Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers
Abstract
:1. Introduction
2. PET-MRI Scanner
3. PET-MRI Imaging Protocols
4. Esophageal Cancer
5. Stomach Cancer
6. Colorectal Cancer
7. Pancreatic Cancer
8. Conclusions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2016; American Cancer Society: Atlanta, GA, USA, 2016. [Google Scholar]
- Kuehl, K.; Veit, P.; Rosenbaum, S.J.; Bockisch, A.; Antoch, G. Can PET/CT replace separate diagnostic CT for cancer imaging? Optimizing CT protocols for imaging cancer of the chest and abdomen. J. Nucl. Med. 2007, 48, 45S–57S. [Google Scholar] [PubMed]
- Ong, L.C.; Jin, Y.; Song, I.C.; Yu, S.; Zhang, K.; Chow, P.K. 2-[18F]-2-deoxy-d-glucose (FDG) uptake in human tumor cells is related to the expression of GLUT-1 and hexokinase II. Acta Radiol. 2008, 49, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Gorospe, L.; Raman, S.; Echeveste, J.; Avril, N.; Herrero, Y.; Herna Ndez, S. Whole-body PET/CT: Spectrum of physiological variants, artifacts and interpretative pitfalls in cancer patients. Nucl. Med. Commun. 2005, 26, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Fletcher, J.W. PET/CT standardized uptake values (SUVs) in clinical practice and assessing response to therapy. Semin. Ultrasound CT MRI 2010, 31, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Even-Sapir, E. Increased 18F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): Accumulated data from four years of experience with PET/CT. Semin. Nucl. Med. 2007, 37, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, L.K. Use of PET/CT scanning cancer patients: Technical and practical considerations. Proceedings 2005, 18, 321–330. [Google Scholar] [PubMed]
- Kelloff, G.; Hoffman, J.M.; Johnson, B.; Scher, H.I.; Siegel, B.A.; Cheng, E.Y.; Cheson, B.D.; O’shaughnessy, J.; Guyton, K.Z.; Mankoff, D.A.; et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin. Cancer Res. 2005, 11, 2785–2808. [Google Scholar] [CrossRef] [PubMed]
- Antoch, G.; Stattaus, J.; Nemat, A.T.; Marnitz, S.; Beyer, T.; Kuehl, H.; Bockisch, A.; Debatin, J.F.; Freudenberg, L.S. Non-small cell lung cancer: Dual modality PET/CT in preoperative staging. Radiology 2003, 229, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.A.; Gu, M.; Gould, E.; Teng, M.; Baker, K.; Matthews, R. Utility of positron emission tomography-magnetic resonance imaging in musculoskeletal imaging. World J. Radiol. 2016, 8, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Friedman, K.; Chandarana, H.; Melsaether, A.; Moy, L.; Ding, Y.S.; Jhaveri, K.; Beltran, L.; Jain, R. Current status of hybrid PET/MRI in oncologic imaging. Am. J. Roentgenol. 2016, 296, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Heusch, O.; Nensa, F.; Schaarschmidt, B.; Beiderwellen, K.; Gomez, B.; Köhler, J.; Reis, H.; Ruhlmann, V.; Buchbender, C. Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.M.; Grueneisen, J.; Schaarschmidt, B.M.; Buchbender, C.; Nagarajah, J.; Umutlu, L.; Antoch, G.; Kinner, S. Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur. J. Radiol. 2016, 85, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.C.; Paldino, M.J.; Dodge, C.; Krishnamurthy, R.; Krishnamurthy, R.; Rohren, E.M. Assessment of sequential PET/MRI in comparison with PET/CT of pediatric lymphoma: A prospective study. Am. J. Roentgenol. 2016, 26, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Ma, Q.; Sun, H.; Zhang, S.; Liu, C.; Zhai, W. PET/MRI with diagnostic MR sequences vs PET/CT in the detection of abdominal and pelvic cancer. Eur. J. Radiol. 2016, 85, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Fde, G.; von Schulthess, G.; Veit-Haibach, P. Workflow in simultaneous PET/MRI. Semin. Nucl. Med. 2015, 45, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, P.S.; van Hillegersberg, R.; Lever, F.M.; Lips, I.M.; van Lier, A.L.; Meijer, G.J.; van Leeuwen, M.S.; van Vulpen, M.; Ruurda, J.P. Imaging strategies in the management of oesophageal cancer: What’s the role of MRI? Eur. Radiol. 2013, 23, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, P.S.; van Lier, A.L.; Lips, I.M.; Meijer, G.J.; Reerink, O.; van Vulpen, M.; Lam, M.G.; van Hillegersberg, R.; Ruurda, J.P. Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clin. Radiol. 2015, 70, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; I, H.; Kim, S.J.; Jeong, Y.J.; Kim, I.J.; Pak, K.; Park, D.Y.; Kim, H. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: Comparison with PET/CT, endoscopic ultrasound, and CT. J. Nucl. Med. 2014, 55, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Shuto, K.; Okazumi, S.; Shimada, H.; Kazama, T.; Matsubara, H. Apparent diffusion coefficient values measured by diffusion-weighted imaging predicts chemoradiotherapeutic effect for advanced esophageal cancer. Dig. Surg. 2011, 28, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.S.; Triantafyllou, M.; Triantafyllou, K.; Rosch, T. EUS in the management of gastric cancer. Ann. Gastroenterol. 2011, 24, 9–15. [Google Scholar] [PubMed]
- Lim, J.S.; Yun, M.J.; Kim, M.J.; Hyung, W.J.; Park, M.S.; Choi, J.Y.; Kim, T.S.; Lee, J.D.; Noh, S.H.; Kim, K.W. CT and PET in stomach cancer: Preoperative staging and monitoring of response to therapy. Radiographics 2006, 26, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Lim, J.D.; Noh, S.H.; Hyung, W.J.; Cheong, J.H.; Bong, J.K.; Cho, A.; Lee, J.D. Lymph node staging of gastric cancer using 18F-FDG PET: A comparison study with CT. J. Nucl. Med. 2005, 10, 1582–1588. [Google Scholar]
- Huang, Z.; Xie, D.H.; Guo, L.; Hu, H.; Fang, X.; Meng, Q.; Ping, X.X.; Lu, Z.W. The utility of MRI for pre-operative T and N staging of gastric carcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20140552. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; De Cobelli, F.; Canevari, C.; Gallivanone, F.; Esposito, A.; Castiglioni, I.; Ambrosi, A.; Albarello, L.; Mazza, E.; et al. Response of chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and 18F-FDG-PET/CT: Correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value in histological tumor regression grade. J. Magn. Reson. Imaging 2014, 40, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Zieker, D.; Konigsrainer, I.; Weinreich, J.; Bechert, S.; Glatzle, J.; Nieselt, K.; Bühler, S.; Löffler, M.; Gaedcke, J.; Northoff, H.; et al. Phosphoglycerate kinase 1 promoting tumor progression and metastasis in gastric cancer—Detected in a tumor mouse model using positron emission tomography/magnetic resonance imaging. Cell. Physiol. Biochem. 2010, 26, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, S.H.; Im, S.A.; Oh, D.Y.; Kim, Y.T.; Han, J.K. Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: A preliminary study. Eur. Radiol. 2016, 26, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Vitola, J.V.; Sandler, M.P.; Arildsen, R.C.; Powers, T.A.; Wright, J.K., Jr.; Chapman, W.C.; Pinson, C.W. Staging of recurrent metastatic colorectal carcinoma with PET. J. Nucl. Med. 1997, 38, 1196–1201. [Google Scholar] [PubMed]
- Mainenti, P.P.; Romano, F.; Pizzuti, L.; Segreto, S.; Storto, G.; Mannelli, L.; Imbriaco, M.; Camera, L.; Maurea, S. Non-invasive diagnostic imaging of colorectal liver metastases. World J. Radiol. 2015, 7, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Martin, D.R.; Saini, S. Imaging of liver metastases: MRI. Cancer Imaging 2007, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Oh, S.N.; Yeo, D.M.; Kang, W.K.; Jung, C.K.; Kim, S.W.; Park, M.Y. Computed tomography and magnetic resonance imaging evaluation of lymph node metastasis in early colorectal cancer. World J. Gastroenterol. 2015, 21, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Lee, J.M.; Song, Y.S.; Woo, S.; Hur, B.Y.; Jeon, J.H.; Paeng, J.C. Added value of integrated whole-body PET/MRI for evaluation of colorectal cancer: Comparison with contrast-enhanced MDCT. Am. J. Roentgenol. 2016, 1, W10–W20. [Google Scholar] [CrossRef] [PubMed]
- Paspulati, R.M.; Partovi, S.; Herrmann, K.A.; Krishnamurthi, S.; Delaney, C.P.; Nguyen, N.C. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: A pilot study. Abdom. Imaging 2015, 40, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Chen, Y.; Fishman, E.K. Evolution of imaging in rectal cancer: Multimodality imaging with MDCT, MRI, and PET. J. Gastrointest. Oncol. 2015, 6, 172–184. [Google Scholar] [PubMed]
- Jha, P.; Bijan, B.; Melendres, G.; Shelton, D.K. Hybrid imaging for pancreatic malignancy: Clinical applications, merits, limitations, and pitfalls. Clin. Nucl. Med. 2015, 40, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Mergo, P.J.; Helmberger, T.K.; Buetow, P.C.; Helmberger, R.C.; Ros, P.R. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics 1997, 17, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, S.; Nishii, R.; Wakamatsu, H.; Mizutani, Y.; Kiyohara, S.; Fujita, S.; Futami, S.; Sakae, T.; Furukoji, E.; Tamura, S.; et al. The usefulness of 18F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: Comparison with 18F-FDG PET/CT. Ann. Nucl. Med. 2013, 27, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Isohashi, K.; Onishi, H.; Hori, M.; Kim, T.; Higuchi, I.; Inoue, A.; Shimosegawa, E.; Takeda, Y.; Hatazawa, J. 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: Comparison to PET/CT. Int. J. Clin. Oncol. 2011, 16, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Tien, Y.W.; Chang, M.C.; Cheng, M.F.; Chang, Y.T.; Wu, C.H.; Chen, X.J.; Kuo, T.C.; Yang, S.H.; Shih, I.L. PET/MRI in pancreatic and periampullary cancer: Correlating diffusion-weighted imaging, MR spectroscopy and glucose metabolic activity with clinical stage and prognosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
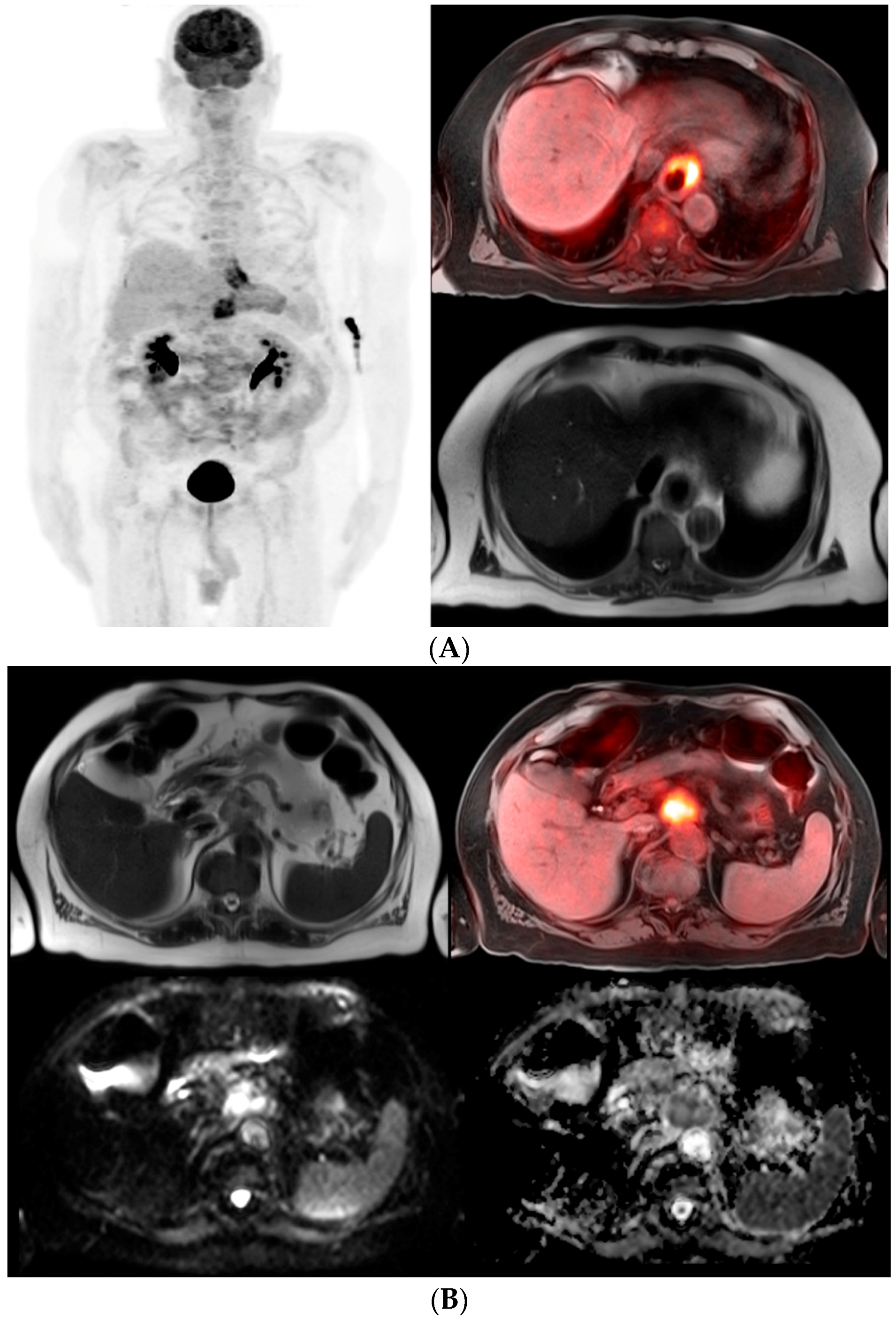
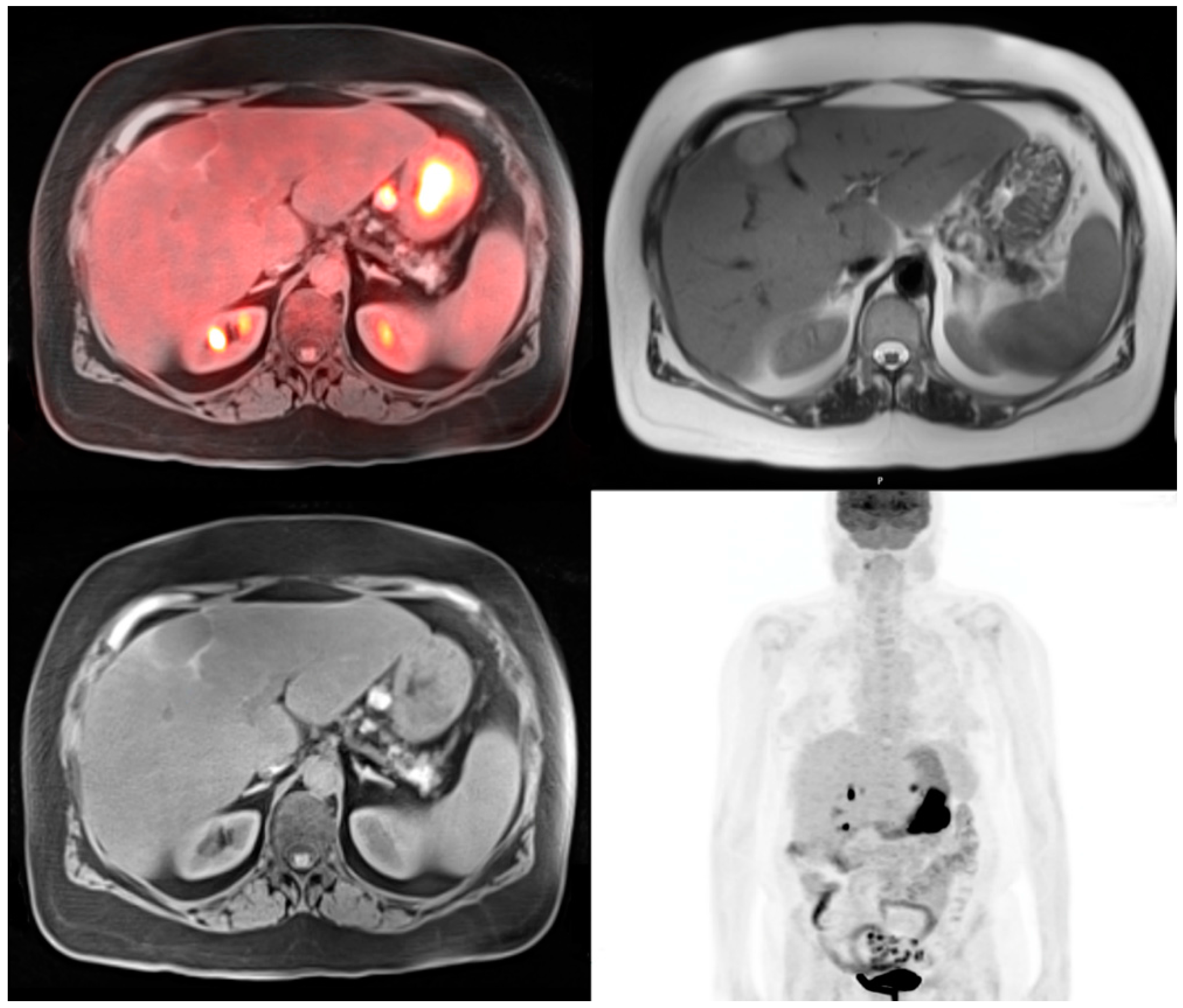

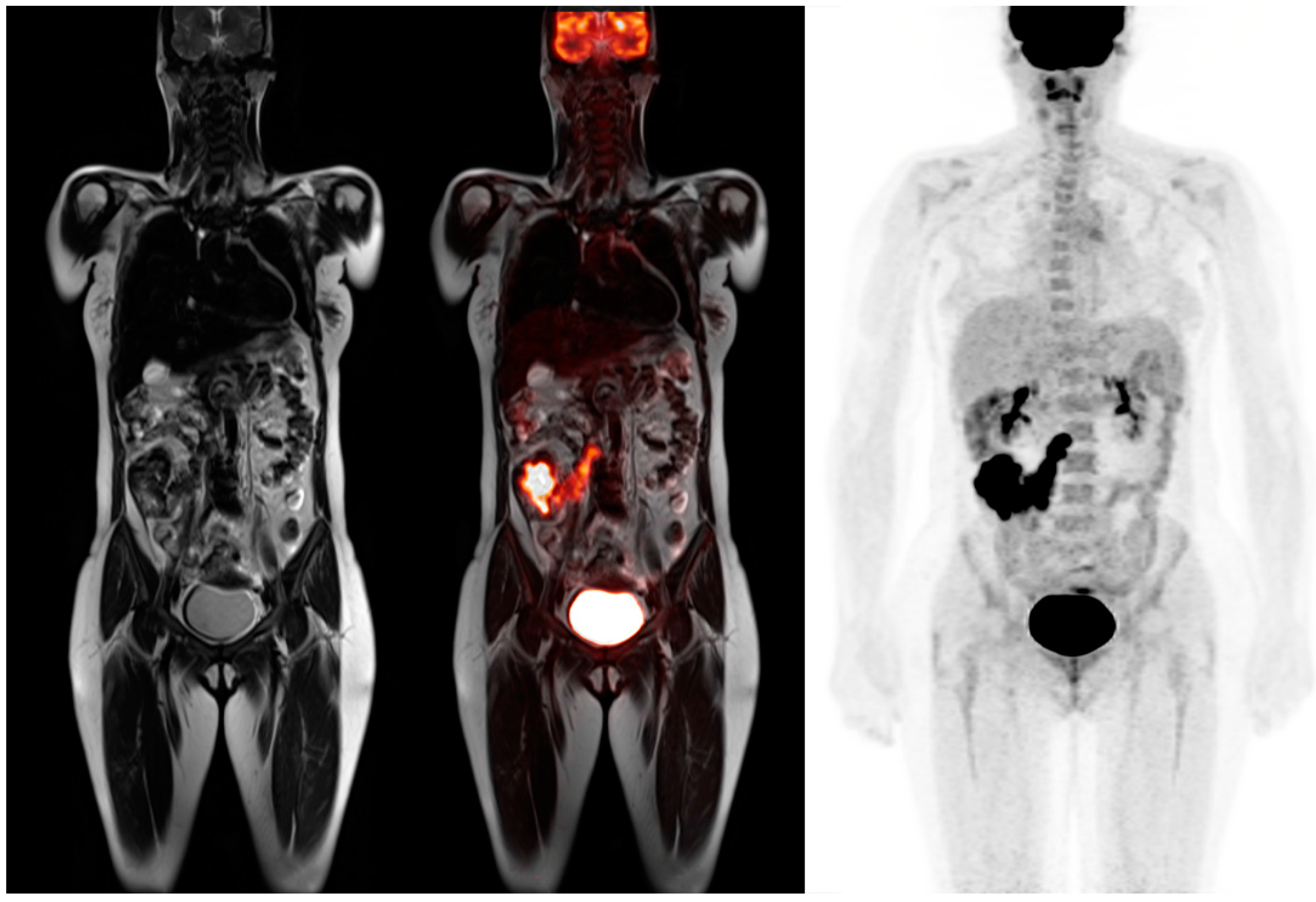
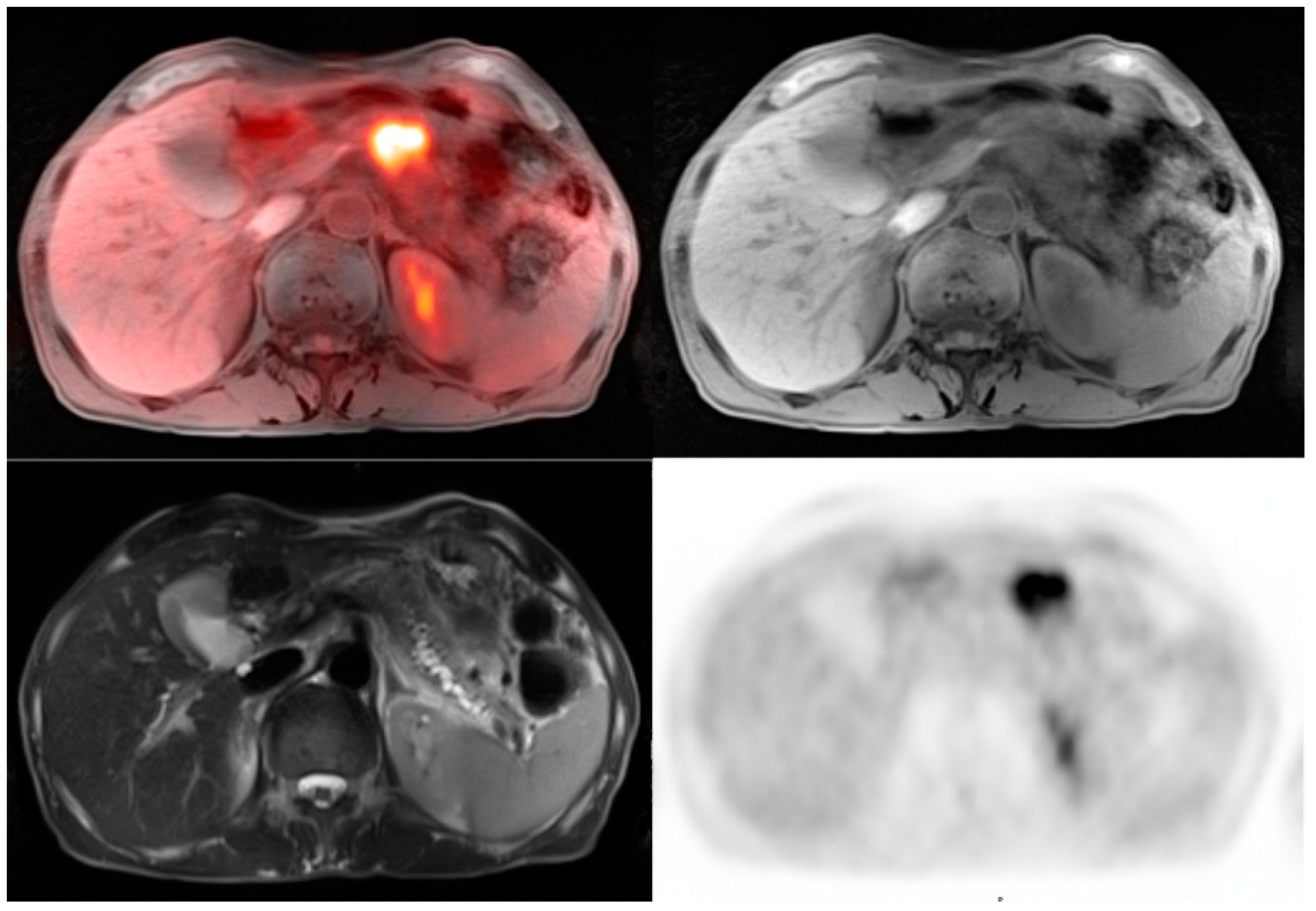
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, R.; Choi, M. Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers. Diagnostics 2016, 6, 35. https://doi.org/10.3390/diagnostics6030035
Matthews R, Choi M. Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers. Diagnostics. 2016; 6(3):35. https://doi.org/10.3390/diagnostics6030035
Chicago/Turabian StyleMatthews, Robert, and Minsig Choi. 2016. "Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers" Diagnostics 6, no. 3: 35. https://doi.org/10.3390/diagnostics6030035
APA StyleMatthews, R., & Choi, M. (2016). Clinical Utility of Positron Emission Tomography Magnetic Resonance Imaging (PET-MRI) in Gastrointestinal Cancers. Diagnostics, 6(3), 35. https://doi.org/10.3390/diagnostics6030035





