1. Introduction
Idiopathic pulmonary fibrosis (IPF) remains the prototypical fibrosing interstitial lung disease (ILD), with a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) and histopathology. Contemporary guidelines emphasize multidisciplinary diagnosis and increasingly recognize the construct of progressive pulmonary fibrosis (PPF) across non-IPF ILDs, underscoring shared pathobiology and trajectories of decline [
1,
2].
Beyond respiratory morbidity, chronic lung disease has been linked to neurocognitive dysfunction through convergent mechanisms that include sustained hypoxemia, systemic inflammation, oxidative stress, and microvascular injury. Reviews synthesize evidence for structural and functional brain changes in chronic respiratory disease—ranging from white-matter abnormalities to deficits in attention, executive function, and memory—suggesting a lung–brain axis that may accelerate cognitive aging [
3,
4].
Epidemiologic and imaging studies increasingly connect reduced pulmonary function to cerebral small-vessel disease (CSVD)—a major substrate for vascular cognitive impairment. In population cohorts, lower spirometric indices correlate with magnetic resonance imaging (MRI)-defined lacunes and white-matter lesions, independent of smoking, supporting a vascular pathway by which impaired pulmonary physiology may compromise cerebral perfusion and oxygen delivery [
5]. Authoritative reviews of Cerebral Small Vessel Disease (CSVD) detail how endothelial dysfunction, hypoperfusion, and inflammatory cascades drive cognitive decline, motor slowing, and gait instability, providing a biologically plausible framework for pulmonary-to-cerebral signaling [
6].
Although cognitive impairment has been extensively characterized in obstructive lung diseases, ILD-specific data are emergent. Early case–control work in IPF demonstrated lower performance across attention, processing speed, and learning domains compared with healthy controls, particularly in those with advanced diffusion impairment [
7]. Subsequent studies across idiopathic interstitial pneumonia (IIP) phenotypes corroborated decrements in multiple domains—often in association with reduced DLCO and exercise desaturation—yet sample sizes were modest and instruments varied [
8]. A recent scoping review highlighted the paucity of high-level evidence, identified DLCO, hypoxemia, and disease phenotype (e.g., IPF/UIP) among the factors most consistently linked to worse cognition, and called for multidimensional analyses integrating pulmonary physiology, disease severity indices, and comorbidities [
9].
Sleep-disordered breathing (SDB) represents a further, potentially modifiable contributor to neurocognitive risk in ILD. Obstructive sleep apnea (OSA) is highly prevalent in IPF—prospective and retrospective series and a recent meta-analysis estimate that roughly two-thirds of patients have OSA—and has been associated with poorer neurocognitive performance and lower nocturnal oxygen saturations [
10,
11,
12]. These data suggest that intermittent hypoxemia and sleep fragmentation may compound daytime cognitive deficits in fibrosing ILDs and strengthen the rationale for routine screening and targeted management of SDB within comprehensive ILD care pathways [
10,
11,
12].
Risk-stratification frameworks such as the GAP (Gender–Age–Physiology) and ILD-GAP models capture survival risk across IPF and non-IPF ILDs and can be augmented by comorbidity indices to refine prognosis [
13,
14,
15]. Yet whether these validated staging tools parallel neurocognitive vulnerability—and how they interact with physiologic gas-exchange metrics, systemic inflammatory markers, and multimorbidity—remains insufficiently defined. Accordingly, we hypothesize that ILD is independently associated with worse global cognitive performance relative to non-ILD comparators, and that greater restriction and gas-exchange impairment (particularly lower DLCO), higher disease-severity stage (GAP/ILD-GAP), and higher systemic inflammatory burden will be associated with lower cognitive scores. Our objective is to provide a multidimensional assessment of pulmonary, cognitive, and clinical correlates to clarify which physiologic and clinical factors most robustly predict neurocognitive dysfunction in fibrosing ILD. According to the European Respiratory Society (ERS)/American Thoracic Society (ATS) 2025 update on multidisciplinary classification of the Interstitial Pneumonias, fibrotic ILDs are divided based on radiological–pathological criteria into UIP, NSIP, Bronchiolocentric interstitial pneumonia (BIP), Diffuse alveolar damage (DAD), Pleuroparenchymal fibroelastosis (PPFE), Lymphoid interstitial pneumonia (LIP), combined pattern, and unclassifiable pattern [
16].
2. Materials and Methods
2.1. Study Design and Setting
We conducted a single-center, observational cross-sectional study at the ‘Victor Babeș’ University of Medicine and Pharmacy Timișoara, Romania, using patients evaluated in the university-affiliated pulmonology clinics. Participants were consecutively enrolled between October 2022 and September 2025, after ethics approval and until the planned sample size was reached. Recruitment and assessments were coordinated through the university’s respiratory research units and conducted in the same academic clinical environment described in prior institutional work, with standardized procedures harmonized across clinics and laboratories. The study period encompassed consecutive enrollments until the planned sample size was attained.
PICO statement: The study population consisted of adults evaluated in university pulmonology clinics, including patients with fibrotic ILD [classified by multidisciplinary discussion (MDD) into IPF, connective-tissue disease-associated fibrotic ILD, hypersensitivity pneumonitis (HP), idiopathic nonspecific interstitial pneumonia (iNSIP), sarcoidosis-associated pulmonary fibrosis (SAPF), and unclassifiable ILD] and a contemporaneous non-ILD comparator group. The “intervention/exposure” was the presence of fibrosing ILD (and, secondarily, ILD subtype and GAP severity), contrasted with non-ILD participants as the comparator. The primary outcome was global cognitive performance (MMSE total score, with secondary analyses of MMSE domains and MMSE < 24). The overarching objective was to quantify differences in cognition between ILD and non-ILD groups, to explore cognitive heterogeneity across ILD subtypes, and to identify clinical and physiologic predictors—particularly diffusing capacity (DLCO%) and GAP severity—associated with low cognitive performance.
2.2. Legal and Ethical Considerations
The protocol was reviewed and approved by the Local Commission of Ethics for Scientific Research in accordance with Romanian Law no. 95/2006, Order 904/2006, the EU Good Clinical Practice Directive 2005/28/EC, ICH–GCP, and the Declaration of Helsinki. All participants provided written informed consent prior to any study procedure. Data were pseudonymized at source, stored on secure institutional servers, and analyzed only in aggregate form; no identifiable information was retained in the analytic datasets.
2.3. Inclusion and Exclusion Criteria
Eligible participants were adults (≥18 years) able to complete neurocognitive testing in Romanian and to undergo standardized lung function testing within the same evaluative visit. The fibrosing interstitial lung disease (ILD) group comprised patients with a multidisciplinary diagnosis of ILD established according to contemporaneous ATS/ERS guidance (integrating clinical evaluation, HRCT patterning, respiratory functional testing, serology, and depending on the case, bronchoalveolar lavage or lung biopsy) [
16]. ILD subtypes recorded a priori were UIP, NSIP, BIP, sarcoidosis-associated pulmonary fibrosis, and unclassifiable/mixed pattern. The comparator group included adults without clinical or radiologic evidence of interstitial disease, evaluated contemporaneously in the same university-affiliated pulmonology clinics for non-fibrotic respiratory complaints (e.g., chronic cough, suspected obstructive airway disease, or unexplained dyspnea) and meeting the same eligibility criteria. Although all comparators were free of ILD, their specific non-fibrotic diagnoses were not retained as a structured variable in the analytic dataset.
Exclusion criteria applicable to both groups were any acute exacerbation of respiratory disease within four weeks; acute or chronic hypoxemia, decompensated cardiac, renal, or hepatic failure precluding testing; current delirium or acute confusional state; prior neurological disorders known to impair global cognition (dementia, major stroke with residual aphasia); uncorrected visual or hearing deficits interfering with testing; and inability to complete the Mini-Mental State Examination (MMSE). For analytic consistency, participants with incomplete primary outcome data (MMSE total score) or key pulmonary function indices (DLCO%, total lung capacity—TLC%) were excluded.
2.4. Procedures and Measurements
All evaluations were completed during a single visit whenever feasible. Trained clinicians administered the MMSE in Romanian under standardized, distraction-free conditions. In addition to the total MMSE score (0–30), we recorded domain subscores (orientation, registration, attention/calculation, recall, and language) using the canonical scoring rubric. Pulmonary function was assessed in accordance with ATS/ERS standards. Spirometry provided Forced Vital Capacity (FVC) and Forced Expiratory Volume in 1 s (FEV1), and the FEV1/FVC ratio was calculated from best acceptable maneuvers. Body plethysmography yielded TLC% and residual volume (RV%). Single-breath diffusing capacity for carbon monoxide (DLCO%) and transfer coefficient (KCO%) were measured with correction for the participant’s hemoglobin; percent predicted values were derived from reference equations implemented by the institutional laboratory. Oxygen saturation at rest on room air was documented.
Peripheral venous blood was sampled on the same day for routine biomarkers, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin, differential leukocyte count (eosinophils), urea, and creatinine. Assays were performed in the hospital’s ISO-certified core laboratory using validated methods with ongoing internal quality control. Comorbidity burden was quantified by the Charlson Comorbidity Index (CCI). Among ILD participants, disease severity was indexed with the GAP index using sex, age, FVC%, and DLCO%; GAP stage and a dichotomized severity threshold (GAP ≥ 4 vs. < 4) were pre-specified. Smoking status (never, former, current) was ascertained by structured interview and chart verification.
2.5. Study Variables and Outcomes
The primary outcome was global cognitive performance by MMSE analyzed as a continuous score and, in secondary analyses, as a binary endpoint using the customary impairment threshold MMSE < 24 [
17]. Key pulmonary predictors were DLCO%, KCO%, TLC%, RV%, FVC%, and the FEV1/FVC ratio. Inflammatory and hematologic biomarkers (CRP, ESR, eosinophils, hemoglobin, urea, creatinine) were secondary predictors. The principal exposure was diagnostic group (fibrotic ILD vs. non-ILD). Within the ILD group, exploratory prespecified subgroup analyses contrasted UIP, NSIP, BIP, sarcoidosis-associated pulmonary fibrosis and unclassifiable ILD. Additional exploratory analyses examined GAP severity (GAP ≥ 4) in relation to cognitive outcomes.
We did not systematically collect data on educational attainment, detailed sleep metrics, mood symptoms, or psychotropic medication use in a structured format suitable for analysis; these potentially important determinants of cognitive performance are therefore not included as covariates in the present models.
2.6. Definitions
ILD subtypes followed multidisciplinary discussion, integrating clinical–radiologic–pathologic data; morphological pattern was defined on HRCT and histology (where needed). Restrictive ventilatory impairment was inferred from reduced TLC% with preserved or elevated FEV1/FVC ratio; gas-exchange impairment was indexed by reduced DLCO% and KCO%. Low cognitive performance was defined as MMSE < 24, recognizing that this threshold indicates at least mild global impairment in clinical and research settings. CRP and ESR were interpreted as systemic inflammation markers; eosinophils were analyzed as a percentage of leukocytes. GAP staging categorized severity as Stage I–III and was additionally dichotomized at ≥4 points to denote more advanced disease.
2.7. Sample Size and Power Considerations
Before enrollment, we targeted a medium standardized difference in MMSE between ILD and non-ILD groups (Cohen’s d ≈ 0.6), which under α = 0.05 and power = 0.80 yields a minimum total sample of nearly 72 participants for two-group comparisons; to accommodate for potential missingness and ensure adequate precision for subgroup and multivariable analyses, we planned approximately 75–80 participants. The final analytic sample comprised 77 individuals (45 ILD and 32 non-ILD), aligning with the original target and ensuring ≥80% power to detect clinically meaningful between-group MMSE differences, given observed variances.
2.8. Statistical Analysis
Continuous variables are reported as mean ± standard deviation (SD) if normally distributed, or median with interquartile range (IQR) if skewed. Categorical variables are shown as frequencies or percentages. Between-group comparisons (ILD vs. non-ILD) used independent-samples t-tests or Mann–Whitney U tests, as appropriate. One-way ANOVA or Kruskal–Wallis tests evaluated differences among three or more subgroups (e.g., UIP, NSIP, other). When overall tests were significant, we conducted post hoc pairwise comparisons via Bonferroni correction to control for type I error. We performed Pearson’s or Spearman’s correlations (depending on normality) to assess relationships between MMSE scores and numeric parameters (DLCO%, CRP, etc.). Multiple logistic regression was applied to identify independent predictors of MMSE < 24, with age, sex, ILD diagnosis, DLCO%, CRP, and CCI as covariates. Continuous predictors were entered as continuous terms without categorization; DLCO% was scaled per 10-percentage-point decrement and CRP per 5 mg/L increase to aid interpretability of odds ratios. The dependent variable in these models was binary (MMSE < 24 vs. ≥ 24. Statistical significance was set at two-sided p < 0.05.
3. Results
Table 1 offers a comparative overview of demographic characteristics and key comorbidities in the ILD (
n = 45) and non-ILD (
n = 32) cohorts. The mean age is slightly higher in ILD at 65.2 years, yet this did not reach statistical significance (
p = 0.19). A near-equal proportion of men appears in both groups (58% vs. 59%), indicating no major sex imbalance. The prevalence of smoking history—known to influence both pulmonary function and extra-pulmonary outcomes—was somewhat greater in ILD (42% vs. 34%), though not significantly (
p = 0.43). Comorbidity burdens, reflected by the CCI, are modestly higher in ILD (3.7 ± 2.1 vs. 3.1 ± 1.9), but again no statistical difference emerges (
p = 0.25). Hypertension (HBP) and diabetes rates are also similar across the two populations, suggesting that common cardiometabolic conditions are comparably distributed. Dyslipidemia was more frequently recorded among ILD participants (26.7% vs. 18.8%), yet the difference is not conclusive (
p = 0.42). Non-ILD participants were primarily evaluated for non-fibrotic respiratory complaints (such as chronic cough, suspected obstructive airway disease, or unexplained dyspnea) and had no clinical or radiologic evidence of interstitial lung disease, but their specific non-fibrotic diagnoses were heterogeneous and not tabulated as a separate analytic variable.
Table 2 illustrates the ILD cohort stratified by subtype, focusing on markers of disease severity. The GAP index (Gender, Age, Physiology) median values show moderate severity overall. The UIP subgroup (n = 15) exhibits the highest GAP index median at 4 (IQR 3–5), suggesting greater disease burden than NSIP (3 [
2,
3,
4]) or sarcoidosis-associated pulmonary fibrosis (2 [
1,
2,
3]). A one-way ANOVA comparing FVC% among these subgroups yielded an overall
p < 0.05, indicating that at least one pairwise contrast is significant. Post hoc Bonferroni analysis reveals that UIP patients have significantly lower FVC% (66.2 ± 14.5) than NSIP patients (72.3 ± 12.1,
p = 0.041), consistent with UIP being the more fibrotic and functionally limiting phenotype. FEV1% tends to mirror FVC% patterns, with UIP showing the lowest mean (68.7 ± 13.2) and sarcoidosis registering the highest mean (78.3 ± 9.1), although not statistically significant.
Both DLCO (46.8 ± 15.7 vs. 71.4 ± 18.2) and KCO are lower in the ILD group compared with the non-ILD group (68.5 ± 15.2 vs. 86.9 ± 14.3,
p = 0.002), reinforcing that alveolar membrane pathology affects gas-transfer efficiency. Total lung capacity (TLC%) shows a classic restrictive pattern in ILD (67.3 ± 12.0) relative to non-ILD (83.6 ± 14.2,
p < 0.001). Residual volume (RV%) is also diminished in ILD, reflecting reduced lung volumes overall (
p = 0.007). Notably, FVC% (forced vital capacity) is significantly compromised in ILD (69.7 ± 13.4 vs. 82.4 ± 14.5,
p = 0.001), though the FEV1/FVC ratio remains normal or even slightly elevated (81.6 ± 8.2 vs. 78.9 ± 7.5,
p = 0.13). This is typical for restrictive diseases, wherein both FEV1 and FVC decrease proportionally (
Table 3).
Table 3.
Pulmonary function test comparison: ILD vs. non-ILD.
Table 3.
Pulmonary function test comparison: ILD vs. non-ILD.
| Parameter | ILD (n = 45) | Non-ILD (n = 32) | p-Value |
|---|
| DLCO% | 46.8 ± 15.7 | 71.4 ± 18.2 | <0.001 |
| KCO% | 68.5 ± 15.2 | 86.9 ± 14.3 | 0.002 |
| TLC% | 67.3 ± 12.0 | 83.6 ± 14.2 | <0.001 |
| RV% | 59.1 ± 18.1 | 71.9 ± 19.7 | 0.007 |
| FVC% | 69.7 ± 13.4 | 82.4 ± 14.5 | 0.001 |
| FEV1/FVC Ratio | 81.6 ± 8.2 | 78.9 ± 7.5 | 0.13 |
Table 4 presents cognitive performance, measured by MMSE, stratified by broad diagnostic categories (ILD vs. non-ILD) and further broken down by ILD subtypes. A striking difference emerges: the mean MMSE for the ILD group is 23.9 ± 3.6, significantly lower than 26.8 ± 2.8 in the non-ILD cohort (
p < 0.001 by
t-test), and nearly one-third (33.3%) of ILD patients fall below the <24 cutoff consistent with at least mild cognitive impairment. Among ILD subtypes, those with the UIP pattern show the lowest average MMSE (22.8 ± 3.5) and the highest frequency (46.7%) of scores <24. By contrast, NSIP patients exhibit a somewhat higher mean MMSE (24.9 ± 3.2) and fewer individuals below the cutoff (25%). The one-way ANOVA across ILD subtypes is significant (
p = 0.02), and Bonferroni post hoc testing indicates a significant difference between UIP and NSIP (
p = 0.04). Other fibrotic ILD patterns (e.g., Bronchiolocentric interstitial pneumonia) cluster closer to NSIP in cognitive scores. The small sarcoidosis-associated pulmonary fibrosis subgroup (n = 3) had numerically higher mean MMSE scores (25.7), but this subgroup is too small for reliable inference, and these values should be interpreted as purely descriptive.
Table 5 presents individual MMSE domains, providing insight into which cognitive aspects are disproportionately impacted in ILD versus non-ILD. Orientation scores are marginally lower in ILD (9.3 vs. 9.7,
p = 0.05), hinting at early deficits in time and place awareness. Registration (i.e., immediate memory) also shows a mild but significant decline (2.3 vs. 2.7,
p = 0.02). Strikingly, the largest differences lie in attention/calculation (2.9 ± 1.4 in ILD vs. 4.2 ± 1.0 in non-ILD,
p < 0.001) and recall (1.4 ± 0.9 vs. 2.2 ± 0.8,
p < 0.001). Language scores, however, do not differ significantly (7.9 vs. 8.0,
p = 0.62).
Table 6 compares laboratory parameters between ILD and non-ILD participants, offering potential insights into systemic inflammation, oxygen transport capacity, and metabolic status. Hemoglobin levels average around 13.9 g/dL in ILD, slightly lower but not statistically different from the non-ILD mean of 14.3 g/dL. Eosinophil percentages appear higher in ILD (2.4 vs. 1.9), yet this did not reach significance (
p = 0.16), suggesting that eosinophilic inflammation is not a universal driver of ILD (though it can be relevant in specific entities such as eosinophilic pneumonia). CRP and ESR, broad markers of inflammation, trend higher in ILD (14.2 vs. 8.5 mg/L and 19.7 vs. 13.5 mm/h, respectively), with borderline significance (CRP
p = 0.06, ESR
p = 0.07).
Table 7 underscores the relationships between cognitive performance (MMSE) and various physiologic or inflammatory markers. Pearson’s correlation coefficients demonstrate a moderate positive link between MMSE and DLCO% (r = 0.44,
p = 0.0005), KCO% (r = 0.37,
p = 0.002), TLC% (r = 0.34,
p = 0.006), and FVC% (r = 0.31,
p = 0.01). In essence, better pulmonary function—particularly higher diffusing capacity (DLCO, KCO)—is associated with better cognitive scores. These findings bolster the hypothesis that alveolar gas-exchange capacity may influence cerebral oxygenation and thus cognitive processes.
The analysis also presents a post hoc evaluation comparing individuals with MMSE < 24 to those scoring ≥24, illustrating more severe physiologic compromise in the cognitively impaired group. For instance, their mean DLCO% is 38.4 versus 52.1 (p = 0.002), representing a substantial decrement in diffusing capacity. Similar patterns emerge for KCO% (62.1 vs. 72.4, p = 0.01), TLC% (61.0 vs. 72.7, p = 0.008), and FVC% (64.2 vs. 72.3, p = 0.03). CRP, while weakly negatively correlated (r = −0.22, p = 0.07), does show a trend toward higher values in those with lower MMSE (17.5 vs. 11.1 mg/L, p = 0.09), but the difference lacks strict significance.
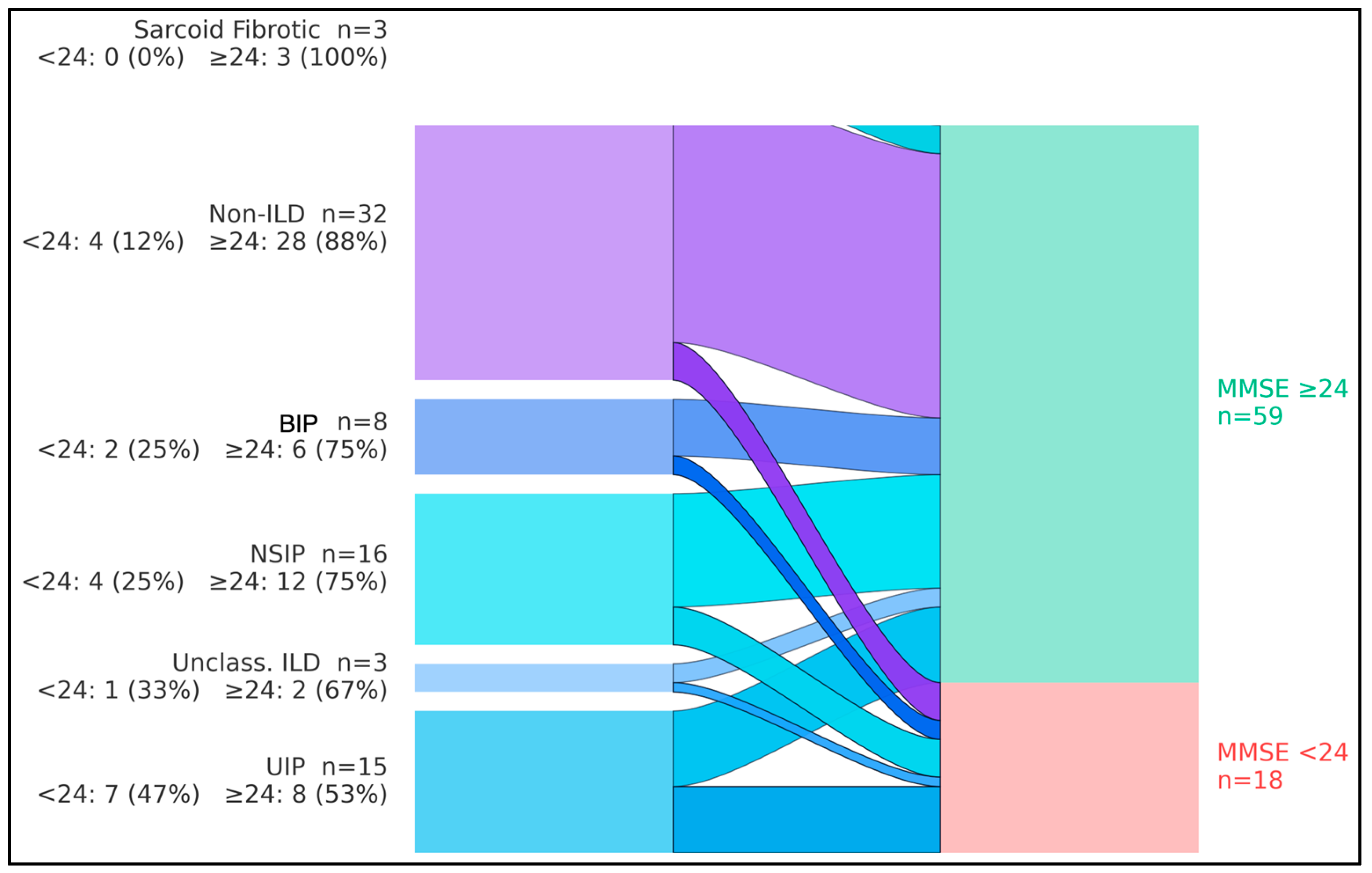
The flows showed that, overall, 18/77 participants (23.4%) had MMSE < 24 and 59/77 (76.6%) had MMSE ≥ 24. By diagnostic group, cognitive impairment (MMSE < 24) occurred in 7/15 with UIP (46.7%), 4/16 with NSIP (25.0%), 2/8 with BIP pattern (25.0%), 1/3 with unclassifiable/combined pattern ILD (33.3%), 0/3 with sarcoidosis-associated pulmonary fibrosis (0%), and 4/32 among non-ILD comparators (12.5%). Consequently, UIP contributed the largest share of impaired cases (7/18, 38.9%), whereas non-ILD participants predominantly exhibited preserved cognition (28/32, 87.5%). The aggregate streams converged to a larger node for MMSE ≥ 24 (n = 59) and a smaller node for MMSE < 24 (n = 18), indicating that most groups retained normal cognition while impairment clustered in fibrotic ILD—particularly UIP (
Figure 1).
Table 8 presents a multivariate logistic regression model identifying predictors of cognitive impairment (MMSE < 24) across the entire sample (n = 77). Even after adjusting for age, comorbidity burden (CCI), DLCO%, and CRP, having an ILD diagnosis is significantly associated with increased odds of cognitive impairment (OR = 2.72, 95% CI 1.14–6.48,
p = 0.024). This finding highlights that ILD status itself confers an independent contribution to poorer cognitive outcomes.
Among continuous predictors, each 10% decrement in DLCO% elevates the risk of low MMSE by approximately 42% (OR = 1.42, p = 0.008), emphasizing the importance of gas-exchange capacity. CRP was not a statistically significant predictor in this model (p = 0.10), and any apparent association should be considered exploratory; these data do not support a strong independent effect of CRP on MMSE < 24 once pulmonary physiology and comorbidity are taken into account. Age, expressed per five-year increment, shows a borderline effect (p = 0.09), reflecting the general principle that advancing age can modestly increase the odds of cognitive decline.
We also included GAP ≥ 4 as a dichotomous variable for ILD participants, representing more advanced disease. ILD patients with GAP ≥ 4 have nearly triple the odds of impaired cognition (OR = 2.91, p = 0.026) compared to those with lower GAP scores, underscoring that disease severity is a key driver. Overall, this model supports the notion that ILD severity and reduced DLCO% are robust predictors of cognitive risk, reinforcing calls for integrated pulmonary and neurocognitive assessment in patients with fibrotic lung disease.
Standardized mean differences (Hedges g; impaired minus unimpaired) indicated that the cognitively impaired group had markedly lower lung volumes and gas-transfer capacity, alongside somewhat higher systemic inflammation. Effect sizes for TLC%, DLCO%, KCO%, and FVC% ranged from −0.61 to −0.99, corresponding to moderate-to-large deficits in pulmonary function among participants with MMSE < 24 (
Figure 2). In contrast, C-reactive protein (CRP) showed a moderate positive effect size (g = 0.57, 95% CI 0.04 to 1.09), indicating higher inflammatory burden in the impaired group, although this finding should be interpreted cautiously and in conjunction with regression results, where CRP did not independently predict MMSE < 24.