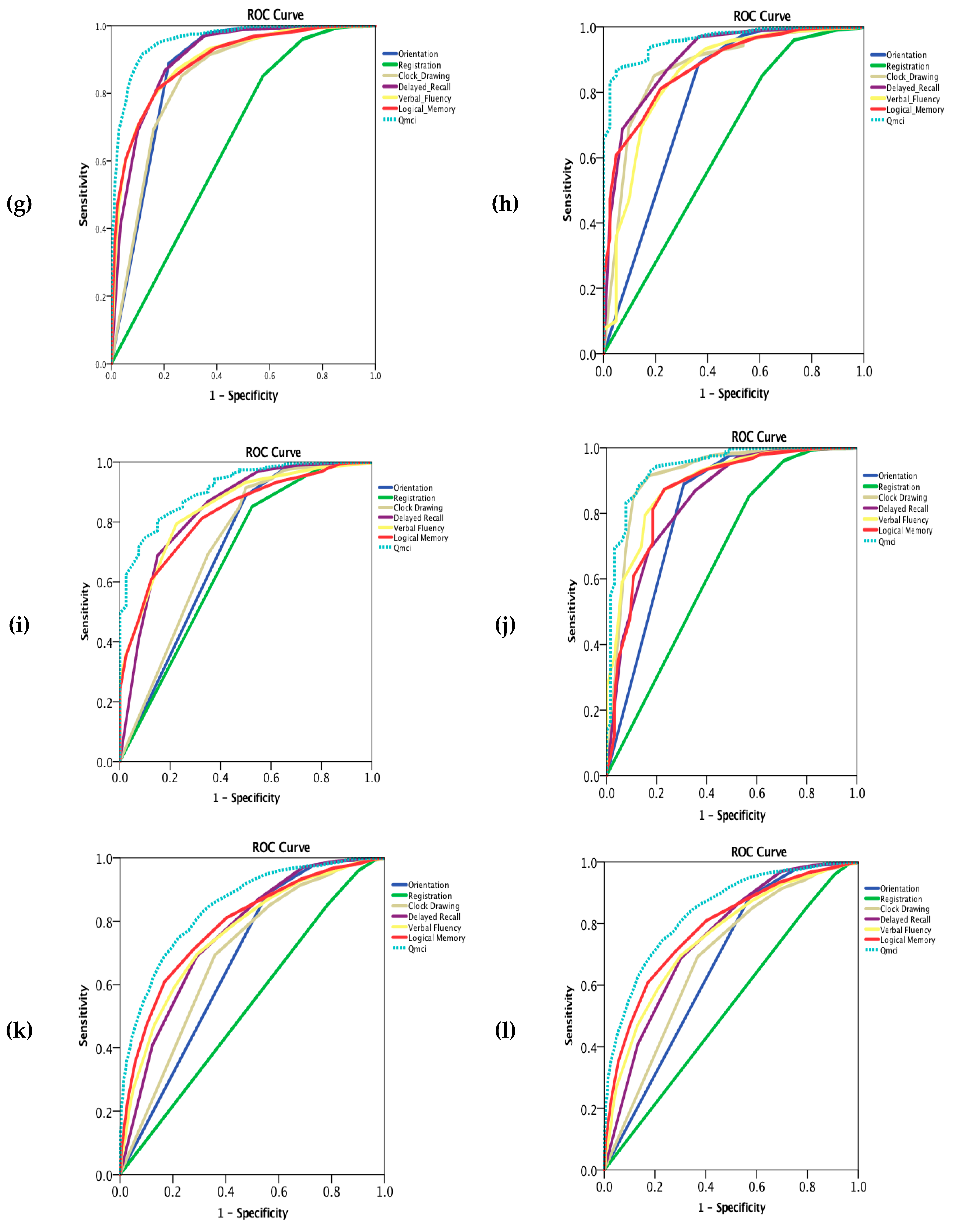
| Diagnosis | N = X | Age (Median & IQR) | Education (Median & IQR) | Gender (% Male) | SMMSE (Median & IQR) | Qmci (Median & IQR) | SMMSE AUC NC v CI (95% Confidence Intervals) | Qmci AUC NC v CI (95% Confidence Intervals) | p = x |
|---|---|---|---|---|---|---|---|---|---|
| Total (All including co-morbid depression & MCI) | 3020 | 77 (81 − 72 = 9) | 12 (14 − 9 = 5) | 1537/2997 * (51%) | 25 (28 − 20 = 8) | 43 (56 − 29 = 27) | 0.87 (0.85–0.88) | 0.93 (0.92–0.94) | z = 11.6 p < 0.001 |
| Dementia (All including co-morbid depression) | 2160 | 77 (82 − 73 = 9) | 12 (14 − 9 = 5) | 1087/2145 * (51%) | 23 (26 − 18 = 8) | 38 (50 − 24 = 26) | 0.92 (0.91–0.93) | 0.96 (0.95–0.96) | z = −8.6 p < 0.001 |
| Dementia (All excluding co-morbid depression) | 1879 | 78 (82 − 74 = 8) | 11 (14 − 9 = 5) | 971/1864 * (52%) | 23 (26 − 18 = 8) | 37 (49 − 24 = 25) | 0.92 (0.91–0.93) | 0.96 (0.95–0.97) | z = −8.3 p < 0.001 |
| AD | 1483 | 78 (83 − 74 = 9) | 12 (14 − 9 = 5) | 651/1475 * (44%) | 23 (25.5 − 18 = 7.5) | 37 (48 − 23 = 25) | 0.94 (0.93–0.95) | 0.97 (0.96–0.97) | z = −5.9 p < 0.001 |
| VaD | 130 | 74 (79 − 69 = 10) | 12 (14 − 10 = 4) | 95 (73%) | 27 (29 − 25 = 4) | 53 (64 − 40 = 24) | 0.74 (0.68–0.79) | 0.87 (0.84–0.91) | z = −6.3 p < 0.001 |
| Mixed (AD/VaD) | 400 | 77 (81 − 74 = 7) | 11 (13 − 9 = 4) | 256/393 * (65%) | 22 (27 − 19 = 8) | 36 (51 − 23 = 28) | 0.91 (0.89–0.93) | 0.95 (0.94–0.96) | z = −5.7 p < 0.001 |
| AD, VaD and Mixed | 2013 | 78 (82 − 74 = 4) | 12 (14 − 9 = 5) | 1002/1998 * (50%) | 23 (26 − 18 = 8) | 38 (50 − 24 = 26) | 0.92 (0.91–0.93) | 0.96 (0.95–0.97) | z = 8.4 p < 0.001 |
| FTD | 41 | 69 (71 − 62 = 9) | 12 (14 − 10 = 4) | 25/41 (61%) | 23 (27 − 19 = 8) | 42 (52 − 28 = 24) | 0.90 (0.85–0.96) | 0.96 (0.94–0.98) | z = −2.5 p = 0.01 |
| PDD | 41 | 75 (77 − 71 = 6) | 12 (12 − 9.5 = 2.5) | 28/41 (68%) | 26 (29 − 21 = 8) | 46 (61 − 32 = 29) | 0.81 (0.72–0.90) | 0.92 (0.88–0.95) | z = −3.1 p = 0.002 |
| LBD | 65 | 78 (82 − 73 = 9) | 10 (14 − 8 = 6) | 32/65 (49%) | 24 (27 − 18 = 9) | 37 (54 − 24 = 30) | 0.94 (0.92–0.97) | 0.94 (0.91–0.97) | z = 0.11 p = 0.91 |
| MCI | 860 | 75 (80 − 70 = 10) | 12 (14 − 10 = 4) | 450/852 * (53%) | 28 (29 − 25 = 4) | 56 (66 − 46 = 20) | 0.73 (0.71–0.75) | 0.85 (0.83–0.87) | z = −10.8 p < 0.001 |
| Co-morbid depression | 281 | 75 (81 − 70 = 11) | 12 (14 − 10 = 4) | 116/281 (41%) | 25 (27 − 21 = 6) | 44 (56 − 30 = 26) | 0.88 (0.86–0.91) | 0.93 (0.91–0.95) | z = −4.2 p < 0.001 |
| MCI with co-morbid depression | 94 | 73 (75 − 68 = 7) | 12 (14 − 9 = 5) | 50/92 * (54%) | 26 (29 − 23 = 6) | 52 (62 − 40 = 22) | 0.79 (0.73–0.85) | 0.90 (0.86–0.93) | z = −4.4 p < 0.001 |
| MCI without co-morbid depression | 766 | 76 (80 − 70 = 10) | 12 (14 − 10 = 4) | 400/760 * (53%) | 28 (29 − 26 = 3) | 57 (66 − 46 = 20) | 0.72 (0.70–0.75) | 0.84 (0.82–0.86) | z = −10.4 p < 0.001 |
| Controls | 875 | 70 (76 − 62 = 14) | 13 (16 − 12 = 4) | 372/875 (43%) | 29 (30 − 28 = 2) | 74 (81 − 66 = 15) | NA | NA | NA |
| Diagnosis | N = X | Orientation (Median and IQR) | Registration (Median and IQR) | Clock Drawing (Median and IQR) | Delayed Recall (Median and IQR) | Verbal Fluency (Median and IQR) | Logical Memory (Median and IQR) | Qmci Total (Median and IQR) |
|---|---|---|---|---|---|---|---|---|
| Total (All) | 3020 | 8 (10 − 6 = 4) | 5 (5 − 4 = 1) | 12 (14 − 5 = 9) | 4 (12 − 0 = 12) | 5 (7 − 3 = 4) | 8 (12 − 4 = 8) | 43 (56 − 29 = 27) |
| Dementia (All including co-morbid depression) | 2160 | 7 (9 − 5 = 4) | 5 (5 − 3 = 2) | 11 (14 − 3 = 11) | 4 (8 − 0 = 8) | 5 (7 − 3 = 4) | 8 (12 − 4 = 8) | 38 (50 − 24 = 26) |
| Dementia (All excluding co-morbid depression) | 1879 | 7 (9 − 5 = 4) | 5 (5 − 3 = 2) | 11 (13 − 3 = 10) | 0 (8 − 0 = 8) | 4 (6 − 3 = 3) | 8 (12 − 4 = 8) | 37 (49 − 24 = 25) |
| AD | 1483 | 7 (9 − 5 = 4) | 5 (5 − 3 = 2) | 11 (13 − 3 = 10) | 0 (8 − 0 = 8) | 4 (6 − 3 = 3) | 8 (12 − 4 = 8) | 37 (48 − 23 = 25) |
| VaD | 130 | 9 (10 − 7 = 3) | 5 (5 − 4 = 1) | 14 (15 − 11 = 4) | 8 (12 − 0 = 12) | 7 (9 − 4 = 5) | 10 (14 − 8 = 6) | 53 (64 − 40 = 24) |
| Mixed (AD/VaD) | 400 | 7 (10 − 5 = 5) | 5 (5 − 3 = 2) | 10 (14 − 2 = 12) | 0 (8 − 0 = 8) | 5 (7 − 3 = 4) | 8 (12 − 4 = 8) | 35.5 (51 − 23 = 28) |
| AD, VaD and Mixed | 2013 | 7 (9 − 5 = 4) | 5 (5 − 3 = 2) | 11 (14 − 3 = 11) | 0 (8 − 0 = 8) | 5 (7 − 3 = 4) | 8 (12 − 4 = 8) | 38 (50 − 24 = 26) |
| FTD | 41 | 9 (10 − 6 = 4) | 5 (5 − 3 = 2) | 12 (13 − 5 = 8) | 4 (8 − 0 = 8) | 4 (7 − 3 = 4) | 8 (12 − 4 = 8) | 43 (55 − 30 = 25) |
| PDD | 41 | 9.5 (10 − 7 = 3) | 5 (5 − 4 = 1) | 12.5 (15 − 7 = 8) | 8 (12 − 0 = 12) | 5.5 (7 − 4 = 3) | 10 (14 − 8 = 6) | 46 (61 − 32 = 29) |
| LBD | 65 | 8 (10 − 6 = 4) | 5 (5 − 3 = 2) | 7 (12 − 1 = 11) | 8 (12 − 0 = 12) | 4 (6 − 3 = 3) | 8 (10 − 4 = 6) | 37 (54 − 24 = 30) |
| MCI | 860 | 10 (10 − 8 = 2) | 5 (5 − 5 = 0) | 14 (15 − 12 = 3) | 12 (16 − 4 = 12) | 7 (9 − 5 = 4) | 12 (16 − 8 = 8) | 56 (66 − 46 = 20) |
| Co-morbid depression | 281 | 8 (10 − 6 = 4) | 5 (5 − 4 = 1) | 11 (14 − 4 = 10) | 4 (12 − 0 = 12) | 6 (8 − 3 = 5) | 10 (14 − 4 = 10) | 44 (56 − 30 = 26) |
| MCI with co-morbid depression | 94 | 9 (10 − 7 = 3) | 5 (5 − 4 = 1) | 13 (15 − 10 = 5) | 8 (12 − 4 = 8) | 8 (8 − 4 = 4) | 12 (14 − 8 = 6) | 51.5 (62 − 40 = 22) |
| MCI without co-morbid depression | 766 | 10 (10 − 8 = 2) | 5 (5 − 5 = 0) | 14 (15 − 12 = 3) | 12 (16 − 4 = 12) | 7 (9 − 5 = 4) | 12 (16 − 8 = 8) | 57 (66 − 46 = 20) |
| Controls | 875 | 10 (10 − 10 = 0) | 5 (5 − 5 = 0) | 15 (15 − 14 = 1) | 16 (20 − 12 = 8) | 10 (13 − 8 = 5) | 18 (22 − 14 = 8) | 74 (81 − 66 = 15) |
| Diagnosis | N = X | Orientation AUC (95% CI) | Registration AUC (95% CI) | Clock Drawing AUC (95% CI) | Delayed Recall AUC (95% CI) | Verbal Fluency AUC (95% CI) | Logical Memory AUC (95% CI) | Qmci Total AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Total (All) | 3020 | 0.81 (0.79–0.82) | 0.62 (0.60–0.64) | 0.80 (0.79–0.82) | 0.86 (0.85–0.87) | 0.85 (0.84–0.87) | 0.87 (0.85–0.88) | 0.93 (0.92–0.94) |
| Dementia (All including co-morbid depression) | 2160 | 0.86 (0.85–0.87) | 0.65 (0.63–0.67) | 0.85 (0.83–0.86) | 0.90 (0.89–0.91) | 0.89 (0.88–0.90) | 0.89 (0.88–0.91) | 0.96 (0.95–0.96) |
| Dementia (All excluding co-morbid depression) | 1879 | 0.87 (0.85–0.88) | 0.66 (0.64–0.68) | 0.85 (0.84–0.87) | 0.90 (0.89–0.92) | 0.90 (0.88–0.91) | 0.90 (0.89–0.91) | 0.96 (0.95–0.97) |
| AD | 1483 | 0.88 (0.87–0.90) | 0.66 (0.64–0.68) | 0.85 (0.84–0.87) | 0.91 (0.90–0.93) | 0.91 (0.89–0.92) | 0.91 (0.89–0.92) | 0.97 (0.96–0.97) |
| VaD | 130 | 0.71 (0.66–0.77) | 0.56 (0.50–0.61) | 0.72 (0.67–0.77) | 0.80 (0.75–0.84) | 0.80 (0.77–0.84) | 0.81 (0.77–0.85) | 0.87 (0.84–0.91) |
| Mixed (AD/VaD) | 400 | 0.84 (0.82–0.87) | 0.65 (0.61–0.68) | 0.85 (0.82–0.87) | 0.90 (0.88–0.92) | 0.89 (0.87–0.91) | 0.89 (0.87–0.91) | 0.95 (0.94–0.96) |
| AD, VaD & Mixed | 2013 | 0.86 (0.85–0.88) | 0.65 (0.63–0.67) | 0.84 (0.83–0.86) | 0.90 (0.89–0.91) | 0.90 (0.88–0.91) | 0.90 (0.88–0.91) | 0.96 (0.95–0.97) |
| FTD | 41 | 0.78 (0.69–0.87) | 0.63 (0.53–0.73) | 0.88 (0.81–0.94) | 0.90 (0.87–0.95) | 0.85 (0.78–0.92) | 0.88 (0.83–0.93) | 0.96 (0.94–0.98) |
| PDD | 41 | 0.71 (0.61–0.81) | 0.68 (0.58–0.77) | 0.73 (0.63–0.82) | 0.84 (0.77–0.91) | 0.85 (0.79–0.91) | 0.83 (0.77–0.88) | 0.92 (0.88–0.95) |
| LBD | 65 | 0.82 (0.74–0.88) | 0.66 (0.58–0.74) | 0.92 (0.87–0.96) | 0.84 (0.79–0.90) | 0.89 (0.85–0.93) | 0.86 (0.81–0.92) | 0.94 (0.91–0.97) |
| MCI | 860 | 0.68 (0.65–0.70) | 0.54 (0.51–0.57) | 0.69 (0.67–0.72) | 0.76 (0.74–0.78) | 0.76 (0.74–0.78) | 0.80 (0.77–0.81) | 0.85 (0.83–0.87) |
| Co-morbid depression | 281 | 0.80 (0.77–0.84) | 0.62 (0.57–0.66) | 0.81 (0.78–0.84) | 0.86 (0.84–0.89) | 0.84 (0.82–0.87) | 0.84 (0.82–0.87) | 0.93 (0.91–0.95) |
| MCI with co-morbid depression | 94 | 0.75 (0.69–0.82) | 0.59 (0.52–0.65) | 0.75 (0.70–0.81) | 0.82 (0.78–0.87) | 0.80 (0.76–0.85) | 0.80 (0.75–0.84) | 0.90 (0.86–0.93) |
| MCI without co-morbid depression | 766 | 0.67 (0.64–0.69) | 0.53 (0.50–0.56) | 0.69 (0.66–0.71) | 0.75 (0.73–0.77) | 0.76 (0.73–0.78) | 0.80 (0.76–0.81) | 0.84 (0.82–0.86) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Caoimh, R.; Molloy, D.W. Comparing the Diagnostic Accuracy of Two Cognitive Screening Instruments in Different Dementia Subtypes and Clinical Depression. Diagnostics 2019, 9, 93. https://doi.org/10.3390/diagnostics9030093
O’Caoimh R, Molloy DW. Comparing the Diagnostic Accuracy of Two Cognitive Screening Instruments in Different Dementia Subtypes and Clinical Depression. Diagnostics. 2019; 9(3):93. https://doi.org/10.3390/diagnostics9030093
Chicago/Turabian StyleO’Caoimh, Rónán, and D. William Molloy. 2019. "Comparing the Diagnostic Accuracy of Two Cognitive Screening Instruments in Different Dementia Subtypes and Clinical Depression" Diagnostics 9, no. 3: 93. https://doi.org/10.3390/diagnostics9030093
APA StyleO’Caoimh, R., & Molloy, D. W. (2019). Comparing the Diagnostic Accuracy of Two Cognitive Screening Instruments in Different Dementia Subtypes and Clinical Depression. Diagnostics, 9(3), 93. https://doi.org/10.3390/diagnostics9030093






