First Clinical Experiences with the Ultra-Fast Time-of-Flight BIOGRAPH One Next-Generation Hybrid PET/MRI System
Abstract
1. Introduction
2. Materialsand Methods
2.1. Image Reading
2.2. Image Analysis
2.3. Statistics
3. Results
3.1. Image Reading
3.2. Image Metrics
4. Discussion
4.1. PET Image Quality
4.2. PET Quantification
4.3. Brain Applications
4.4. Non-Brain [18F]FDG Oncology Applications
4.5. MRI
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delso, G.; Furst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Drzezga, A.; Souvatzoglou, M.; Eiber, M.; Beer, A.J.; Furst, S.; Martinez-Moller, A.; Nekolla, S.G.; Ziegler, S.; Ganter, C.; Rummeny, E.J.; et al. First clinical experience with integrated whole-body PET/MR: Comparison to PET/CT in patients with oncologic diagnoses. J. Nucl. Med. 2012, 53, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Singnurkar, A.; Poon, R.; Metser, U. Head-to-Head Comparison of the Diagnostic Performance of FDG PET/CT and FDG PET/MRI in Patients with Cancer: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2024, 223, e2431519. [Google Scholar] [CrossRef]
- Mirshahvalad, S.A.; Kohan, A.; Metser, U.; Hinzpeter, R.; Ortega, C.; Farag, A.; Veit-Haibach, P. Diagnostic performance of whole-body [18F]FDG PET/MR in cancer M staging: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 673–685. [Google Scholar] [CrossRef]
- Virarkar, M.; Ganeshan, D.; Devine, C.; Bassett, R., Jr.; Kuchana, V.; Bhosale, P. Diagnostic value of PET/CT versus PET/MRI in gynecological malignancies of the pelvis: A meta-analysis. Clin. Imaging 2020, 60, 53–61. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Abdlkadir, A.; Herrmann, K.; Bomanji, J.; Jadvar, H.; Shi, H.; Mansour, A.; Paez, D.; Chiti, A.; Scott, A.M. Diagnostic Accuracy of [18F]FDG PET/MRI in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2024, 65, 1533–1539. [Google Scholar] [CrossRef]
- Ling, S.W.; de Jong, A.C.; Schoots, I.G.; Nasserinejad, K.; Busstra, M.B.; van der Veldt, A.A.M.; Brabander, T. Comparison of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2021, 33, 61–71. [Google Scholar] [CrossRef]
- Shen, F.; Liu, Q.; Wang, Y.; Chen, C.; Ma, H. Comparison of [18F] FDG PET/CT and [18F]FDG PET/MRI in the Detection of Distant Metastases in Breast Cancer: A Meta-Analysis. Clin. Breast Cancer 2025, 25, e113–e123.e4. [Google Scholar] [CrossRef]
- Mirshahvalad, S.A.; Metser, U.; Basso Dias, A.; Ortega, C.; Yeung, J.; Veit-Haibach, P. 18F-FDG PET/MRI in Detection of Pulmonary Malignancies: A Systematic Review and Meta-Analysis. Radiology 2023, 307, e221598. [Google Scholar] [CrossRef]
- Tordjman, M.; Yuce, M.; Geahchan, A.; Petralia, G.; Delgado Bolton, R.C.; Wang, K.; Doshi, A.H.; Bolger, I.; Mei, X.; Dercle, L.; et al. Comparison of MRI, [18F]FDG-PET/CT, and [18F]FDG-PET/MRI for Initial Staging of Multiple Myeloma: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2025, 50, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Veit-Haibach, P.; Ahlstrom, H.; Boellaard, R.; Delgado Bolton, R.C.; Hesse, S.; Hope, T.; Huellner, M.W.; Iagaru, A.; Johnson, G.B.; Kjaer, A.; et al. International EANM-SNMMI-ISMRM consensus recommendation for PET/MRI in oncology. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3513–3537. [Google Scholar] [CrossRef]
- Guedj, E.; Varrone, A.; Boellaard, R.; Albert, N.L.; Barthel, H.; van Berckel, B.; Brendel, M.; Cecchin, D.; Ekmekcioglu, O.; Garibotto, V.; et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 632–651. [Google Scholar] [CrossRef]
- Traub-Weidinger, T.; Arbizu, J.; Barthel, H.; Boellaard, R.; Borgwardt, L.; Brendel, M.; Cecchin, D.; Chassoux, F.; Fraioli, F.; Garibotto, V.; et al. EANM practice guidelines for an appropriate use of PET and SPECT for patients with epilepsy. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1891–1908. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Prakken, N.H.J.; Besson, F.L.; Borra, R.J.H.; Buther, F.; Buechel, R.R.; Catana, C.; Chiti, A.; Dierckx, R.; Dweck, M.R.; Erba, P.A.; et al. PET/MRI in practice: A clinical centre survey endorsed by the European Association of Nuclear Medicine (EANM) and the EANM Forschungs GmbH (EARL). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2927–2934. [Google Scholar] [CrossRef]
- Cabello, J.; Jurkiewicz, M.T.; Andrade, A.; Benzinger, T.L.S.; An, H.; Anazodo, U.C. Evaluation of an MRI-Guided PET Image Reconstruction Approach With Adaptive Penalization Strength. IEEE Trans. Radiat. Plasma Med. Sci. 2024, 8, 277–286. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Hansen, A.E.; Henriksen, O.M.; Bruun, F.J.; Eikenes, L.; Oen, S.K.; Karlberg, A.; Hojgaard, L.; Law, I.; Andersen, F.L. AI-driven attenuation correction for brain PET/MRI: Clinical evaluation of a dementia cohort and importance of the training group size. Neuroimage 2020, 222, 117221. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, C.N.; Andersen, F.L.; Andersen, T.L.; Anderberg, L.; Engkebolle, C.; Madsen, K.; Hojgaard, L.; Henriksen, O.M.; Law, I. DeepDixon synthetic CT for [18F]FET PET/MRI attenuation correction of post-surgery glioma patients with metal implants. Front. Neurosci. 2023, 17, 1142383. [Google Scholar] [CrossRef]
- Langen, K.J.; Galldiks, N.; Mauler, J.; Kocher, M.; Filss, C.P.; Stoffels, G.; Regio Brambilla, C.; Stegmayr, C.; Willuweit, A.; Worthoff, W.A.; et al. Hybrid PET/MRI in Cerebral Glioma: Current Status and Perspectives. Cancers 2023, 15, 3577. [Google Scholar] [CrossRef]
- Vestergaard, M.B.; Calvo, O.P.; Hansen, A.E.; Rosenbaum, S.; Larsson, H.B.W.; Henriksen, O.M.; Law, I. Validation of kinetic modeling of [15O]H2O PET using an image derived input function on hybrid PET/MRI. Neuroimage 2021, 233, 117950. [Google Scholar] [CrossRef]
- Fan, A.P.; Khalighi, M.M.; Guo, J.; Ishii, Y.; Rosenberg, J.; Wardak, M.; Park, J.H.; Shen, B.; Holley, D.; Gandhi, H.; et al. Identifying Hypoperfusion in Moyamoya Disease with Arterial Spin Labeling and an [15O]-Water Positron Emission Tomography/Magnetic Resonance Imaging Normative Database. Stroke 2019, 50, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, P.; Vangel, M.G.; Lahoud, R.M.; Furtado, F.S.; Rosen, B.R.; Groshar, D.; Canamaque, L.G.; Umutlu, L.; Zhang, E.W.; Mahmood, U.; et al. PET/MRI assessment of lung nodules in primary abdominal malignancies: Sensitivity and outcome analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
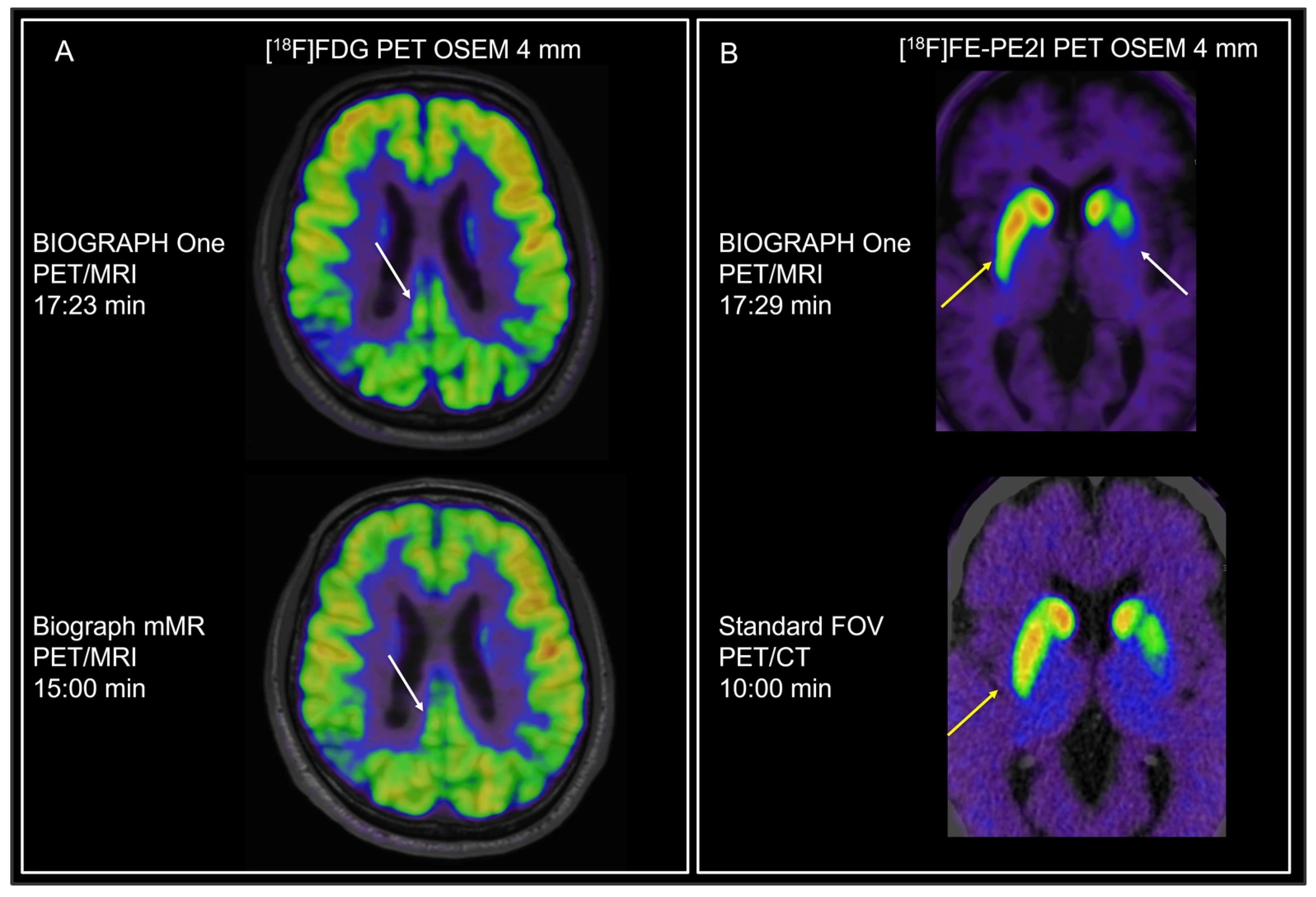

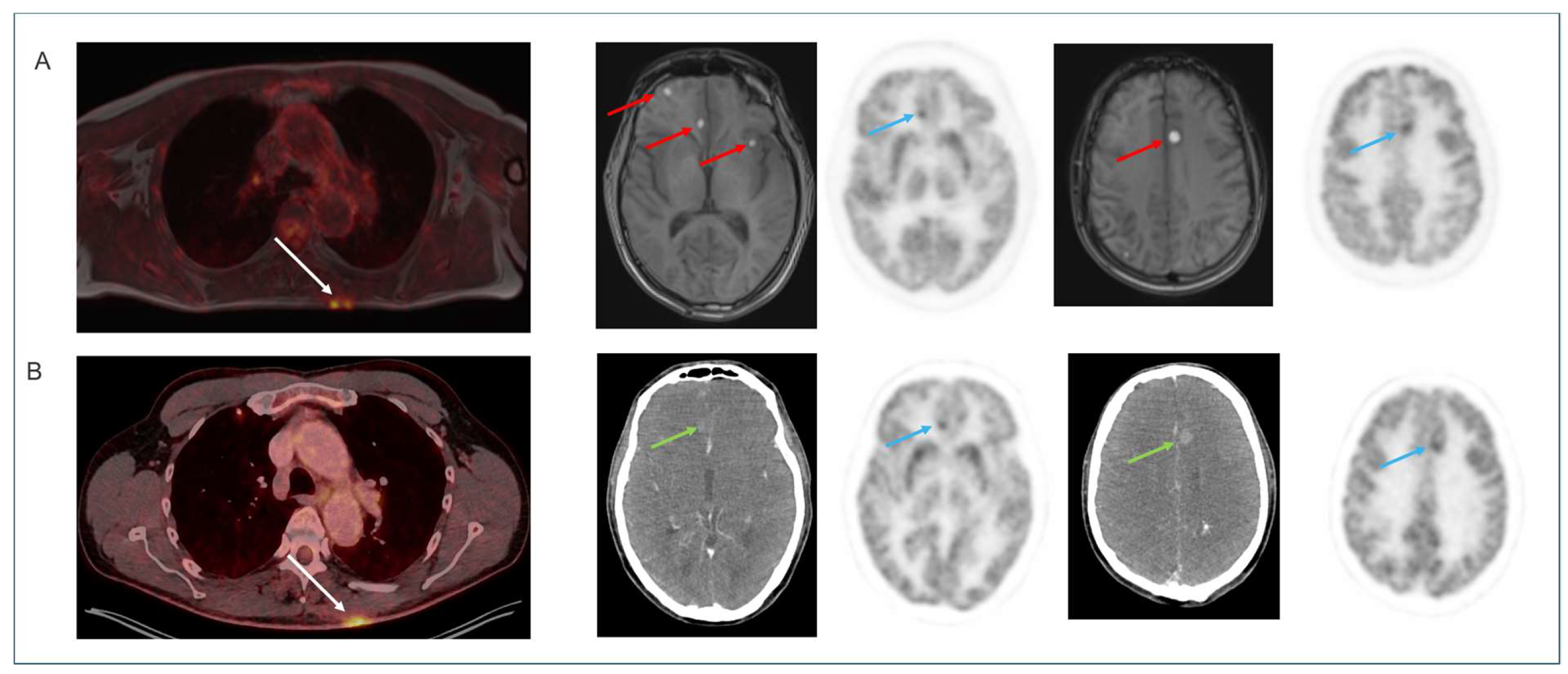
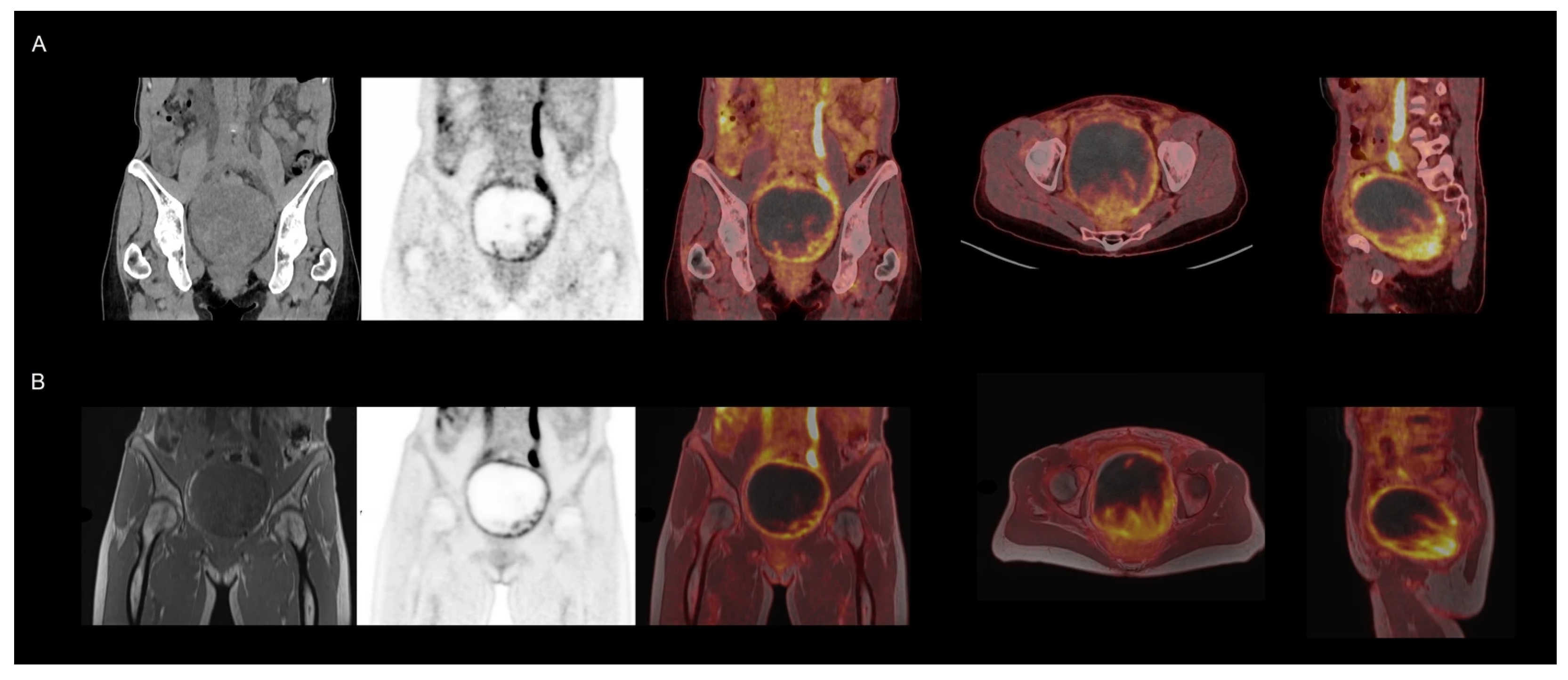



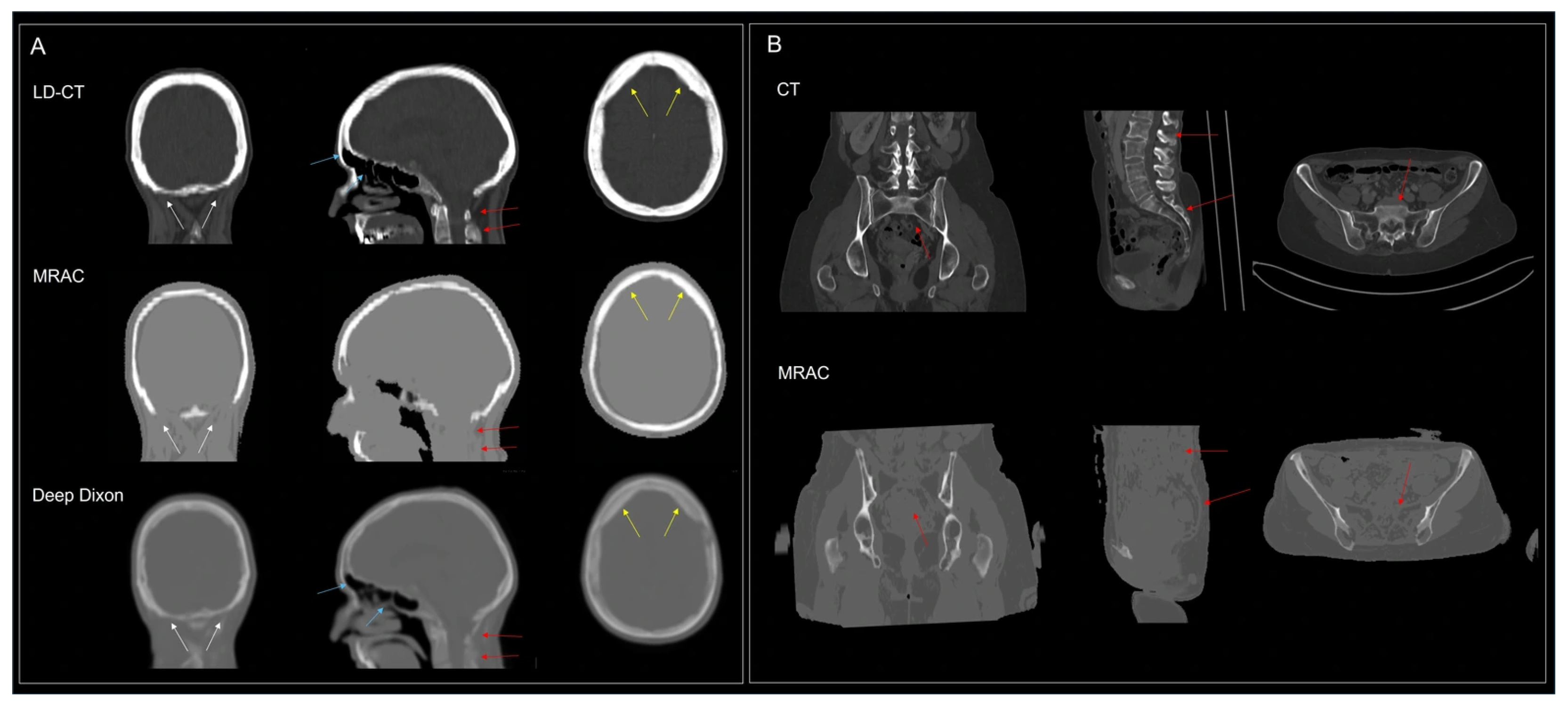
| Protocol | Tracer | Patients, n | Indication |
|---|---|---|---|
| Multi -bed | |||
| Whole body, single pass a | [18F]FDG | 5 | Lymphoma or sarcoma |
| Whole body, multi pass b | [18F]FDG | 5 | Lymphoma or sarcoma |
| Total body (vertex to toe) | [18F]FDG | 5 | Melanoma, sarcoidosis, or sarcoma |
| Single-bed | |||
| Head/neck | [18F]FDG | 5 | Head and neck squamous-cell carcinoma |
| Female pelvis | [18F]FDG | 10 | Cervical and ovarian cancer |
| Brain neuro | [18F]FDG | 5 | Dementia |
| Brain neuro | [18F]FE-PE2I | 10 | Parkinson’s disease/Parkinsonism |
| Brain onco | [18F]FET | 4 | Glioma or pituitary adenoma |
| Brain onco | [68Ga]Ga-DOTATOC | 10 | Meningioma |
| Clinical Scan Protocol | Activity | Standard FOV PET/CT | LAFOV PET/CT | mMR PET/MRI | BIOGRAPH One PET/MRI |
|---|---|---|---|---|---|
| Scan Start | |||||
| Whole body [18F]FDG | 3 MBq/kg 60 min p.i. | 1.5 mm/s a PSF + TOF 2 mm Gauss 4 iteration/5 subsets | 5 min PSF + TOF 2 mm Gauss 4 iteration/5 subsets | - | WB/TB: 3:00/3:03 min b Head/neck: 4:00/20:07 Female pelvis: 4:00/23:02 PSF + TOF 2 mm Gauss 3 iteration/5 subsets |
| Brain neuro [18F]FDG | 200 MBq 40 min p.i. | OSEM + TOF 4 mm Gauss 12 iterations/5 subsets | - | 15 min OSEM 4 mm Gauss 4 iterations /21 subsets | 10:00/17:26 min b OSEM + TOF 4 mm Gauss 12 iterations/5 subsets |
| Brain neuro [18F]FE-PE2I | 200 MBq 30 min p.i. | 10 min OSEM + TOF 4 mm Gauss 12 iterations/5 subsets | - | 10 min PSF 4 mm Gauss 4 iterations/21 subsets | 10:00/18:07 min b OSEM + TOF 4 mm Gauss 12 iterations/5 subsets |
| Brain onco [18F]FET | 200 MBq 20 min p.i. | 20 min OSEM + TOF 2 mm Gauss 8 iterations/5 subsets | 20 min OSEM + TOF 5.3 mm Gauss 4 iterations/5 subsets | 20 min OSEM 5 mm Gauss 4 iterations/21 subsets | 7:00/8:19 min b OSEM + TOF 5 mm Gauss 3 iterations/5 subsets |
| Brain onco [68Ga]Ga-DOTATOC | 100 MBq 50 min p.i. | 10 min PSF + TOF No post filter 12 iterations/5 subsets | - | - | 7:00/8:14 min b PSF + TOF No post filter 8 iterations/5 subsets |
| Protocol | Delay (min) a | Median Exam Duration (min) b | Median [IQR] PET Quality Score c | Median [IQR] MRI Quality Score c | Median [IQR] Diagnostic Score d |
|---|---|---|---|---|---|
| Multi-bed ([18F]FDG) | |||||
| Whole body, single pass c | 41:30 | 20:28 | 1.3 [1.1;1.3] | 2.9 [2.8;3] | 2.5 [2;3] |
| Whole body, multi-pass d | 33:08 | 27:29 | 1.6 [1,4;1.8] | 2.9 [2.9;3] | 2.5 [2;2.5] |
| Total body (vertex to toe) | 28:40 | 35:32 | 1.6 [1.6;2.0] | 2.8 [2.8;3] | 2.5 [2;2.5] |
| Single-bed | |||||
| Head/neck ([18F]FDG) | 27:40 | 28:22 | 1.9 [1.8;2.0] | 2.8 [2.7;2.9] | 2 [2;2] |
| Female pelvis ([18F]FDG) | 29:17 | 28:38 | 1.6 [1.5;1.9] | 2.9 [2.5;2.9] | 2.5 [2;3] |
| Brain neuro ([18F]FDG) | 34.20 | 22:49 | 1.6 [1.6;1.6] | 2.9 [2.7;2.9] | 3 [2.5;3] |
| Brain neuro ([18F]PE2I) | 40:53 | 24:02 | 1.7 [1.6;1.8] | 2.6 [2.6;2.8] | 3 [3;3] |
| Brain onco ([18F]FET) | 41:55 | 12:52 | 1.6 [1.6;1.7] | 2.2 [2.1;2.2] | 3 [3;3] |
| Brain onco ([68Ga]Ga-DOTATOC) | 28:41 | 11:47 | 2.0 [1.9;2.3] | 2.7 [2.6;2.8] | 3 [3;3] |
| BIOGRAPH One | Biograph mMR | Standard FOV PET/CT | LAFOV PET/CT | |
|---|---|---|---|---|
| Brain | ||||
| COV WM (%) | 9.6 [9;11.5] | 11.7 [10.9;16.0] | 10.7 | - |
| SUVr Frontal | 1.22 [1.19;1.38] | 1.06 [0.96;1.20] | 1.21 | - |
| SUVr Occipital | 1.34 [1.18;1.45] | 1.13 [1.08;1.41] | 1.26 | - |
| SUVr Striate | 1.38 [1.24;1.49] | 1.22 [1.10;1.36] | 1.28 | - |
| Body | ||||
| COV liver (%) | 17.2 [16.5;17.3] | - | 15.5 [13.2;16.2] | 8.1 [7.3;8.8] ‡ |
| SUVpeak [a.u.] | 9.51 [6.81;11.73] | - | 7.54 [7.42;9.22] | 10.6 [7.25;10.95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Henriksen, O.M.; Korsholm, K.; Loft, A.; Hall, J.M.; Langkilde, A.R.; Larsen, V.A.; Kristensen, T.S.; Ewertsen, C.; Høi-Hansen, F.E.; Lehmann, P.M.; et al. First Clinical Experiences with the Ultra-Fast Time-of-Flight BIOGRAPH One Next-Generation Hybrid PET/MRI System. Diagnostics 2026, 16, 398. https://doi.org/10.3390/diagnostics16030398
Henriksen OM, Korsholm K, Loft A, Hall JM, Langkilde AR, Larsen VA, Kristensen TS, Ewertsen C, Høi-Hansen FE, Lehmann PM, et al. First Clinical Experiences with the Ultra-Fast Time-of-Flight BIOGRAPH One Next-Generation Hybrid PET/MRI System. Diagnostics. 2026; 16(3):398. https://doi.org/10.3390/diagnostics16030398
Chicago/Turabian StyleHenriksen, Otto M., Kirsten Korsholm, Annika Loft, Johanna M. Hall, Annika R. Langkilde, Vibeke A. Larsen, Thomas S. Kristensen, Caroline Ewertsen, Frederikke E. Høi-Hansen, Patrick M. Lehmann, and et al. 2026. "First Clinical Experiences with the Ultra-Fast Time-of-Flight BIOGRAPH One Next-Generation Hybrid PET/MRI System" Diagnostics 16, no. 3: 398. https://doi.org/10.3390/diagnostics16030398
APA StyleHenriksen, O. M., Korsholm, K., Loft, A., Hall, J. M., Langkilde, A. R., Larsen, V. A., Kristensen, T. S., Ewertsen, C., Høi-Hansen, F. E., Lehmann, P. M., Kettless, K., Andersen, F. L., Andersen, T. L., & Law, I. (2026). First Clinical Experiences with the Ultra-Fast Time-of-Flight BIOGRAPH One Next-Generation Hybrid PET/MRI System. Diagnostics, 16(3), 398. https://doi.org/10.3390/diagnostics16030398








