Serum P-Cresyl Sulfate Levels Correlate with Peripheral Arterial Disease in Hypertensive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Determination of Serum PCS Levels by High-Performance Liquid Chromatography–Mass Spectrometry
2.5. ABI Measurements
2.6. Strategies to Avoid Bias
2.7. Statistical Analysis
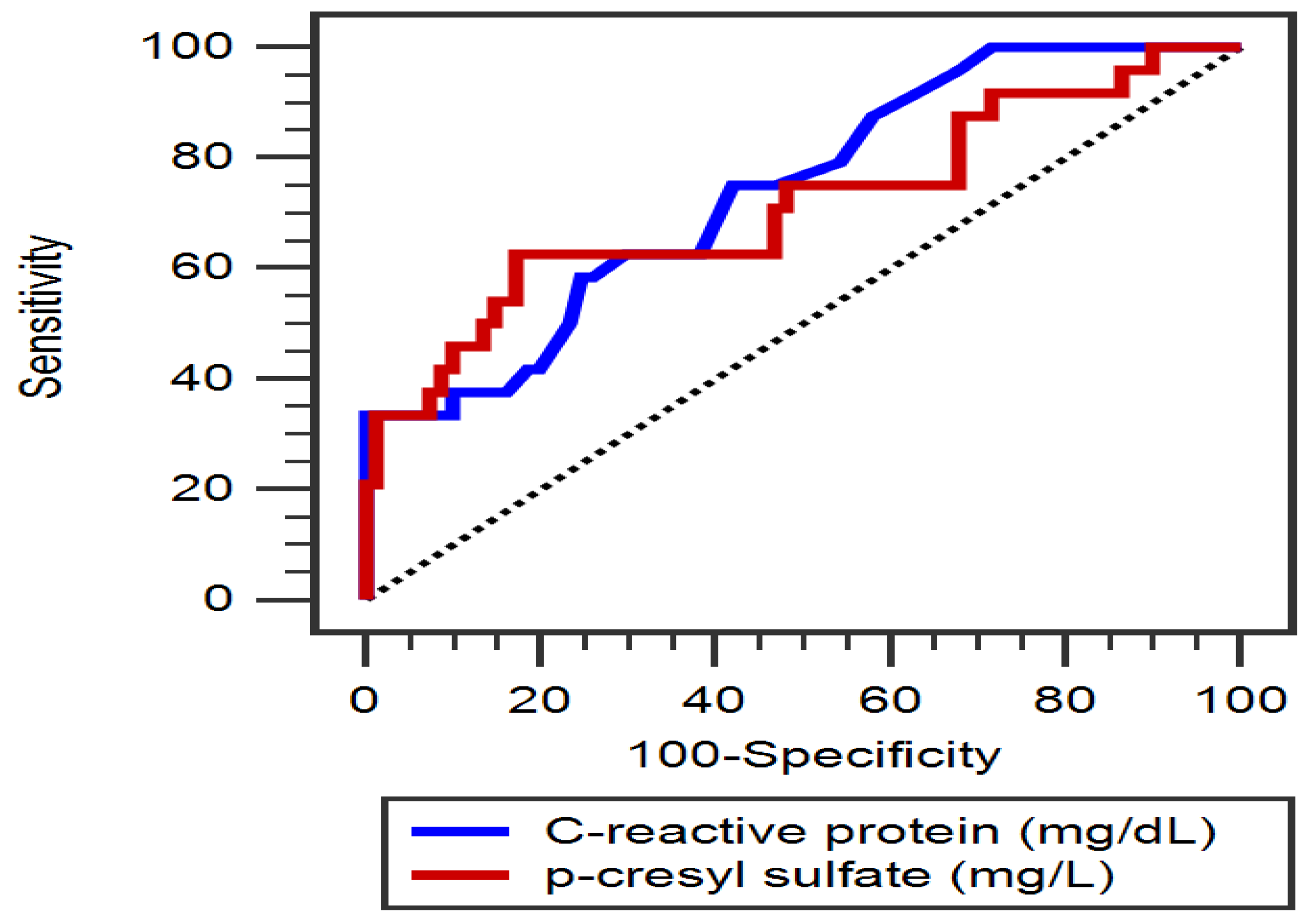
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, A.T.; Mojaddedi, S.; Loseke, I.H.; Bray, C. Hypertension in patients with peripheral artery disease: An updated literature review. Cureus 2024, 16, e62246. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Aronow, H.D.; Goodney, P.P.; Arya, S.; Brewster, L.P.; Byrd, L.; Chandra, V.; Drachman, D.E.; Eaves, J.M.; Ehrman, J.K.; et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2024, 149, e1313–e1410. [Google Scholar] [PubMed]
- Kharawala, A.; Nagraj, S.; Pargaonkar, S.; Seo, J.; Kokkinidis, D.G.; Altin, S.E. Hypertension management in peripheral artery disease: A mini review. Curr. Hypertens. Rev. 2024, 20, 1–9. [Google Scholar] [CrossRef]
- Poredoš, P.; Mikhailidis, D.P.; Paraskevas, K.I.; Blinc, A.; Antignani, P.L.; Stanek, A.; Mansilha, A.; Cevc, M. Management of arterial hypertension in patients with peripheral arterial disease. Int. Angiol. 2024, 43, 541–547. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gu, D.; Chen, J.; Wu, X.; Kelly, T.N.; Huang, J.F.; Chen, J.C.; Chen, C.S.; Bazzano, L.A.; Reynolds, K.; et al. Premature deaths attributable to blood pressure in China: A prospective cohort study. Lancet 2009, 374, 1765–1772. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, Y.; Zhang, H.; Ba, M.; Chen, P.; Li, H.; Chen, K.; Sha, W.; Zhang, C.; Chen, H. Association between high blood pressure and long term cardiovascular events in young adults: Systematic review and meta-analysis. BMJ 2020, 370, m3222. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. P-cresyl sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Shafi, T.; Meyer, T.W.; Hostetter, T.H.; Melamed, M.L.; Parekh, R.S.; Hwang, S.; Banerjee, T.; Coresh, J.; Powe, N.R. Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: Results from the retained organic solutes and clinical outcomes (ROSCO) investigators. PLoS ONE 2015, 10, e0126048. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Vanholder, R.; Van Biesen, W.; Pletinck, A.; Schepers, E.; Neirynck, N.; Speeckaert, M.; De Bacquer, D.; Verbeke, F. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, Y.L.; Lai, Y.H.; Wang, C.H.; Hsu, B.G. Serum p-cresyl sulfate level is an independent marker of peripheral arterial stiffness as assessed using brachial-ankle pulse wave velocity in patients with non-dialysis chronic kidney disease stage 3 to 5. Toxins 2022, 14, 287. [Google Scholar] [CrossRef]
- Hsu, H.J.; Yen, C.H.; Wu, I.W.; Hsu, K.H.; Chen, C.K.; Sun, C.Y.; Chou, C.C.; Chen, C.Y.; Tsai, C.J.; Wu, M.S.; et al. The association of uremic toxins and inflammation in hemodialysis patients. PLoS ONE 2014, 9, e102691. [Google Scholar] [CrossRef]
- Wang, C.P.; Lu, L.F.; Yu, T.H.; Hung, W.C.; Chiu, C.A.; Chung, F.M.; Yeh, L.R.; Chen, H.J.; Lee, Y.J.; Houng, J.Y. Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis 2010, 211, 579–583. [Google Scholar] [CrossRef]
- Chiu, C.A.; Lu, L.F.; Yu, T.H.; Hung, W.C.; Chung, F.M.; Tsai, I.T.; Yang, C.Y.; Hsu, C.C.; Lu, Y.C.; Wang, C.P.; et al. Increased levels of total p-cresylsulphate and indoxyl sulphate are associated with coronary artery disease in patients with diabetic nephropathy. Rev. Diabet. Stud. 2010, 7, 275–284. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, C.P.; Yu, T.H.; Hung, W.C.; Chung, F.M.; Lu, Y.C.; Hsu, C.C.; Lu, L.F.; Huang, L.L.; Lee, Y.J.; et al. Serum total p-cresylsulfate level is associated with abnormal QTc interval in stable angina patients with early stage of renal failure. Clin. Chim. Acta 2014, 437, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Tang, W.H.; Hsu, C.C.; Tsai, I.T.; Hung, W.C.; Yu, T.H.; Wu, C.C.; Chung, F.M.; Lu, Y.C.; Lee, Y.J.; et al. Associations among chronic kidney disease, high total p-cresylsulfate and left ventricular systolic dysfunction. Clin. Chim. Acta 2016, 457, 63–68. [Google Scholar] [CrossRef]
- Lin, C.J.; Pan, C.F.; Liu, H.L.; Chuang, C.K.; Jayakumar, T.; Wang, T.J.; Chen, H.H.; Wu, C.J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 2012, 225, 173–179. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, P.Y.; Tsai, J.P.; Wang, J.H.; Hsu, B.G. Serum osteoprotegerin levels and the vascular reactivity index in patients with hypertension. Medicina 2023, 59, 1794. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.T.; Lin, L.; Lin, H.J.; Lai, Y.H.; Hsu, B.G. Positive correlation of serum indoxyl sulfate level with peripheral arterial disease in hemodialysis patients. Vascular 2022, 30, 928–933. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Fiore, V.; Malaponte, G. Inflammation and Peripheral Arterial Disease: The Value of Circulating Biomarkers (Review). Int. J. Mol. Med. 2014, 33, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Celebi, S.; Berkten, B.; Amasyali, B. The Association between Thrombotic and Inflammatory Biomarkers and Lower-Extremity Peripheral Artery Disease. Int. Wound J. 2020, 17, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Joosten, M.M.; Pai, J.K.; Bertoia, M.L.; Rimm, E.B.; Spiegelman, D.; Mittleman, M.A.; Mukamal, K.J. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012, 308, 1660–1667. [Google Scholar] [CrossRef]
- Ridker, P.M.; Stampfer, M.J.; Rifai, N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001, 285, 2481–2485. [Google Scholar] [CrossRef]
- De Haro, J.; Acin, F.; Medina, F.J.; Lopez-Quintana, A.; March, J.R. Relationship between the plasma concentration of C-reactive protein and severity of peripheral arterial disease. Clin. Med. Cardiol. 2008, 3, 1–7. [Google Scholar] [CrossRef]
- Kim, B.G.; Seo, J.; Kim, G.S.; Jin, M.N.; Lee, H.Y.; Byun, Y.S.; Kim, B.O. Elevated C-reactive protein/albumin ratio is associated with lesion complexity, multilevel involvement, and adverse outcomes in patients with peripheral artery disease. Angiology 2022, 73, 843–851. [Google Scholar] [CrossRef]
- Sharma, P.; Klarin, D.; Voight, B.F.; Tsao, P.S.; Levin, M.G.; Damrauer, S.M. Evaluation of plasma biomarkers for causal association with peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1114–1123. [Google Scholar] [CrossRef]
- Saenz-Pipaon, G.; Martinez-Aguilar, E.; Orbe, J.; González Miqueo, A.; Fernandez-Alonso, L.; Paramo, J.A.; Roncal, C. The role of circulating biomarkers in peripheral arterial disease. Int. J. Mol. Sci. 2021, 22, 3601. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, Y.; Zhu, Z.; Su, X.; Ni, J.; Du, R.; Zhang, R.; Jin, W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front. Med. 2016, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef]
- Wu, P.H.; Lin, Y.T.; Chiu, Y.W.; Baldanzi, G.; Huang, J.C.; Liang, S.S.; Lee, S.C.; Chen, S.C.; Hsu, Y.L.; Kuo, M.C.; et al. The relationship of indoxyl sulfate and p-cresyl sulfate with target cardiovascular proteins in hemodialysis patients. Sci. Rep. 2021, 11, 3786. [Google Scholar] [CrossRef]
- Chang, J.F.; Hsieh, C.Y.; Liou, J.C.; Liu, S.H.; Hung, C.F.; Lu, K.C.; Lin, C.C.; Wu, C.C.; Ka, S.M.; Wen, L.L.; et al. Scavenging intracellular ros attenuates p-cresyl sulfate-triggered osteogenesis through MAPK signaling pathway and NF-κB activation in human arterial smooth muscle cells. Toxins 2020, 12, 472. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease (Medika study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Evenepoel, P. The gut-kidney axis: Indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol. Dial. Transplant. 2011, 26, 759–761. [Google Scholar] [CrossRef]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Kuypers, D.; Augustijns, P.; Meijers, B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1136–1144. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 105) | Control Group (n = 81) | Low ABI Group (n = 24) | p Value |
|---|---|---|---|---|
| Age (years) | 65.07 ± 10.41 | 64.51 ± 9.80 | 66.96 ± 12.29 | 0.313 |
| Height (cm) | 161.01 ± 8.63 | 161.41 ± 8.18 | 159.67 ± 10.08 | 0.386 |
| Body weight (kg) | 69.53 ± 12.81 | 68.93 ± 12.23 | 71.58 ± 14.71 | 0.375 |
| Body mass index (kg/m2) | 26.73 ± 3.82 | 26.36 ± 3.60 | 27.96 ± 4.35 | 0.071 |
| Left ankle-brachial index | 1.09 (1.01–1.13) | 1.11 (1.07–1.16) | 0.89 (0.83–0.94) | <0.001 * |
| Right ankle-brachial index | 1.07 (1.00–1.13) | 1.09 (1.05–1.14) | 0.87 (0.83–0.93) | <0.001 * |
| Systolic blood pressure (mmHg) | 131.95 ± 16.70 | 132.02 ±17.13 | 131.71 ± 15.52 | 0.936 |
| Diastolic blood pressure (mmHg) | 74.50 ± 10.17 | 74.60 ± 9.85 | 74.13 ± 11.40 | 0.840 |
| Total cholesterol (mg/dL) | 170.94 ± 41.04 | 167.83 ± 40.10 | 181.46 ± 43.31 | 0.154 |
| Triglyceride (mg/dL) | 128.00 (91.00–172.50) | 119.00 (88.50–179.50) | 141.50 (122.50–164.25) | 0.262 |
| HDL-C (mg/dL) | 44.52 ± 12.59 | 44.32 ± 12.51 | 45.21 ± 13.09 | 0.763 |
| LDL-C (mg/dL) | 99.88 ± 31.83 | 98.04 ± 32.70 | 106.08 ± 28.47 | 0.279 |
| Fasting glucose (mg/dL) | 107.00 (95.00–133.50) | 106.00 (94.50–131.50) | 114.00 (97.00–163.50) | 0.312 |
| Blood urea nitrogen (mg/dL) | 17.02 ± 5.07 | 16.79 ± 4.11 | 17.79 ± 7.52 | 0.398 |
| Creatinine (mg/dL) | 1.13 ± 0.30 | 1.10 ± 0.27 | 1.23 ± 0.39 | 0.054 |
| eGFR (mL/min) | 66.89 ± 18.94 | 68.69 ± 17.75 | 60.80 ± 21.84 | 0.073 |
| Total calcium (mg/dL) | 9.14 ± 0.37 | 9.14 ± 0.39 | 9.13 ± 0.30 | 0.919 |
| Phosphorus (mg/dL) | 3.49 ± 0.52 | 3.50 ± 0.52 | 3.48 ± 0.51 | 0.860 |
| iPTH (pg/mL) | 48.10 (33.75–65.25) | 47.30 (33.35–62.25) | 51.50 (34.68–75.08) | 0.296 |
| CRP (mg/dL) | 0.20 (0.15–0.27) | 0.19 (0.14–0.25) | 0.26 (0.20–0.81) | <0.001 * |
| Total p-cresyl sulfate (mg/L) | 6.91 (5.02–10.55) | 6.59 (4.53–8.32) | 11.02 (5.70–17.93) | 0.002 * |
| Male, n (%) | 71 (67.6) | 56 (69.1) | 15 (62.5) | 0.542 |
| Diabetes mellitus, n (%) | 33 (31.4) | 21 (25.9) | 12 (50.0) | 0.026 * |
| Coronary artery disease, n (%) | 70 (66.7) | 52 (64.2) | 18 (75.0) | 0.324 |
| Hyperlipidemia | 86 (81.9) | 68 (84) | 18 (75) | 0.319 |
| Stable congestive heart failure | 16 (15.2) | 11 (13.6) | 5 (20.8) | 0.388 |
| Stroke | 5 (4.8) | 3 (3.7) | 2 (8.3) | 0.352 |
| ACE inhibitor use, n (%) | 34 (32.5) | 28 (34.6) | 6 (25.0) | 0.379 |
| ARB use, n (%) | 49 (46.7) | 37 (45.7) | 12 (50.0) | 0.709 |
| β-blocker use, n (%) | 57 (54.3) | 42 (51.90) | 15 (62.5) | 0.358 |
| CCB use, n (%) | 40 (38.1) | 33 (40.7) | 7 (29.2) | 0.305 |
| Statin use, n (%) | 60 (57.1) | 46 (56.8) | 14 (58.3) | 0.893 |
| Fibrate use, n (%) | 21 (20.0) | 14 (17.3) | 7 (29.2) | 0.201 |
| Aspirin, n (%) | 63 (60.0) | 48 (59.3) | 15 (62.5) | 0.776 |
| Clopidogrel, n (%) | 30 (28.6) | 25 (30.9) | 5 (20.8) | 0.339 |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Total p-cresyl sulfate, 1 mg/L | 1.154 | 1.013–1.315 | 0.031 * |
| C-reactive protein, 0.1 mg/dL | 1.649 | 1.138–2.389 | 0.008 * |
| Diabetes mellitus, present | 2.095 | 0.609–7.199 | 0.240 |
| Body mass index, 1 kg/m2 | 1.098 | 0.936–1.288 | 0.249 |
| eGFR, 1 mL/min | 0.995 | 0.960–1.030 | 0.766 |
| Total cholesterol, 1 mg/dL | 1.004 | 0.991–1.017 | 0.547 |
| Variables | Left Log-ABI | Right Log-ABI | Log-PCS (mg/L) | |||
|---|---|---|---|---|---|---|
| Spearman Coefficient of Correlation | p Value | Spearman Coefficient of Correlation | p Value | Spearman Coefficient of Correlation | p Value | |
| Age (years) | –0.112 | 0.255 | –0.187 | 0.056 | 0.031 | 0.752 |
| Body mass index (kg/m2) | –0.139 | 0.157 | –0.057 | 0.561 | 0.049 | 0.621 |
| Left ABI | — | — | 0.690 | <0.001 * | –0.321 | 0.001 * |
| Right ABI | 0.690 | <0.001 * | — | — | –0.281 | 0.004 * |
| Log-PCS (mg/L) | –0.321 | 0.001 * | –0.281 | 0.004 * | — | — |
| SBP (mmHg) | 0.042 | 0.669 | –0.036 | 0.718 | 0.011 | 0.910 |
| DBP (mmHg) | 0.060 | 0.545 | 0.040 | 0.685 | –0.009 | 0.926 |
| Total cholesterol (mg/dL) | –0.179 | 0.068 | –0.056 | 0.574 | 0.104 | 0.292 |
| Log-Triglyceride (mg/dL) | –0.035 | 0.723 | 0.024 | 0.811 | 0.093 | 0.347 |
| HDL-C (mg/dL) | –0.092 | 0.349 | –0.120 | 0.224 | –0.079 | 0.421 |
| LDL-C (mg/dL) | –0.125 | 0.203 | 0.006 | 0.948 | 0.100 | 0.308 |
| Log-Glucose (mg/dL) | 0.004 | 0.972 | –0.042 | 0.673 | –0.005 | 0.961 |
| eGFR (mL/min) | 0.149 | 0.129 | 0.199 | 0.042 * | –0.325 | 0.001 * |
| Total calcium (mg/dL) | –0.005 | 0.964 | –0.041 | 0.675 | 0.139 | 0.159 |
| Phosphorus (mg/dL) | 0.001 | 0.992 | –0.022 | 0.824 | 0.156 | 0.111 |
| Log-iPTH (pg/mL) | –0.153 | 0.119 | –0.051 | 0.605 | –0.061 | 0.576 |
| Log-CRP (mg/L) | –0.382 | <0.001 * | –0.446 | <0.001 * | 0.220 | 0.024 * |
| Age (years) | –0.112 | 0.255 | –0.187 | 0.056 | 0.031 | 0.752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-B.; Tsai, J.-P.; Hsu, B.-G.; Liu, C.-H.; Wang, J.-H. Serum P-Cresyl Sulfate Levels Correlate with Peripheral Arterial Disease in Hypertensive Patients. Diagnostics 2025, 15, 1097. https://doi.org/10.3390/diagnostics15091097
Chern Y-B, Tsai J-P, Hsu B-G, Liu C-H, Wang J-H. Serum P-Cresyl Sulfate Levels Correlate with Peripheral Arterial Disease in Hypertensive Patients. Diagnostics. 2025; 15(9):1097. https://doi.org/10.3390/diagnostics15091097
Chicago/Turabian StyleChern, Yahn-Bor, Jen-Pi Tsai, Bang-Gee Hsu, Chin-Hung Liu, and Ji-Hung Wang. 2025. "Serum P-Cresyl Sulfate Levels Correlate with Peripheral Arterial Disease in Hypertensive Patients" Diagnostics 15, no. 9: 1097. https://doi.org/10.3390/diagnostics15091097
APA StyleChern, Y.-B., Tsai, J.-P., Hsu, B.-G., Liu, C.-H., & Wang, J.-H. (2025). Serum P-Cresyl Sulfate Levels Correlate with Peripheral Arterial Disease in Hypertensive Patients. Diagnostics, 15(9), 1097. https://doi.org/10.3390/diagnostics15091097








