Improved Quantification of ICG Perfusion Through Motion Compensation in Fluorescence-Guided Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging Data
2.2. Applications of Quantitative ICG Assessment (q-ICG)

2.3. Data Acquisition and Region of Interest Selection (Annotation)
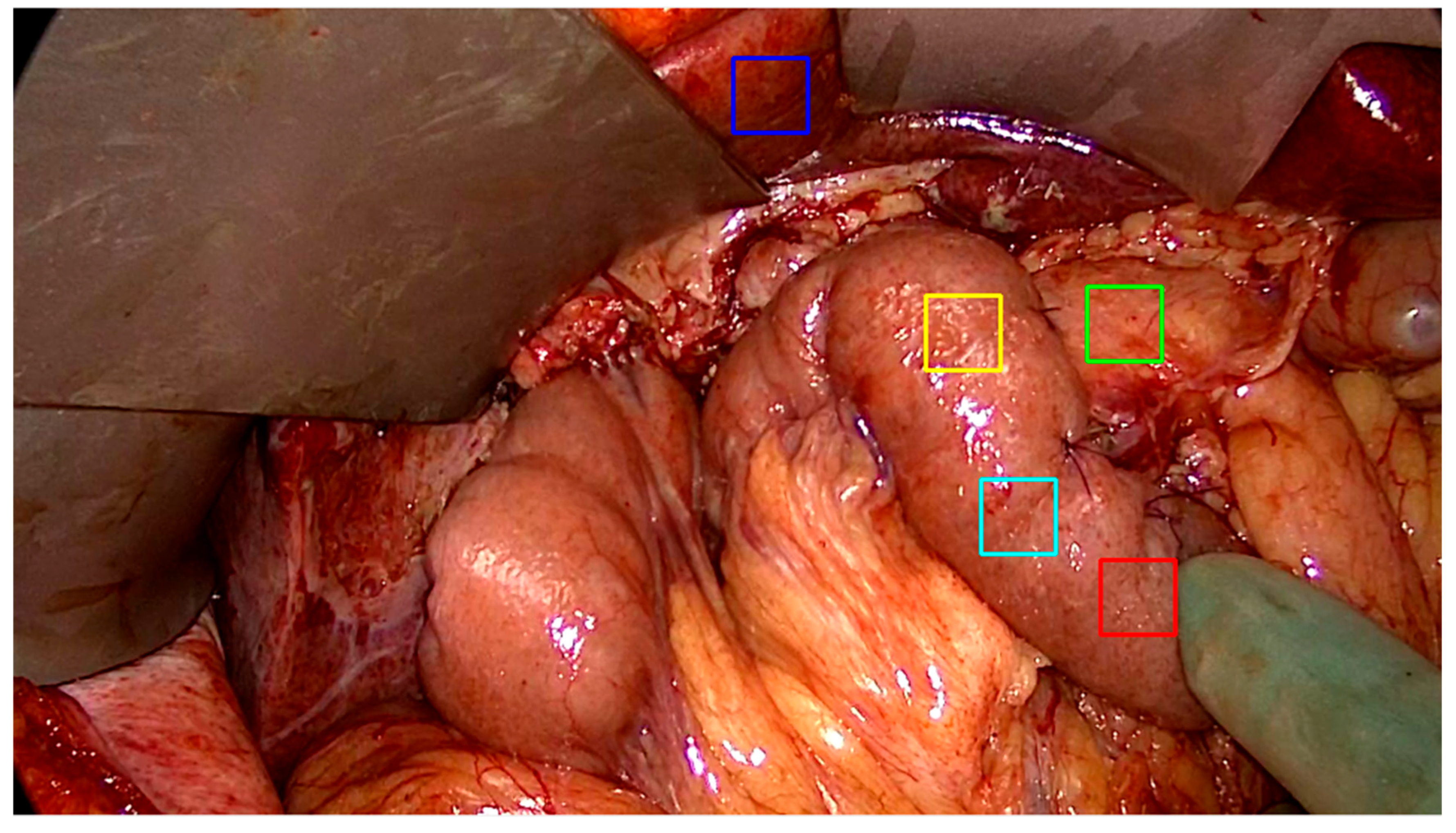
2.4. Motion Compensation

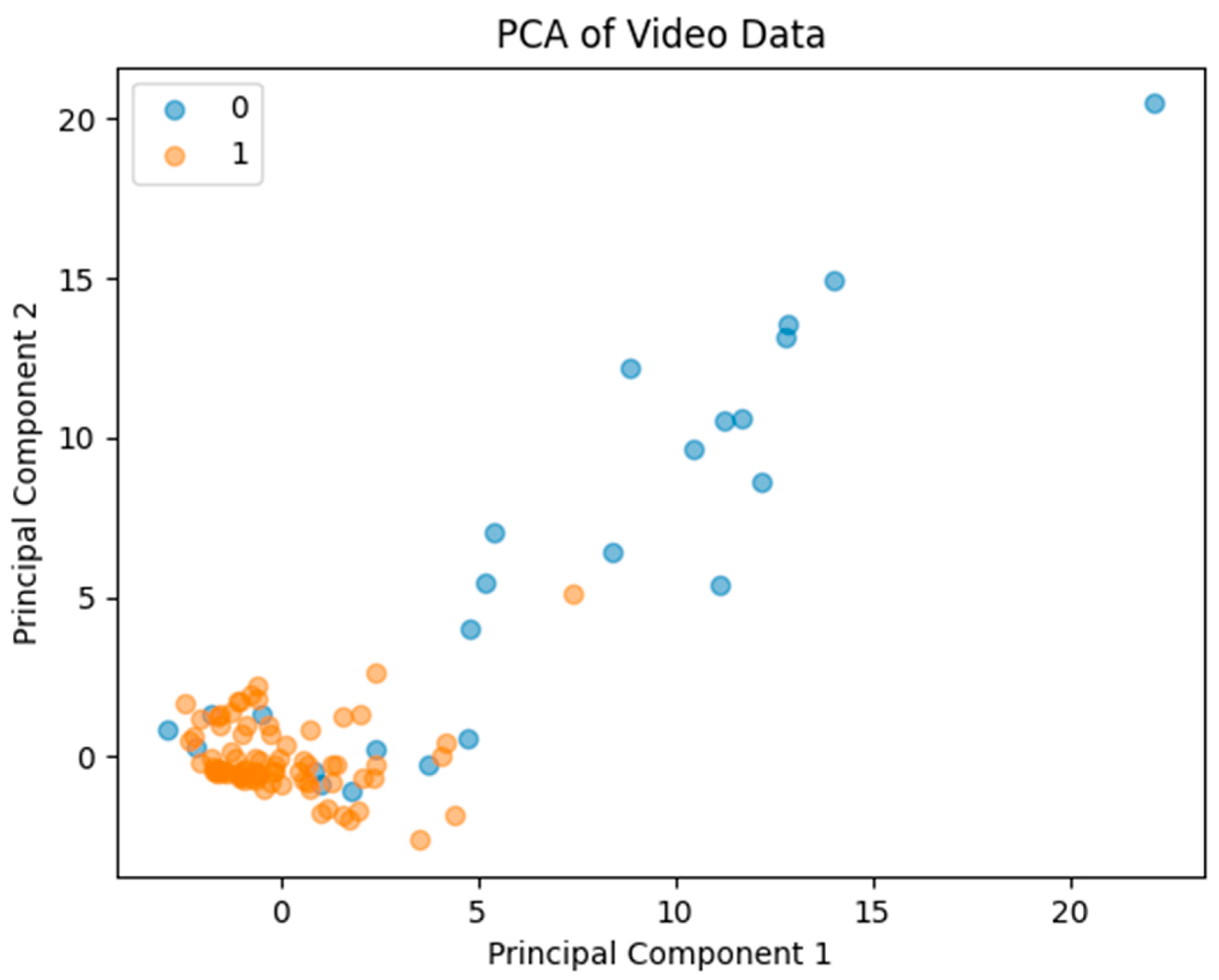
2.5. Motion Compensation Evaluation
2.6. Clinical Relevance
2.7. Statistical Analysis
3. Results
3.1. Summary

3.2. Implications on Perfusion Measurements
3.2.1. Slope
3.2.2. Time-to-Peak
4. Discussion
4.1. Quantitative Effects of Motion Compensation
4.2. Interpretation and Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryski, M.G.; Frenzel Sulyok, L.G.; Kaplan, L.; Singhal, S.; Keating, J.J. Techniques for intraoperative evaluation of bowel viability in mesenteric ischemia: A review. Am. J. Surg. 2020, 220, 309–315. [Google Scholar] [CrossRef]
- Turrentine, F.E.; Denlinger, C.E.; Simpson, V.B.; Garwood, R.A.; Guerlain, S.; Agrawal, A.; Friel, C.M.; LaPar, D.J.; Stukenborg, G.J.; Jones, S.R. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J. Am. Coll. Surg. 2015, 220, 195–206. [Google Scholar] [CrossRef]
- Boni, L.; David, G.; Dionigi, G.; Rausei, S.; Cassinotti, E.; Fingerhut, A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg. Endosc. 2016, 30, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Joosten, J.J.; Longchamp, G.; Khan, M.F.; Lameris, W.; Henegouwen, M.I.v.B.; Bemelman, W.A.; Cahill, R.A.; Hompes, R.; Ris, F. The use of fluorescence angiography to assess bowel viability in the acute setting: An international, multi-centre case series. Surg. Endosc. 2022, 36, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Liot, E.; Assalino, M.; Buchs, N.C.; Schiltz, B.; Douissard, J.; Morel, P.; Ris, F. Does near-infrared (NIR) fluorescence angiography modify operative strategy during emergency procedures? Surg. Endosc. 2018, 32, 4351–4356. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, I.; Keese, M.; Jakob, J.; Stasiunaitis, V.; Gerken, A.; Attenberger, U.; Post, S.; Kienle, P.; Nowak, K. Indocyanine Green Tissue Angiography Can Reduce Extended Bowel Resections in Acute Mesenteric Ischemia. J. Gastrointest. Surg. 2018, 22, 2117–2124. [Google Scholar] [CrossRef]
- Watanabe, J.; Takemasa, I.; Kotake, M.; Noura, S.; Kimura, K.; Suwa, H.; Tei, M.; Takano, Y.; Munakata, K.; Matoba, S.; et al. Blood Perfusion Assessment by Indocyanine Green Fluorescence Imaging for Minimally Invasive Rectal Cancer Surgery (EssentiAL trial): A Randomized Clinical Trial. Ann. Surg. 2023, 278, e688–e694. [Google Scholar] [CrossRef]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: Results of a multicenter randomized controlled trial. Surg. Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, K.Y.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Prognostic impact of fluorescent lymphography on gastric cancer. Int. J. Surg. 2023, 109, 2926–2933. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Cheon, H.; Kim, S.A.; Kim, B.; Jeon, J.Y. Investigation of optimizing indocyanine green solution for in vivo lymphatic research using near-infrared fluorescence indocyanine green lymphangiography. Sci. Rep. 2023, 13, 14966. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Joosten, J.J.; Jindal, A.; Hardy, N.P.; Camilleri-Brennan, J.; Andrejevic, P.; Hompes, R.; Cahill, R.A. Impact of standardising indocyanine green fluorescence angiography technique for visual and quantitative interpretation on interuser variability in colorectal surgery. Surg. Endosc. 2023, 38, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.O.; Nerup, N.; Andersen, J.; Dohrn, N.; Klein, M.F.; Brisling, S.; Salomon, S.; Andersen, P.V.; Möller, S.; Svendsen, M.B.S.; et al. Anastomotic perfusion assessment with indocyanine green in robot-assisted low-anterior resection, a multicenter study of interobserver variation. Surg. Endosc. 2023, 37, 3602–3609. [Google Scholar] [CrossRef]
- Nerup, N.; Svendsen, M.B.S.; Ronn, J.H.; Konge, L.; Svendsen, L.B.; Achiam, M.P. Quantitative fluorescence angiography aids novice and experienced surgeons in performing intestinal resection in well-perfused tissue. Surg. Endosc. 2022, 36, 2373–2381. [Google Scholar] [CrossRef]
- Nerup, N.; Svendsen, M.B.S.; Svendsen, L.B.; Achiam, M.P. Feasibility and usability of real-time intraoperative quantitative fluorescent-guided perfusion assessment during resection of gastroesophageal junction cancer. Langenbeck’s Arch. Surg. 2020, 405, 215–222. [Google Scholar] [CrossRef]
- Lutken, C.D.; Achiam, M.P.; Svendsen, M.B.; Boni, L.; Nerup, N. Optimizing quantitative fluorescence angiography for visceral perfusion assessment. Surg. Endosc. 2020, 34, 5223–5233. [Google Scholar] [CrossRef]
- Nerup, N.; Andersen, H.S.; Ambrus, R.; Strandby, R.B.; Svendsen, M.B.S.; Madsen, M.H.; Svendsen, L.B.; Achiam, M.P. Quantification of fluorescence angiography in a porcine model. Langenbeck’s Arch. Surg. 2017, 402, 655–662. [Google Scholar] [CrossRef]
- Faber, R.A.; Tange, F.P.; Galema, H.A.; Zwaan, T.C.; Holman, F.A.; Peeters, K.C.M.J.; Tanis, P.J.; Verhoef, C.; Burggraaf, J.; Mieog, J.S.D.; et al. Quantification of indocyanine green near-infrared fluorescence bowel perfusion assessment in colorectal surgery. Surg. Endosc. 2023, 37, 6824–6833. [Google Scholar] [CrossRef]
- Gomez-Rosado, J.C.; Valdes-Hernandez, J.; Cintas-Catena, J.; Cano-Matias, A.; Perez-Sanchez, A.; del Rio-Lafuente, F.-J.; Torres-Arcos, C.; Lara-Fernandez, Y.; Capitan-Morales, L.-C.; Oliva-Mompean, F. Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg. Endosc. 2022, 36, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Matsuda, K.; Hayami, S.; Tamura, K.; Mitani, Y.; Mizumoto, Y.; Nakamura, Y.; Murakami, D.; Ueno, M.; Yokoyama, S.; et al. Quantitative Indocyanine Green Fluorescence Imaging Used to Predict Anastomotic Leakage Focused on Rectal Stump During Laparoscopic Anterior Resection. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 542–546. [Google Scholar] [CrossRef]
- Son, G.M.; Kwon, M.S.; Kim, Y.; Kim, J.; Kim, S.H.; Lee, J.W. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg. Endosc. 2019, 33, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Matsuda, K.; Iwamoto, H.; Ueno, M.; Kawai, M.; Hirono, S.; Okada, K.; Miyazawa, M.; Tamura, K.; Mitani, Y.; et al. Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech. Coloproctol. 2019, 23, 973–980. [Google Scholar] [CrossRef]
- De Simone, B.; Abu-Zidan, F.M.; Boni, L.; Castillo, A.M.G.; Cassinotti, E.; Corradi, F.; Di Maggio, F.; Ashraf, H.; Baiocchi, G.L.; Tarasconi, A.; et al. Indocyanine green fluorescence-guided surgery in the emergency setting: The WSES international consensus position paper. World J. Emerg. Surg. 2025, 20, 13. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. JMLR 2011, 12, 2825–2830. [Google Scholar]
- Fok, K.Y.; Toh, J.W. Indocyanine green and height of anastomosis in colorectal surgery- a network meta-analysis. Langenbeck’s Arch. Surg. 2025, 410, 187. [Google Scholar] [CrossRef]
- Tang, Y.; Gitajn, I.L.; Cao, X.; Han, X.; Elliott, J.T.; Yu, X.; Bateman, L.M.; Malskis, B.S.; Fisher, L.A.; Sin, J.M.; et al. Automated motion artifact correction for dynamic contrast-enhanced fluorescence imaging during open orthopedic surgery. Proc. SPIE Int. Soc. Opt. Eng. 2023, 12361, 1236104. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoven, P.; Weller, F.S.; Van De Bent, M.; Goncalves, L.N.; Ruig, M.; Berg, S.D.V.D.; Ooms, S.; Mieog, J.; Van De Bogt, K.E.; Van Schaik, J.; et al. Near-infrared fluorescence imaging with indocyanine green for quantification of changes in tissue perfusion following revascularization. Vascular 2022, 30, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoven, P.; Verduijn, P.S.; Van Capelle, L.; Tange, F.; Michi, M.; Corion, L.; Mulder, B.S.; Mureau, M.; Vahrmeijer, A.; Van Der Vorst, J. Quantification of near-infrared fluorescence imaging with indocyanine green in free flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1820–1825. [Google Scholar] [CrossRef]
- Hardy, N.P.; Dalli, J.; Khan, M.F.; Andrejevic, P.; Neary, P.M.; Cahill, R.A. Inter-user variation in the interpretation of near infrared perfusion imaging using indocyanine green in colorectal surgery. Surg. Endosc. 2021, 35, 7074–7081. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.S.; Clancy, N.T.; Bano, S.; Raza, I.; Diana, M.; Lovat, L.B.; Stoyanov, D.; Chand, M. Interobserver Variability in the Assessment of Fluorescence Angiography in the Colon. Surg. Innov. 2023, 30, 45–49. [Google Scholar] [CrossRef] [PubMed]
- McEntee, P.D.; Singaravelu, A.; McCarrick, C.A.; Murphy, E.; Boland, P.A.; Cahill, R.A. Quantification of indocyanine green fluorescence angiography in colorectal surgery: A systematic review of the literature. Surg. Endosc. 2025, 39, 2677–2691. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Nicolae, S.E.; Piper, T.B.; Nerup, N.A.; Achiam, M.P.; Svendsen, M.B.S. Improved Quantification of ICG Perfusion Through Motion Compensation in Fluorescence-Guided Surgery. Diagnostics 2026, 16, 176. https://doi.org/10.3390/diagnostics16020176
Nicolae SE, Piper TB, Nerup NA, Achiam MP, Svendsen MBS. Improved Quantification of ICG Perfusion Through Motion Compensation in Fluorescence-Guided Surgery. Diagnostics. 2026; 16(2):176. https://doi.org/10.3390/diagnostics16020176
Chicago/Turabian StyleNicolae, Sermed Ellebæk, Thomas Baastrup Piper, Nikolaj Albeck Nerup, Michael Patrick Achiam, and Morten Bo Søndergaard Svendsen. 2026. "Improved Quantification of ICG Perfusion Through Motion Compensation in Fluorescence-Guided Surgery" Diagnostics 16, no. 2: 176. https://doi.org/10.3390/diagnostics16020176
APA StyleNicolae, S. E., Piper, T. B., Nerup, N. A., Achiam, M. P., & Svendsen, M. B. S. (2026). Improved Quantification of ICG Perfusion Through Motion Compensation in Fluorescence-Guided Surgery. Diagnostics, 16(2), 176. https://doi.org/10.3390/diagnostics16020176






