Immunohistochemical Characterization of the Immune Response in Chronic Endometritis Caused by Chlamydia trachomatis
Abstract

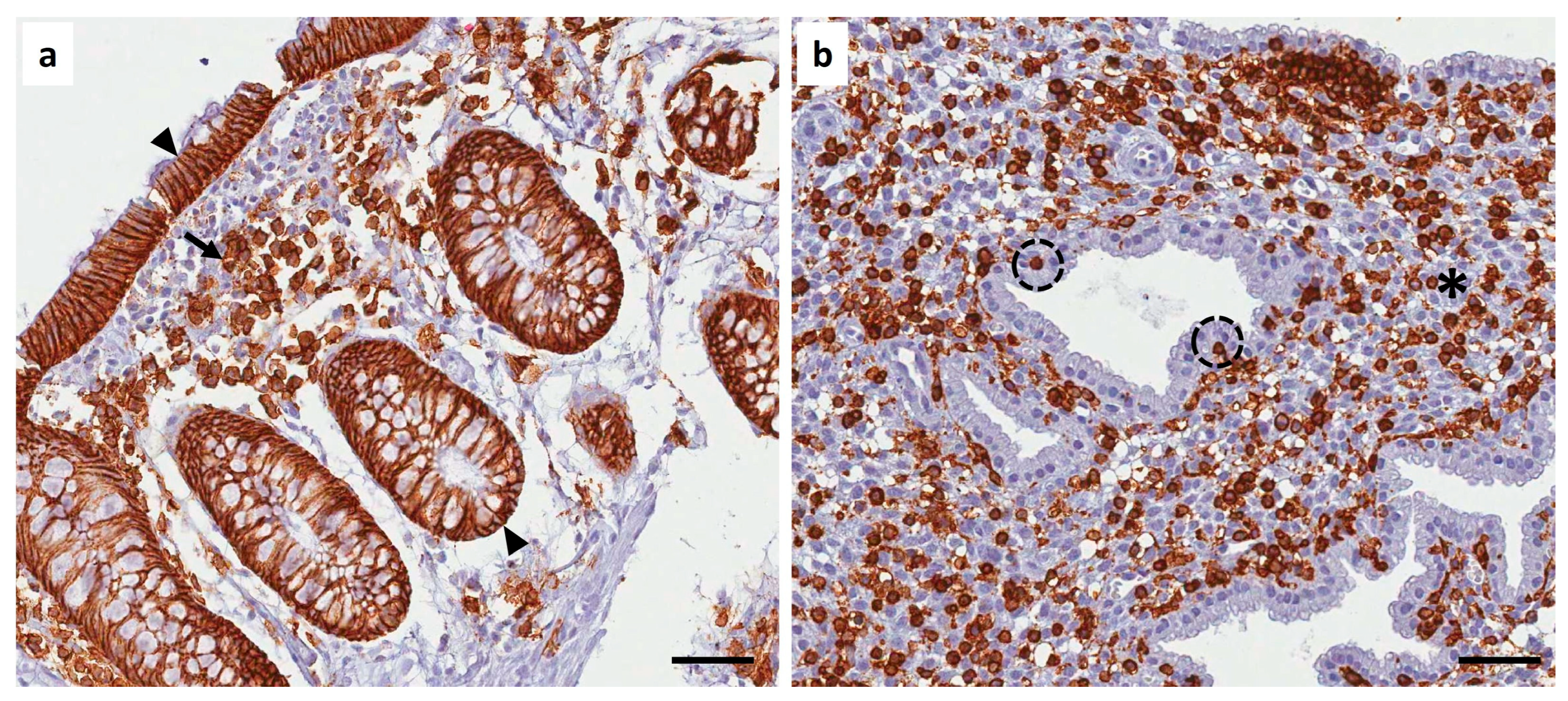
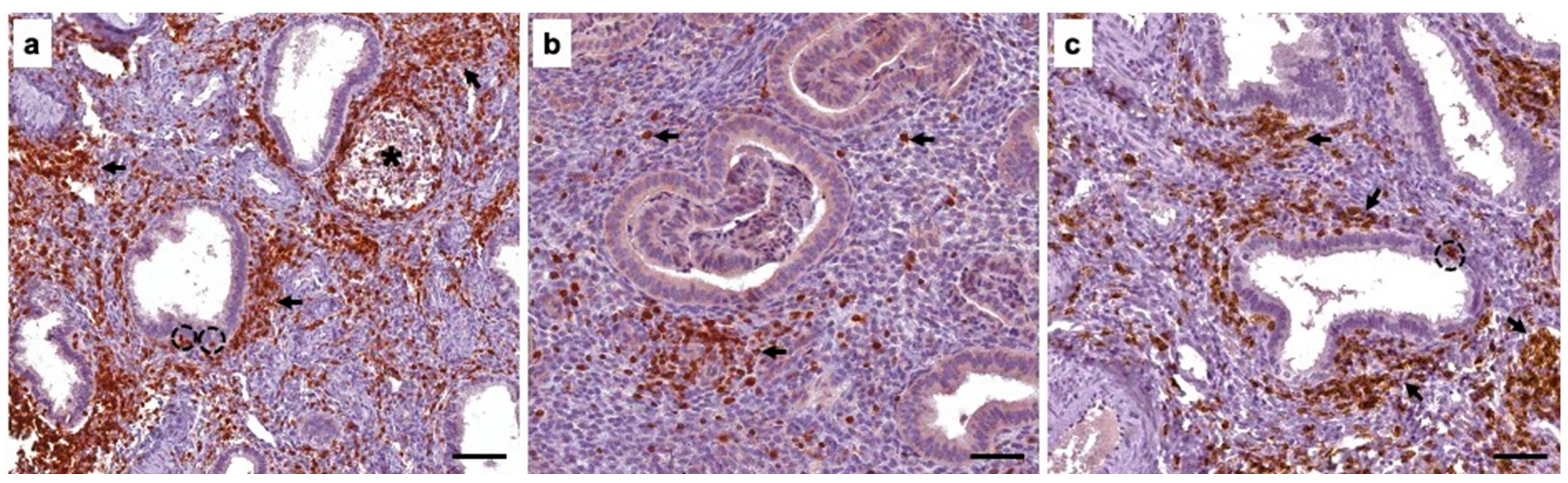
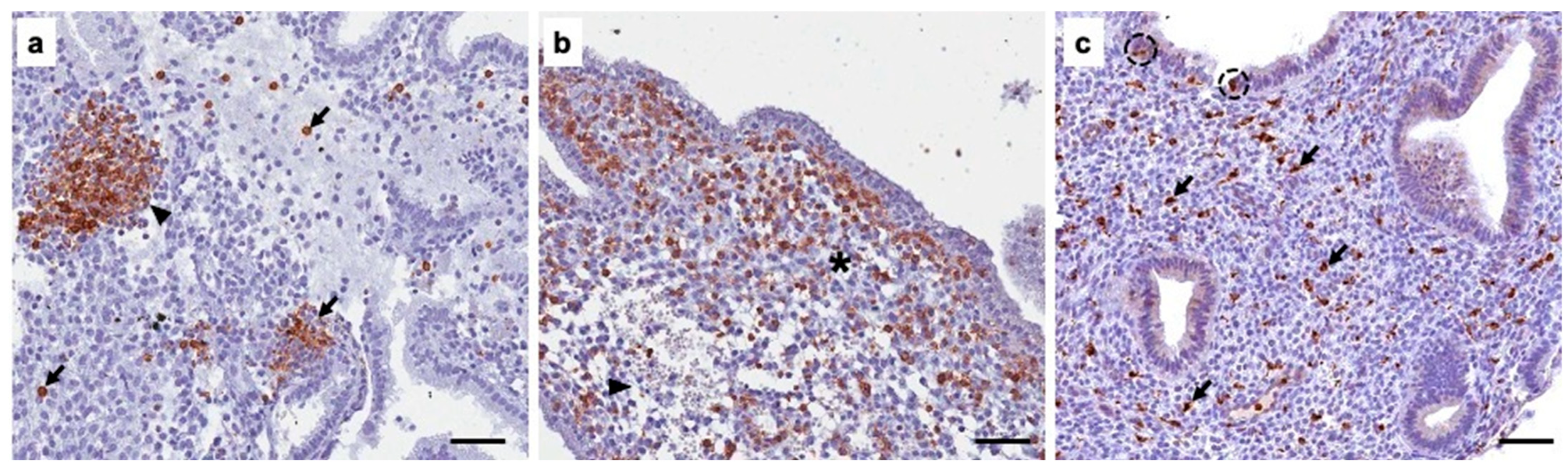
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| µm | Micrometers |
| H&E | Hematoxylin and eosin |
| IF | Immunofluorescence |
| PCR | Polymerase chain reaction |
References
- Yan, X.; Jiao, J.; Wang, X. The pathogenesis, diagnosis, and treatment of chronic endometritis: A comprehensive review. Front. Endocrinol. 2025, 16, 1603570. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sethi, A. Endometritis—Diagnosis, treatment and its impact on fertility—A scoping review. JBRA Assist. Reprod. 2022, 26, 538–546. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Yoon, T.K.; Lee, W.S. Chronic endometritis and infertility. Clin. Exp. Reprod. Med. 2016, 43, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lucan, M.; Sandor, M.; Bodog, A.; Mocuta, D.; Aur, C.D.; Sachelarie, L.; Huniadi, A. Chronic endometritis: A silent contributor to infertility and reproductive failure—A comprehensive review. Reprod. Med. 2025, 6, 14. [Google Scholar] [CrossRef]
- Ortega-Martínez, M.; Gopar-Cuevas, Y.; García-Aguilar, K.; Chávez-Briones, M.D.; Miranda-Maldonado, I.; Ancer-Arellano, A.; García-Juárez, J.; Ancer-Rodríguez, J.; Jaramillo-Rangel, G. Analysis of cell turnover in the macula densa through the normal aging process. Biomed. Rep. 2025, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, T.; Kitaya, K. Challenges in clinical diagnosis and management of chronic endometritis. Diagnostics 2022, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Klimaszyk, K.; Svarre Nielsen, H.; Wender-Ozegowska, E.; Kedzia, M. Chronic endometritis—Is it time to clarify diagnostic criteria? Ginekol. Pol. 2023, 94, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Reighard, S.D.; Sweet, R.L.; Vicetti Miguel, C.; Vicetti Miguel, R.D.; Chivukula, M.; Krishnamurti, U.; Cherpes, T.L. Endometrial leukocyte subpopulations associated with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis genital tract infection. Am. J. Obstet. Gynecol. 2011, 205, 324.e1–324.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, L.; Pei, T.; Liu, D.; Liu, C.; Luo, B.; Xiao, L.; Li, Y.; Wang, R.; Ouyang, Y.; et al. Single-cell transcriptome analysis reveals endometrial immune microenvironment in minimal/mild endometriosis. Clin. Exp. Immunol. 2023, 212, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kisovar, A.; Becker, C.M.; Granne, I.; Southcombe, J.H. The role of CD8+ T cells in endometriosis: A systematic review. Front. Immunol. 2023, 14, 1225639. [Google Scholar] [CrossRef] [PubMed]
- Pirtea, P.; Cicinelli, E.; De Nola, R.; de Ziegler, D.; Ayoubi, J.M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021, 115, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mariee, N.; Jiang, L.; Liu, Y.; Wang, C.C.; Li, T.C.; Laird, S. Measurement of uterine natural killer cell percentage in the periimplantation endometrium from fertile women and women with recurrent reproductive failure: Establishment of a reference range. Am. J. Obstet. Gynecol. 2017, 217, 680.e1–680.e6. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Chiokadze, M.; Bär, C.; Pastuschek, J.; Dons’koi, B.V.; Khazhylenko, K.G.; Schleußner, E.; Markert, U.R.; Favaro, R.R. Beyond uterine natural killer cell numbers in unexplained recurrent pregnancy loss: Combined analysis of CD45, CD56, CD16, CD57, and CD138. Diagnostics 2020, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- NOM-012-SSA3-2012; That Establishes the Criteria for the Execution of Research Projects for Health in Human Beings. Norma Oficial Mexicana: Mexico City, Mexico, 2013.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Miranda-Maldonado, I.; Gopar-Cuevas, Y.; Álvarez-Cuevas, S.; Gallegos-Avila, G.; Ancer-Rodríguez, J.; Ortega-Martínez, M.; Jaramillo-Rangel, G. Immunohistochemical Characterization of the Immune Response in Chronic Endometritis Caused by Chlamydia trachomatis. Diagnostics 2026, 16, 164. https://doi.org/10.3390/diagnostics16010164
Miranda-Maldonado I, Gopar-Cuevas Y, Álvarez-Cuevas S, Gallegos-Avila G, Ancer-Rodríguez J, Ortega-Martínez M, Jaramillo-Rangel G. Immunohistochemical Characterization of the Immune Response in Chronic Endometritis Caused by Chlamydia trachomatis. Diagnostics. 2026; 16(1):164. https://doi.org/10.3390/diagnostics16010164
Chicago/Turabian StyleMiranda-Maldonado, Ivett, Yareth Gopar-Cuevas, Salomón Álvarez-Cuevas, Guadalupe Gallegos-Avila, Jesús Ancer-Rodríguez, Marta Ortega-Martínez, and Gilberto Jaramillo-Rangel. 2026. "Immunohistochemical Characterization of the Immune Response in Chronic Endometritis Caused by Chlamydia trachomatis" Diagnostics 16, no. 1: 164. https://doi.org/10.3390/diagnostics16010164
APA StyleMiranda-Maldonado, I., Gopar-Cuevas, Y., Álvarez-Cuevas, S., Gallegos-Avila, G., Ancer-Rodríguez, J., Ortega-Martínez, M., & Jaramillo-Rangel, G. (2026). Immunohistochemical Characterization of the Immune Response in Chronic Endometritis Caused by Chlamydia trachomatis. Diagnostics, 16(1), 164. https://doi.org/10.3390/diagnostics16010164




