Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021
Abstract
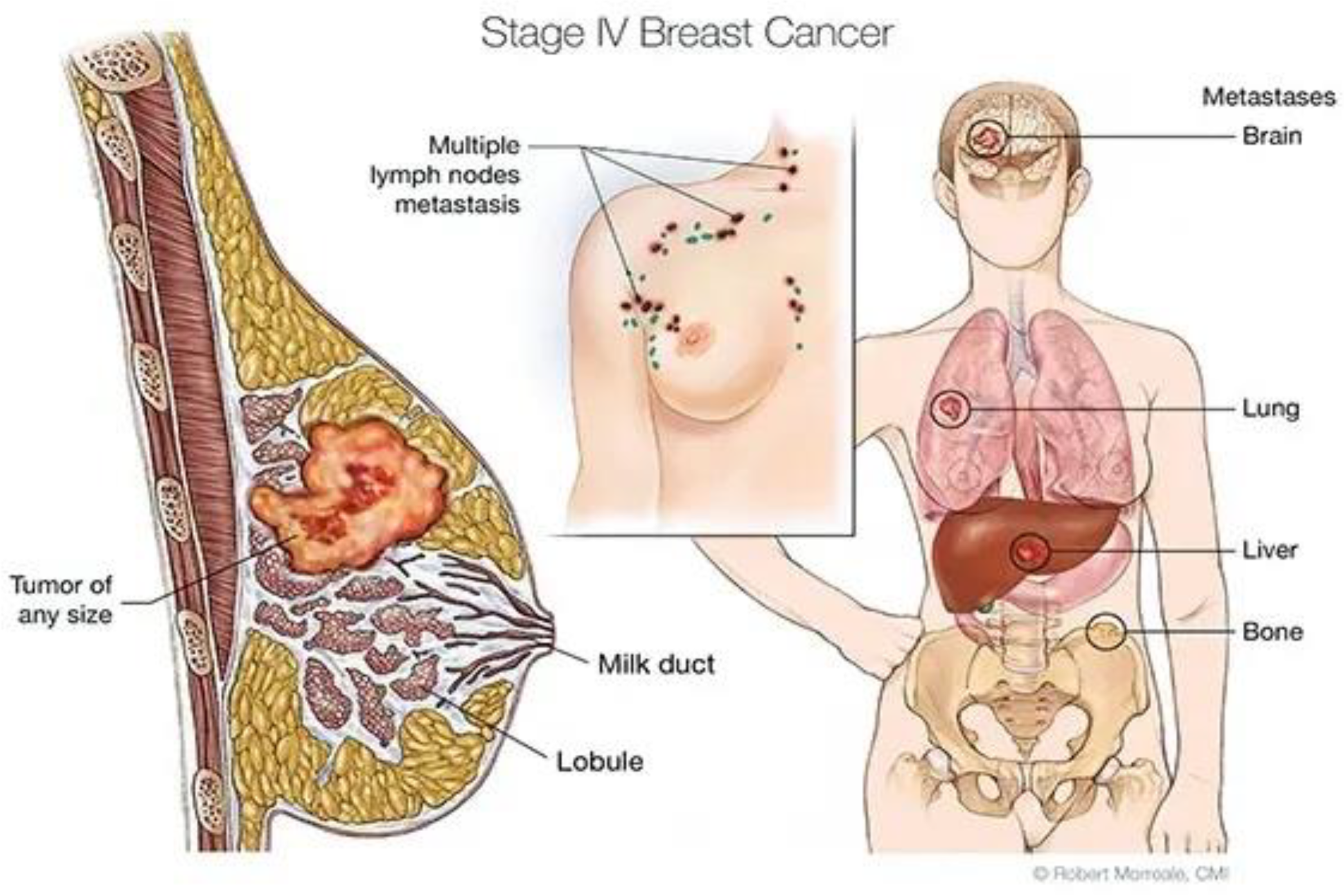
1. Introduction
2. Materials and Methods
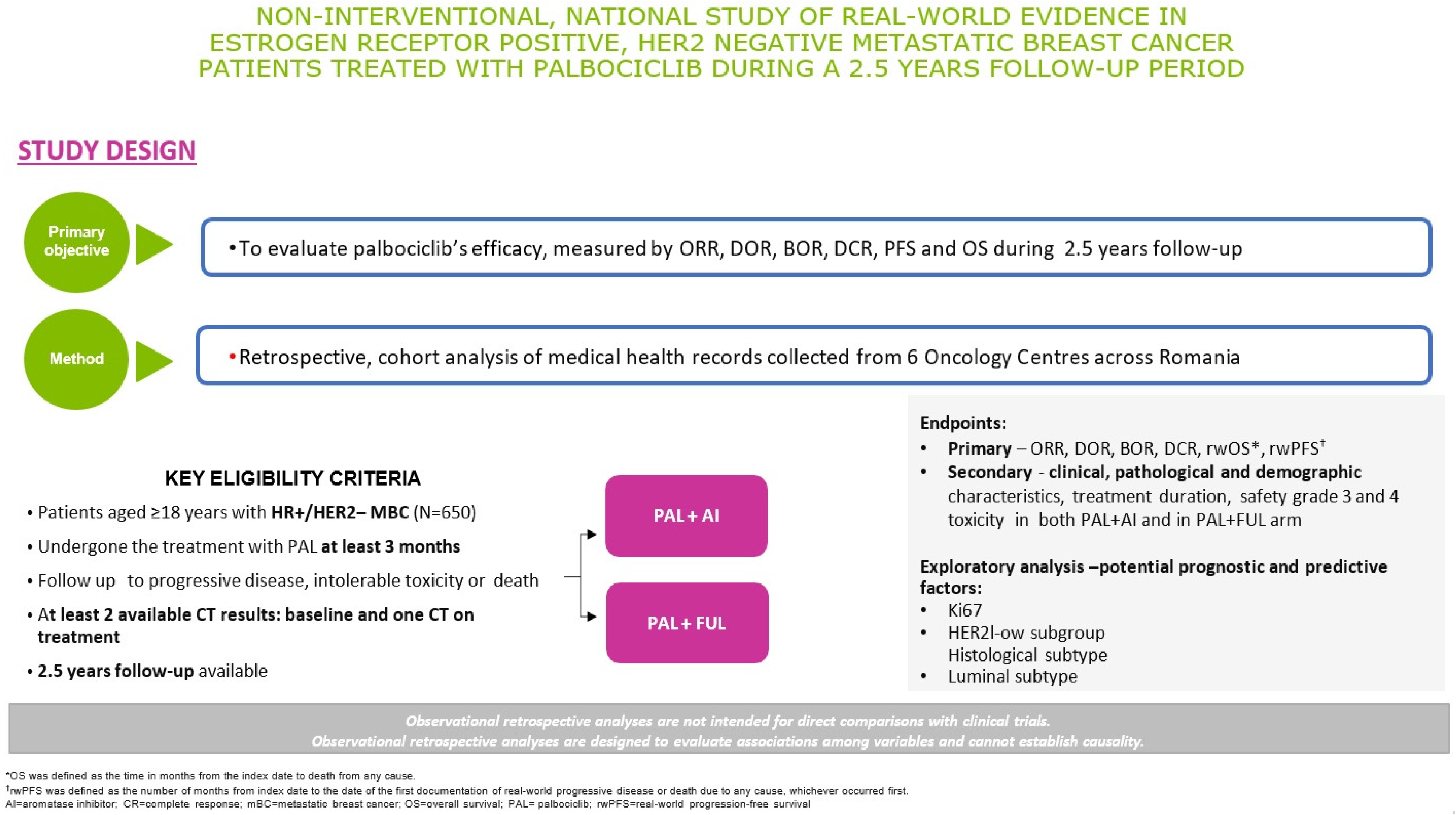
2.1. Trial Design Overview
2.2. Eligibility Criteria
2.3. Sample Size. Procedure and Data Collection
2.3.1. Sample Size
2.3.2. Procedure and Data Collection
2.4. Objectives and Methods of Assessment
2.4.1. Primary Objectives
2.4.2. Secondary Objectives
2.4.3. Safety Objectives
- Date of onset;
- The degree of the event’s severity (Mild/Moderate/Severe);
- If the adverse event is serious (SAE);
- The causality assessment with the drug;
- Any other medical interventions performed by Investigator (e.g., changing concomitant medications);
- Status of the event at the moment of the report (Ongoing, Resolved);
- If resolved, stop date of the event.
2.4.4. Exploratory Variables
2.4.5. Measurement Methods and Outcomes
- ORR is defined as the percentage of patients achieving a confirmed complete response (CR) or partial response (PR);
- DOR is defined as the period of time between the initial documentation of tumour response and the subsequent confirmation of disease progression (PD);
- DCR is defined as the percentage of patients who achieve the best overall response, (CR), (PR), or stable disease (SD), that is maintained for at least 12 weeks;
- BCR is defined as the best imaging response during the treatment time;
- Clinical Benefit Rate (CBR) is defined as the proportion of patients with no disease progression after 6 months of therapy;
- PFS is defined as the time from initiation of treatment to occurrence of PD or death;
- OS is defined as the duration from the start of treatment until death from any cause. For patients who are still alive, follow-up will be censored at the last recorded contact date or the most recent date they were known to be alive. The OS follow-up was conducted at 12, 24, and 30 months (2.5 years). If data were available beyond the 2.5 years, OS was collected also at 33 months from the initiation of Palbociclib.
2.4.6. Laboratory Variables
2.4.7. Demographics and Baseline Characteristics
2.5. Study Drug
2.6. Statistical Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR | Hormone receptor |
| HER2 | Human epidermal grow factor receptor 2 |
| BC | Breast cancer |
| MBC | Metastatic breast cancer |
| CDK 4/6 | Cyclin-dependent kinase 4/6 |
| OS | Overall survival |
| AI | Aromatase inhibitors |
| e-CRF | Electronic case report form |
| EDC | Electronic data capture |
| ITT | Intention-to-Treat |
| PP | Per-Protocol |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| RECIST | Response Evaluation Criteria in Solid Tumours |
| ORR | Overall response rate |
| DOR | Duration of response |
| DCR | Disease control rate |
| BCR | Best clinical response |
| PFS | Progression Free Survival |
| CTCAE | Common Terminology Criteria for Adverse Event |
| SAE | Serious adverse event |
| CR | Complete response |
| PR | Partial response |
| PD | Progression disease |
| SD | Stable disease |
| TNM | Tumour–Nodules–Metastasis |
References
- Metastatic Breast Cancer|Cancer Support Community. Available online: https://www.cancersupportcommunity.org/metastatic-breast-cancer (accessed on 12 April 2025).
- Cardoso, F.; Spence, D.; Mertz, S.; Corneliussen-James, D.; Sabelko, K.; Gralow, J.; Cardoso, M.-J.; Peccatori, F.; Paonessa, D.; Benares, A.; et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005–2015). Breast 2018, 39, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Grover, S.; Sathyanarayana Rao, T.S. Clinical Practice Guidelines for Management of Sexual Dysfunction. Indian J. Psychiatry 2017, 59 (Suppl. S1), S91–S115. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.M.; Negru, S.M.; Popovici, D.I.; Saftescu, S.; Han, R.A.; Dragomir, G.M.; Hoinoiu, T.; Dema, A. Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data. Int. J. Env. Res. Public Health 2020, 17, 8722. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.; Hernando, C.; Martínez, M.T.; Burgués, O.; Tebar-Sánchez, C.; Lameirinhas, A.; Ágreda-Roca, A.; Torres-Ruiz, S.; Garrido-Cano, I.; Lluch, A.; et al. Clinical Impact of New Treatment Strategies for HER2-Positive Metastatic Breast Cancer Patients with Resistance to Classical Anti-HER Therapies. Cancers 2023, 15, 4522. [Google Scholar] [CrossRef]
- Hart, C.D.; Migliaccio, I.; Malorni, L.; Guarducci, C.; Biganzoli, L.; Di Leo, A. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 541–552. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Palbociclib and Letrozole in Advanced Breast Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27959613/ (accessed on 4 March 2025).
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Ettl, J.; Schmidt, M.; Bondarenko, I.M.; Lang, I.; Pinter, T.; Boer, K.; Patel, R.; Randolph, S.; et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. BCR 2016, 18, 67. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Waller, J.; Mitra, D.; Mycock, K.; Taylor-Stokes, G.; Milligan, G.; Zhan, L.; Iyer, S. Real-World Treatment Patterns and Clinical Outcomes in Patients Receiving Palbociclib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced or Metastatic Breast Cancer in Argentina: The IRIS Study. J. Glob. Oncol. 2019, 5, JGO1800239. [Google Scholar] [CrossRef]
- Palmieri, C.; Musson, A.; Harper-Wynne, C.; Wheatley, D.; Bertelli, G.; Macpherson, I.R.; Nathan, M.; McDowall, E.; Bhojwani, A.; Verrill, M.; et al. A real-world study of the first use of palbociclib for the treatment of advanced breast cancer within the UK National Health Service as part of the novel Ibrance® Patient Program. Br. J. Cancer 2023, 129, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, B.; Ling, X.; Li, D.; Zhao, J.; Lv, Y.; Wang, G.; Liu, X.; Li, N.; Yang, J. Palbociclib plus endocrine therapy in hormone receptor-positive and HER2 negative metastatic breast cancer: A multicenter real-world study in the northwest of China. BMC Cancer 2023, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Manso, L.; Hernando, C.; Galán, M.; Oliveira, M.; Cabrera, M.A.; Bratos, R.; Rodríguez, C.A.; Ruiz-Borrego, M.; Blanch, S.; Llombart-Cussac, A.; et al. Palbociclib combined with endocrine therapy in heavily pretreated HR+/HER2- advanced breast cancer patients: Results from the compassionate use program in Spain (PALBOCOMP). Breast 2020, 54, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.L.; Yu-Kite, M.; Mardekian, J.; Cotter, M.J.; Kim, S.; Decembrino, J.; Snow, T.; Carson, K.R.; Motyl Rockland, J.; Gossai, A.; et al. Real-World Data of Palbociclib in Combination With Endocrine Therapy for the Treatment of Metastatic Breast Cancer in Men. Clin. Pharmacol. Ther. 2022, 111, 302–309. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef]
- Gnant, M.; Harbeck, N.; Thomssen, C. St. Gallen/Vienna 2017: A Brief Summary of the Consensus Discussion about Escalation and De-Escalation of Primary Breast Cancer Treatment. Breast Care 2017, 12, 101–106. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- IBRANCE® (palbociclib) Receives Approval in European Union for the Treatment of Women with HR+/HER2- Metastatic Breast Cancer|Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/ibrance_palbociclib_receives_approval_in_european_union_for_the_treatment_of_women_with_hr_her2_metastatic_breast_cancer (accessed on 4 March 2025).
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Rath, S.; Elamarthi, P.; Parab, P.; Gulia, S.; Nandhana, R.; Mokal, S.; Kembhavi, Y.; Perumal, P.; Bajpai, J.; Ghosh, J.; et al. Efficacy and safety of palbociclib and ribociclib in patients with estrogen and/or progesterone receptor positive, HER2 receptor negative metastatic breast cancer in routine clinical practice. PLoS ONE 2021, 16, e0253722. [Google Scholar] [CrossRef]
- Shao, X.; Zheng, Y.; Cao, W.; Shen, X.; Li, G.; Chen, J.; Huang, Y.; Huang, P.; Shi, L.; Ye, W.; et al. Ki67 and progesterone receptor status predicts sensitivity to palbociclib: A real-world study. Ann. Transl. Med. 2021, 9, 707. [Google Scholar] [CrossRef]
- Palleschi, M.; Maltoni, R.; Ravaioli, S.; Vagheggini, A.; Mannozzi, F.; Fanini, F.; Pirini, F.; Tumedei, M.M.; Barzotti, E.; Cecconetto, L.; et al. Ki67 and PR in Patients Treated with CDK4/6 Inhibitors: A Real-World Experience. Diagnostics 2020, 10, 573. [Google Scholar] [CrossRef]

| Inclusion Criteria |
|---|
|
| Exclusion criteria |
|
| Hematology | Clinical Chemistry | Liver Enzymes |
|---|---|---|
| Erythrocytes | Creatinine | Alkaline phosphatase |
| Mean corpuscular volume Hemoglobin Hematocrit | Glucose Urea | Aspartate aminotransferase |
| Neutrophils | Uric acid | Alanine aminotransferase |
| Eosinophils Basophils Lymphocytes | Total Bilirubin | Gamma-glutamyl transferase |
| Monocytes Platelets Leukocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprean, C.M.; Badau, L.M.; Petrita, R.; Median, M.D.; Dema, A. Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021. Diagnostics 2025, 15, 1173. https://doi.org/10.3390/diagnostics15091173
Oprean CM, Badau LM, Petrita R, Median MD, Dema A. Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021. Diagnostics. 2025; 15(9):1173. https://doi.org/10.3390/diagnostics15091173
Chicago/Turabian StyleOprean, Cristina Marinela, Larisa Maria Badau, Ramona Petrita, Mircea Dragos Median, and Alis Dema. 2025. "Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021" Diagnostics 15, no. 9: 1173. https://doi.org/10.3390/diagnostics15091173
APA StyleOprean, C. M., Badau, L. M., Petrita, R., Median, M. D., & Dema, A. (2025). Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021. Diagnostics, 15(9), 1173. https://doi.org/10.3390/diagnostics15091173






