Abstract
Background/Objectives: Symptoms (NPS) in Alzheimer’s disease (AD) have multiple effects in daily living, not only for the patients but for their caregivers too. The present systematic review was performed in order to identify if biomarkers, cognitive functions, and personality traits can be considered as important factors for the development and maintenance of these symptoms. Methods: To achieve that, the existing literature spanning the period from 2018 to 2024 was critically analyzed. To be included in the review, a study had to investigate any of the factors mentioned above. In total, 182 articles were assessed for eligibility, and 50 met the inclusion criteria. Results: Most of the studies were focused on the role of biomarkers and found that amyloid β, tau and phospho-tau protein are closely related to the incidence and the severity of NPS. In fewer studies, cognitive function and personality traits were also associated with NPS. Conclusions: In conclusion, biomarkers, cognitive function and personality traits are associated with NPS, but the underlying mechanisms, still, mostly remain unknown.
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease, characterized by memory loss, deficits in other cognitive functions, and behavioral changes that can ultimately affect daily functioning. The main neuropathological hallmarks of the disease are the accumulation of amyloid plaques and neurofibrillary tangles, which lead to synaptic loss and, ultimately, neurodegeneration [1].
AD diagnosis has been based mainly on clinical manifestations, making it a possible or probable diagnosis rather than a confirmed one. However, in recent years, the development of biomarkers has succeeded in measuring in vivo AD pathophysiology. These biomarkers can be found in blood, in cerebrospinal fluid (CSF) or with the use of Positron Emission Tomography (PET). The identified biomarkers are the amyloid β (Aβ) (A) deposition, the tau protein (T), and the neurodegeneration (N), creating an unbiased descriptive classification scheme [2]. The severity of brain changes is linked to the level of cognitive decline a person experiences [3].
However, AD is not a normal part of aging. According to the World Health Organization, healthy aging is not merely the absence of disease, but rather a lifelong process of maximizing functional abilities to support well-being in older age [4]. Nevertheless, older adults are usually experiencing the preclinical AD “stage” of Mild Cognitive Impairment (MCI), which is characterized by objectively measured deficits in one or more cognitive domains, as demonstrated by standardized neuropsychological testing, while functional independence in daily life is preserved [5]. MCI presents in various subtypes, including amnestic MCI, primarily affecting memory, and non-amnestic MCI, affecting other cognitive functions. These subtypes are further categorized as either single-domain or multi-domain, based on whether a single cognitive function or multiple cognitive functions are impaired, respectively [6]. A meta-analysis of 41 cohort studies found annual conversion rates of MCI to AD dementia of 8.1% and 6.8% in specialist and community settings, respectively [7]. Besides these findings, Davis et al. (2018) found that annual transition probabilities from MCI to more severe states at age 65 were 8% for normal cognition, 22% for MCI due to AD, and 25%, 36%, and 16% for mild, moderate, and severe AD dementia, respectively [8]. The likelihood of progression increased with age, whereas a positive correlation was observed between age, AD severity, and the rates of institutionalization and mortality.
Neuropsychiatric symptoms (NPS) are common in dementia and can be challenging to treat due to their wide range and complex causes. The most common categories of NPS include agitation, aggression, irritability, mood disorders, anxiety, hallucinations, delusions, sleeping disturbances, and eating disorders [9]. The development of NPS is likely caused by a complex interplay of factors. Central to this process is neurodegeneration, which damages circuits involved in emotions, actions, motivation, or perception. This damage can directly trigger NPS or make individuals more susceptible to environmental factors. Additionally, NPS may occur due to challenges individuals face in adjusting to their surroundings as their cognitive abilities decline. Other potential causes include unmet needs, acute illnesses that cause confusion, or environments and caregiving that are not suited to the patient’s current abilities [10]. NPS can have serious consequences if not managed effectively. They can accelerate disease progression, interfere with daily activities, reduce quality of life, and increase caregiver burden and healthcare costs. In severe cases, NPS may lead to hospitalization or admission to a long-term care facility [11].
It is known that the frequency and the severity of NPS increase with the deterioration of cognition, and, for this reason, they may constitute early manifestations of AD [12]. Specifically, 30% of AD cases show NPS in advance of a remarkable cognitive decline [13]. Neuroimaging biomarkers show that grey and white matter structural atrophy is associated with a higher frequency of NPS, especially psychosis, agitation, and apathy [14]. As the disease progresses to MCI, NPS such as agitation, sleep disturbances, and irritability become more frequent and have been linked to an increased risk of progression to dementia [15]. Neurobiological studies indicate that NPS in MCI correlate with amyloid-beta and tau accumulation, neuroinflammation, and structural brain changes affecting the fronto-limbic circuits. The presence of NPS across these early disease stages suggests that they could serve as biomarkers for early diagnosis and targets for intervention to slow the progression to AD [16].
We still do not fully grasp the exact connection between neuropsychiatric symptoms and cognitive impairment. Proposed mechanisms include four possible pathways, which are not mutually exclusive but could potentially work together in various ways: (1) an etiologic pathway, where NPS might initiate pathophysiologic brain changes that ultimately lead to AD pathology, (2) a common neuropathological pathway, where NPS and cognitive deficits might originate from damage to the same brain regions, (3) a psychological reaction, as individuals become aware of their declining cognitive abilities and may experience emotional distress like depression or anxiety, and (4) a combination of neuropsychiatric symptoms and biological factors that might accelerate cognitive decline [17]. Moreover, Young et al. (2018) reported a consistent association between premorbid neuroticism and the development of NPS. In contrast, premorbid conscientiousness, extraversion, openness, and agreeableness may be predictive factors for the development of NPS in the future [18]. These findings suggest that the potential predictive value of various personality traits requires further investigation using more standardized and comprehensive methodologies.
Furthermore, several non-biological factors can contribute to the development of NPS [19]. A large percentage of people with dementia experience NPS triggered by undiagnosed medical issues such as urinary tract infections, thyroid problems, anemia, constipation, and pneumonia [20]. Moreover, drug interactions and inadequate assessment or treatment of pain can lead to NPS [21], or they can be an expression of unmet needs (physical, psychological, social, or emotional) [19]. A lack of communication and activity, either due to verbal difficulties, lack of environmental stimuli, or the negative styles of a carer’s communication, can also lead to NPS [22,23]. However, the present study does not focus on these non-biological factors, because we considered, as the first crucial step, to clarify—at least to some extent—the potential biologically defined substrate that can be associated with specific NPS in AD, something that seems complicated enough in the extant literature.
Objectives
Specifically, the primary objective of this systematic review is to provide a comprehensive analysis of the role of AD-related biomarkers, cognition, and personality traits in the development and maintenance of each of the main NPS in AD. The study is guided by research questions such as: “What is the role and potential interplay of specific biomarkers, cognitive functions, and personality traits in the development of each of the main NPS in AD? Are there any differences in the biologically defined substrate which can be associated with the specific NPS? Are there any common biologically defined factors that can be associated with all or many of the NPS in AD?”. The present, PRISMA-compliant, systematic review aims to critically analyze recent studies published between 2018 and 2024, summarizing how these factors contribute to specific NPS development in AD. Through a thorough examination of existing literature, we seek to clarify whether specific biomarkers, cognitive functions, and personality traits can help explain challenging behaviors in AD.
2. Materials and Methods
2.1. Search Strategy
The systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for the reporting of systematic reviews and meta-analyses [9]. Literature research was conducted in December of 2024 in PubMed (MEDLINE), Scopus, Semantic Scholar, and Google Scholar using the following keywords, which had to be part of the title, abstract, or keywords: (“neuropsychiatric symptoms” OR “behavioral symptoms” OR “psychological symptoms”) AND (“Alzheimer’s Disease” OR AD) AND biomarkers AND (cognition OR “cognitive functions” OR “cognitive abilities”) AND (personality OR “personality traits”).
2.2. Eligibility Criteria
Inclusion criteria for the review required studies to investigate at least the relationship of NPS with AD biomarkers using blood, CSF, or PET. Only recent studies published in English between 1 January 2018 and 20 July 2024 were considered, because there were somewhat similar systematic reviews before. Studies published in English were selected to facilitate accurate comprehension and analysis. The timeframe was also selected to capture the most current research, particularly regarding the increasing integration of biomarkers in diagnostic procedures of AD. The present review focused on primary research studies to ensure the inclusion of original data and analysis. Consequently, previous reviews, meta-analyses, editorials, and chapters were excluded.
2.3. Study Selection
The online database search yielded 135 potential studies. Duplicate removal reduced this to 98 records, which were subsequently screened. Of these, 22 were excluded due to being some type of review, and 30 were excluded for lack of relevance to the aim of the study and the research questions. The excluded studies looked at biomarkers related to AD pathology but did not measure or correlate them with NPS. Consequently, 49 studies met the eligibility criteria and were included in the final analysis. The Newcastle–Ottawa Scale was used to assess the quality of included studies. Scores were assigned for selection, comparability, and outcome/exposure. Only studies with scores above 5 were included in the final analysis (see Appendix A).
2.4. Data Synthesis
A qualitative synthesis was performed to integrate the findings of the included studies. To improve the presentation of the results, the data were organized according to the type of the design of the study (longitudinal or cross-sectional), in order to detect whether there were any systematic differences in the findings of the two types of studies regarding the role of specific biomarkers, cognitive functions, and personality traits, as well as their interplay, in the development of NPS (see Table 1).

Table 1.
Summary of the included studies examining biomarkers, personality traits, and cognitive abilities related to NPS. The studies are presented in two parts based on their design, longitudinal or cross-sectional.
At a second step—after the presentation of the results related to potential common factors and their interplay that can be associated with all or many of the NPS in AD, as these factors are revealed from longitudinal and cross-sectional studies separately—the data were organized on the basis of each NPS, in order to reveal potential specific biologically-defined substrates that can be associated with specific NPS. At this point, it should be mentioned that the present review does not aim to reveal causal relationships between the factors of interest and NPS. Rather, it aims to represent one of the first trials to detect patterns of associations between specific biologically defined factors and specific NPS in AD. This scientific trend, very recently, seems to have already begun to give some interesting research data.
3. Results
3.1. The Included Papers
This review included 50 studies [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] that investigated the role of AD-related biomarkers, cognitive abilities, and personality traits in the development of NPS. The literature search and selection process, adhering to PRISMA 2020 guidelines [74], are depicted in Figure 1. Table 1 provides a detailed summary of the included studies, including sample sizes, study designs, objectives, measures, biomarkers, and key findings.
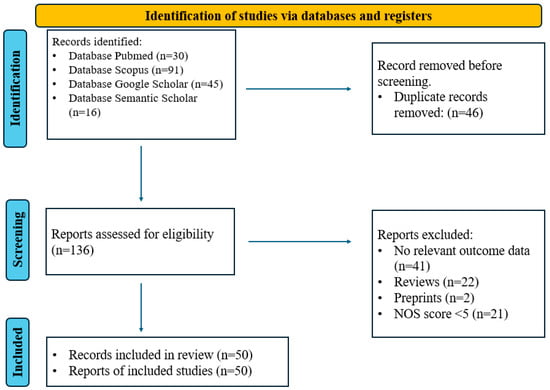
Figure 1.
PRISMA 2020 flow chart for the selection of articles included in the systematic review.
3.2. Characteristics of the Included Studies
Findings from 50 studies included in the review suggest that several biologically defined variables may be responsible for the development and occurrence of NPS (Table 2). However, the relative contribution of each variable appears to vary according to study design, disease stage, and methodological quality. While cross-sectional studies tend to confirm concurrent associations, longitudinal studies yield stronger evidence regarding predictive and dynamic associations. All retrieved articles were written in English, and notably, there was considerable heterogeneity in the sample sizes and the diagnosis of the participants. The majority of studies included patients with dementia and MCI, whereas others included SCI patients and healthy participants. This variability reflects the natural trajectory of Alzheimer’s disease and related dementias. The use of different populations is not a significant bias, but it aligns with the increasingly accepted view of dementia as a continuum, where biological changes precede clinical symptoms by years. Moreover, longitudinal studies involving early-stage participants (cognitively unimpaired or MCI) facilitate the identification of NPS as prodromal features of dementia, rather than consequences of cognitive decline [26,41].

Table 2.
Summary of biomarkers, cognitive functions, imaging data, and personality trait correlates associated with specific NPS.
The NPS in most of the studies were measured with the NPI scale, which is the most widely used neuropsychological tool for the measurement of NPS over the past 25 years. It consists of 12 domains, each containing questions, sub-questions, and ratings of frequency and severity. These domains assess hallucinations, delusions, anxiety, depression, apathy, irritability, agitation, disinhibition, elation, aberrant motor disturbances, appetite/eating behaviors, and night-time sleep disturbances. Moreover, it has been translated into approximately 40 languages, and it is recognized as a valid and reliable tool [75].
To assess cognitive functions, a variety of neuropsychological tools were used. Most studies in this review utilized the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) to measure global cognition and the Clinical Dementia Rating (CDR) scale to evaluate functional abilities. To measure executive functions, Trail Making Tests A and B were mainly used. Additionally, specialized neuropsychological tests were used to examine specific cognitive functions, depending on each study’s objectives and expected outcomes.
For the assessment of personality, the NEO Five-Factor Inventory (FFI) was mainly used. It is a standardized psychometric tool developed to assess the five broad personality factors according to the Five-Factor Model: neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness. It contains 60 items, with 12 items per trait. As it is widely validated, its brevity, ease of administration, and strong psychometric properties, such as high internal consistency and construct validity, make it useful in large-scale or longitudinal studies, including those focused on aging and neurodegenerative diseases. In dementia research, the NEO-FFI has been useful for the assessment of premorbid personality traits that potentially modulate vulnerability to NPS, cognitive decline, or disease progression. However, as a self-report measure, it may be biased, especially when it is administered to individuals with cognitive impairment [76].
For the evaluation of biomarkers, CSF samples were mainly used and specifically amyloid β (Aβ), tau, and p-tau protein. Some studies also examined these biomarkers in plasma. More recent studies used PET (amyloid or FDG) to have more precise measurements of biomarkers and their role in specific brain regions. Additionally, MRI was frequently used to assess structural changes in the brain because of neurodegeneration.
3.3. Findings Related to NPS in General and to Specific Ones
3.3.1. Findings Related to NPS in General
Studies examining NPS globally, often measured with the total NPI, identified biomarkers related to amyloid and tau pathology as key contributors. Lower CSF Aβ42 levels and reduced Aβ42/Aβ40 ratio were repeatedly associated with higher total NPI scores, suggesting a relationship between amyloid burden and the severity of NPS [26,38,44,45,48,49,59,60,70,73]. Longitudinal studies further demonstrated that reduced Aβ42, in combination with elevated total tau and p-tau, predicted the worsening of total NPI scores over time [26,41]. Furthermore, tau/Aβ42 and p-tau/Aβ42 ratios were associated with general NPS burden and progression [26,56,59].
Several studies highlighted the role of neuroinflammation and neurodegeneration biomarkers in NPS. Increased levels of inflammatory markers such as interleukin-6 (IL-6), C-reactive protein (CRP), and soluble intercellular adhesion molecule-1 (sICAM-1) were observed in individuals with higher total NPI scores, indicating systemic inflammatory involvement [33,39]. Moreover, glial activation, measured either through plasma GFAP or PET-based microglial imaging, was associated with higher NPI scores, demonstrating the relevance of innate immune activation in the behavioral phenotype of AD [62,64]. Structural neuroimaging studies supported these findings, linking gray matter atrophy in the hippocampus, anterior cingulate cortex, and medial frontal regions with more severe NPS profiles. In addition, several studies identified widespread white matter damage, particularly in the cingulum, fornix, and frontal associative tracts, as correlates that could be associated with higher NPI scores, suggesting that disrupted connectivity contributes to behavioral dysregulation [59].
Functional imaging provided mixed results. While some PET studies reported associations between increased amyloid or tau binding and general NPI severity [30,40], others found weaker or non-significant links—particularly for amyloid—when tau burden, inflammation, or connectivity disruption were also considered [38,51]. Notably, Dang et al. [55] concluded that tau PET outperformed both amyloid and FDG-PET in predicting total NPI scores, pinpointing the significance of tau pathology in the neurobiology of NPS. Across studies, longitudinal imaging data offered stronger evidence for predictive relationships between biomarker changes and behavioral trajectories compared to cross-sectional associations.
Cognitive impairment also played a role in NPS expression. While findings varied across studies, global cognitive decline and executive dysfunction were the most commonly recognized domains [26,41,53,54,68]. Deficits in cognitive flexibility, attention, and memory were associated with higher total NPI scores, suggesting that broad cognitive dysfunction may amplify or reflect underlying behavioral disturbances. Importantly, longitudinal analyses showed that baseline cognitive impairments could precede increases in NPI scores, supporting a model in which cognitive symptoms and NPS evolve concurrently but are also bidirectionally reinforcing over time [27,33,41].
Only five studies explored the role of personality traits in modulating overall NPS burden. The findings suggest that higher neuroticism may increase general vulnerability to behavioral disturbance, while conscientiousness, extraversion, and openness may have protective effects [30,43]. A longitudinal study by Ronat et al. [43] showed that individuals with higher emotional instability displayed not only higher total NPI scores but also greater hippocampal atrophy, implying a potential pathway linking personality to neurodegeneration and behavior. Moreover, Waschkies et al. [61] demonstrated that combining personality assessments with CSF biomarkers significantly improved the classification of clinical AD stages, suggesting the clinical value of integrating personality assessment into different predictive models.
3.3.2. Depression
Depression was the most frequently studied NPS, with 12 studies examining its associations with biomarkers and cognitive deficits. Depression was associated with AD pathology, with longitudinal studies identifying higher p-tau and low Aβ42 levels as possible factors associated with depressive symptoms [26,36,56]. Some studies further highlighted the role of elevated cortisol and altered HPA axis activity in sustaining depressive symptoms over time [47]. Findings from longitudinal studies, using MRI, revealed prefrontal and hippocampal atrophy associated with depression onset, suggesting that there are overlapping circuits with memory and emotional regulation. Cross-sectional findings supported these associations, particularly linking depression with tau burden and global atrophy in medial temporal and limbic regions [42]. However, these studies often failed to distinguish whether depression preceded, followed, or co-occurred with structural changes, limiting causal inference.
In contrast to apathy, depression was linked not only to executive dysfunction but also to memory and language impairments. Cross-sectional data confirmed these cognitive associations [33], but longitudinal findings provided more precise evidence that baseline depression predicted faster decline in cognition and increased risk of progression to AD [26,36].
3.3.3. Apathy
Ten studies were focused on apathy. Longitudinal studies showed strong associations between tau pathology and executive dysfunction. Elevated p-tau and p-tau/Aβ42 ratios were significantly predictive of worsening apathy over time in multiple longitudinal investigations [26,41,59], particularly in association with degeneration in prefrontal and anterior cingulate regions. Structural imaging from these studies showed that white matter damage, especially in the cingulum and uncinate fasciculus, preceded apathy progression, supporting a model of damage in fronto-limbic circuits. Cross-sectional studies reported similar associations between apathy and tau burden [40,53], but their findings were often limited to concurrent relationships and lacked the temporal specificity that longitudinal studies provided.
Cognitively, apathy was associated with deficits in executive functions, especially in tasks involving initiation and goal setting. Cross-sectional studies often linked apathy to poor performance on executive tasks such as the Stroop and TMT-B [54]. Longitudinal studies confirmed that executive deficits may precede apathy progression [26,27,41].
3.3.4. Anxiety
Anxiety was also found to be associated with biomarkers and cognitive deficits. Longitudinal studies showed that anxiety in cognitively unimpaired individuals was associated with higher plasma NfL and predicted decline in processing speed and attentional control [27,41]. These findings suggest that anxiety may signal early neural injury, even before overt tau or amyloid changes are apparent. Cross-sectional studies identified similar associations [42] and added the role of altered connectivity in salience network [67]. Furthermore, both study types identified neuroinflammatory markers (e.g., IL-6, CRP) as correlates [33]. Cognitively, anxiety was associated with deficits in attention and verbal fluency in both designs, though longitudinal data clarified that such cognitive decline may follow rather than precede anxiety [27].
3.3.5. Agitation and Other Frontal Symptoms
In nine studies, frontal symptoms, including agitation, disinhibition, and irritability, were closely linked with neurodegeneration and inflammatory biomarkers. Longitudinal studies demonstrated that high levels of plasma p-tau181 and neuroinflammatory markers, such as TNF-α, were associated with the development of agitation and disinhibition [25,58] and with accelerated frontal cortical thinning and anterior cingulate degeneration [33,41,53]. Cross-sectional studies associated frontal symptoms with atrophy in the dorsolateral prefrontal cortex and white matter lesions in frontal tracts [59,64]. However, these relationships were correlational, with limited ability to account for disease progression or symptom fluctuation. Both study types supported a strong link between frontal symptoms and executive dysfunction, though only longitudinal analyses indicated that frontal network disruption predicted future behavioral dysregulation [26,41].
3.3.6. Νight-Time Behavior
Analyzing five studies, night-time behavioral disturbances were shown to correlate with tau pathology, salience network dysfunction, and frontal atrophy. Longitudinal studies found that individuals with elevated p-tau/Aβ42 ratios and prefrontal cortical thinning experienced greater worsening of sleep symptoms over time [35,56,67]. APOE ε4 status was found to moderate this relationship, particularly among individuals with existing tau pathology [67]. Cross-sectional studies reported similar biomarkers but failed to prove the directional relationship. For instance, studies linking amyloid burden with fragmented sleep patterns [55] could not confirm whether sleep disturbance was a cause or consequence. In both designs, sleep disturbances were associated with hyperconnectivity in salience and default network.
3.3.7. Psychotic Symptoms
In six studies, psychotic symptoms, including delusions and hallucinations, were associated with more severe AD stages. Longitudinal studies found that elevated p-tau181 and a-synuclein/Aβ42 ratio were associated with psychosis onset [52,72]. Structural MRI showed that psychosis developed in individuals with pronounced prefrontal atrophy and impaired connectivity with limbic circuits [33,41]. Cross-sectional studies confirmed these associations but were less consistent. For example, delusions were sometimes linked with hippocampal atrophy [60], but without clear differentiation between early and late disease stages. Longitudinal data provided stronger evidence that psychosis follows the accumulation of multiple pathologies and genetic susceptibility (e.g., APOE ε4) and is associated with rapid cognitive decline.
3.3.8. Appetite Disorders
Only two studies in this review examined appetite changes or disorders in their outcomes. Krell-Roesch et al. (2022) found that higher levels of plasma-derived p-tau181 and p-tau217 were associated with increased symptoms of disinhibition, agitation, and appetite changes, as measured by the NPI [58]. Furthermore, Jiang et al. (2024) found that NPS can be predictors of decline in nutritional status as higher scores in NPI were longitudinally associated with lower scores in the Mini Nutritional Assessment [47]. This study also supported the idea that NPS may negatively impact eating patterns and overall nutrition, even when they are not limited to appetite-related symptoms. So, NPS such as apathy, agitation, or psychomotor dysfunction may disrupt meal routines, reduce food intake, or lead to caregiver-related feeding challenges.
4. Discussion
The present systematic review provides a comprehensive analysis of recent studies (2018–2024) examining the relationship between NPS in AD and three key factors: biomarkers, cognitive functions, and personality traits. Compared to previous systematic reviews, which primarily explored the role of amyloid and tau biomarkers, this study expands these findings by adding personality traits and cognitive dysfunction as possible contributors to NPS. Earlier reviews established correlations between Aβ pathology and symptoms such as apathy and depression, but they lacked extensive longitudinal evidence to assess the predictive validity of these biomarkers. Furthermore, previous research has attributed NPS to neurotransmitter imbalances, particularly in acetylcholine, serotonin, and dopamine pathways [9]. The review by Showraki et al. (2019) focused exclusively on CSF biomarkers and their association with NPS, particularly in patients with AD and MCI [77]. While agitation and aggression were the most consistently related symptoms to CSF biomarkers, it also reported conflicting results for the other NPS. The current review also includes findings on inflammatory biomarkers such as NfL and GFAP, which were not extensively covered in prior reviews but appear to be associated with NPS, particularly in early AD stages [73]. Moreover, the inclusion of both longitudinal and cross-sectional studies helped for a comparative analysis of the temporal dynamics and predictive strength of various biomarkers and cognitive domains.
Tau pathology, and especially high levels p-tau and p-tau/Aβ42 ratios, emerged as the most consistent biological factor of NPS. These biomarkers were repeatedly associated with the presence and worsening of apathy, depression, agitation, and psychosis, especially in longitudinal studies that captured their progression over time [26,30,41,56,59]. Imaging data found that tau deposition was mainly pointed in fronto-limbic circuits, confirming its role in the neural basis of NPS. In contrast, Aβ42, even though it was commonly examined, demonstrated weaker associations, especially when analyzed independently of tau. Aβ42 appears to play a more prominent role in the early stages of the disease, acting as a background condition that facilitates the emergence of NPS, rather than being a direct cause of their development [40,50,56].
Neuroinflammatory biomarkers in both CSF and plasma were also detected. Specifically, interleukin-6, C-reactive protein (CRP), NfL, and GFAP were linked with the severity of NPS [33,52,62]. Longitudinal evidence further confirmed that these inflammatory markers were not only present in symptomatic individuals but also predicted symptom onset and progression, particularly for agitation, anxiety, and frontal symptoms. Moreover, disruption in the default mode and salience networks was associated with symptoms like sleep disturbances and anxiety, especially in Aβ-positive individuals [35,55,78].
Cognitive impairment consistently related to NPS, with executive dysfunction being the most prominent cognitive dysfunction. Deficits in cognitive flexibility, attention, and inhibitory control—as assessed by tools such as the Trail Making Test-B, Stroop Test, and Symbol Digit Modalities Test—were associated with apathy, agitation, and sleep disturbances [26,41,53,54]. Importantly, longitudinal studies revealed that executive dysfunction often preceded the onset or worsening of NPS, emphasizing its predictive value. Episodic memory deficits were significantly associated with affective and psychotic symptoms [27,33,68].
While the majority of studies support strong biomarker–NPS relationships, inconsistencies have emerged, and multiple factors could explain these contradictions. One potential explanation lies in methodological differences across studies. The measurement of biomarkers varies significantly depending on the sample type (e.g., CSF vs. plasma vs. PET imaging) and the techniques used for analysis. Some studies use CSF biomarkers, which provide a more direct reflection of pathological changes in the brain, but others use plasma biomarkers, which may be affected by peripheral factors such as systemic inflammation or blood–brain barrier integrity. Additionally, variations in imaging modalities can contribute to discrepancies. For example, some studies using PET imaging have found no correlation between amyloid burden and NPS [50], whereas studies using CSF biomarkers have reported strong associations [26].
Another source of inconsistency is related to differences in patient populations. Studies included in this review ranged from individuals with MCI to mild or moderate AD, and it is likely that the role of biomarkers in NPS differs depending on the disease stage. For example, amyloid accumulation may play a more significant role in the early stages of AD, whereas tau pathology and neurodegeneration might be more strongly linked to NPS in later stages. Environmental and psychosocial factors may also account for some of the contradictory findings. NPS do not emerge solely due to underlying neuropathology but can also be influenced by lifestyle factors, social interactions, caregiver burden, and stress levels. Some studies did not control these external influences, which could contribute to variability in results.
Regarding personality traits, the analysis of the five included studies indicated that high neuroticism emerged as a risk factor for depression, anxiety, and irritability, whereas conscientiousness and extraversion demonstrated a protective effect by reducing NPS [30,43,61]. Moreover, these personality traits may interact with underlying neurobiological mechanisms. For instance, neuroticism has been associated with dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis and increased cortisol levels, which have been implicated in both tau phosphorylation and progression of NPS [47]. On the other hand, extraversion and openness may reflect cognitive reserve buffering against the impact of neuropathological burden on behavior [30,61]. These findings support biopsychosocial models of dementia that integrate personality traits into the assessment and holistic treatments.
Symptom-level analysis revealed trends different associations with biomarkers and cognitive functions. Depression was strongly associated with tau pathology, prefrontal atrophy, and elevated cortisol, suggesting a synergistic effect between neurodegeneration and HPA axis dysfunction [26,36,47]. Apathy had a distinct profile, with elevated tau, fronto-limbic atrophy, and disconnection in white matter tracts such as the cingulum and uncinate fasciculus [30,41,59]. Anxiety was characterized by elevated NfL, salience network dysfunction, and neuroinflammation, appearing as both a prodromal and concurrent symptom in various stages of AD [27,41,67]. Agitation and disinhibition were linked to TNF-α, p-tau, and atrophy in the dorsolateral prefrontal cortex, reflecting advanced neurodegeneration [25,33,41]. Sleep disturbances were associated with default mode network dysregulation and tau pathology, particularly among APOE ε4 carriers [35,56]. Psychotic symptoms, including hallucinations and delusions, usually appear later in the course of the disease and were associated with α-synuclein, p-tau, and atrophy in the prefrontal cortex and limbic structures [52,60]. Appetite changes, although under-studied, were related to p-tau217 and orbitofrontal disinhibition [47,58].
A key strength of this review is the direct comparison between longitudinal and cross-sectional studies. Longitudinal designs provided stronger evidence for temporal relationships and predictive value, allowing for more definitive conclusions regarding causality and disease trajectory. Cross-sectional studies, even though they were useful for identifying patterns, were more limited by potential confounders and the inability to establish directionality.
Despite its comprehensive approach, this review has, also, some limitations. One major limitation is its biologically deterministic framework, which assumes that NPS are primarily driven by biological mechanisms such as amyloid and tau pathology, neuroinflammation, and neurotransmitter dysfunction. While biomarkers provide valuable insights into disease progression, this perspective may oversimplify the role of environmental and psychological factors in the manifestation of NPS. They can be influenced by variables such as caregiver interactions, social isolation, stress levels, and pre-existing psychiatric conditions, which were not considered in this review. Furthermore, while personality traits were included as an influencing factor, the complex interplay between psychosocial experiences and biological vulnerability remains underexplored. The timespan of this review (2018–2024) represents another limitation. While focusing on recent literature ensures the inclusion of the latest advancements in biomarker research, neuroimaging techniques, and theoretical models, it may exclude foundational studies that shaped current understanding of NPS. Another limitation relates to the heterogeneity of study methodologies, particularly in the assessment of both biomarkers and NPS. Different studies utilize CSF, PET, or plasma biomarkers, leading to inconsistent findings regarding the relationship between amyloid and tau burden with NPS. Although biomarkers are increasingly used to characterize and predict NPS, their interpretability varies based on the method of measurement (e.g., CSF, PET, plasma), disease stage, and individual-level factors such as cognitive reserve or genetic profile. While this review primarily focused on biological and cognitive correlates of NPS, future studies should adopt multimodal, prospective designs that integrate biomarkers, cognitive batteries, and psychosocial variables to generate individualized predictive models.
5. Conclusions
The present study highlights the complex interplay between biomarkers, cognitive functions, and personality traits in the manifestation and progression of NPS in AD. While tau pathology, neuroinflammation, and executive dysfunction emerged as prominent contributors, the expression of NPS is likely influenced by a broader set of dynamic and interrelated mechanisms. The findings support the need for longitudinal studies that integrate biological and psychosocial perspectives to better understand and manage behavioral disturbances across the continuum of dementia.
Author Contributions
Conceptualization, A.C. and D.M.; methodology, A.C. and D.M.; validation, D.M., V.P. and M.T.; formal analysis, A.C.; investigation, A.C.; data curation, A.C.; writing—original draft preparation, A.C. and D.M.; writing—review and editing, D.M., V.P. and M.T.; supervision, D.M., V.P. and M.T.; project administration, A.C. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The present study is a systematic review and did not involve humans.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Scores of included studies on Newcastle–Ottawa Scale.
Table A1.
Scores of included studies on Newcastle–Ottawa Scale.
| Selection | Comparability | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Representative of the Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcome of the Interest Not Present at the Start of the Study | Main Factor | Additional Factor | Assessment of Outcomes | Sufficient Follow-Up Time | Adequacy of Follow-Up | Total |
| Liguori, 2018 [24] | * | * | * | * | * | * | * | * | * | 9/9 |
| Ruthirakuhan, 2019 [25] | - | - | * | * | * | * | * | * | * | 7/9 |
| Banning, 2020 [26] | * | * | * | * | * | * | * | * | * | 9/9 |
| Burhanullah, 2020 [27] | * | * | * | * | * | * | * | * | * | 9/9 |
| Huang, 2020 [28] | * | * | * | * | * | * | * | * | * | 9/9 |
| Almdahl, 2023 [29] | * | * | * | * | * | * | * | * | * | 9/9 |
| Binette, 2021 [30] | * | * | * | * | * | * | * | * | - | 8/9 |
| Babulal, 2022 [31] | * | * | * | * | * | - | * | * | * | 8/9 |
| Chan, 2022 [32] | * | * | * | * | * | * | * | * | * | 9/9 |
| Clark, 2022 [33] | - | - | * | * | * | * | * | * | * | 7/9 |
| Johansson, 2022 [34] | * | * | * | * | * | * | * | * | * | 9/9 |
| Kim, 2022 [35] | * | * | * | - | * | * | * | * | - | 7/9 |
| Babulal, 2023 [36] | * | * | * | * | * | * | * | * | - | 8/9 |
| Li, 2023 [37] | * | * | * | * | * | * | * | * | * | 9/9 |
| Marquié, 2023 [38] | * | * | * | * | * | * | * | * | * | 9/9 |
| Pink, 2023 [39] | * | - | * | * | * | * | * | * | * | 8/9 |
| Burling, 2024 [40] | * | * | * | * | * | * | * | * | * | 9/9 |
| Guan, 2024 [41] | * | * | * | * | * | * | * | * | * | 9/9 |
| Ronat, 2024 [42] | * | * | * | * | * | * | * | * | * | 9/9 |
| Ronat, 2024 [43] | * | * | * | * | * | * | * | * | * | 9/9 |
| Rabl, 2022 [44] | - | - | * | * | * | * | * | * | * | 7/9 |
| Ismail, 2023 [45] | * | * | * | * | * | * | * | * | * | 9/9 |
| Ghahremani, 2023 [46] | * | * | * | * | * | * | * | * | * | 9/9 |
| Jiang, 2024 [47] | * | * | * | * | * | * | * | * | * | 9/9 |
| Wang, 2019 [48] | * | - | * | - | * | * | * | - | - | 5/9 |
| Banning, 2020 [49] | * | - | * | - | * | * | * | - | - | 5/9 |
| Sannermann, 2020 [50] | * | * | * | - | * | * | * | - | - | 6/9 |
| Cotta Ramusino, 2021 [51] | * | - | * | - | * | * | * | - | - | 5/9 |
| De Oliveira, 2021 [52] | * | * | * | - | * | * | * | - | - | 6/9 |
| Jacobs, 2021 [53] | * | * | * | - | * | * | * | - | - | 6/9 |
| Siafarikas, 2021 [54] | * | * | * | - | * | * | * | - | - | 6/9 |
| Dang, 2022 [55] | * | * | * | - | * | * | * | - | - | 6/9 |
| Henjum, 2022 [56] | * | - | * | - | * | * | * | - | - | 5/9 |
| Kan, 2022 [57] | * | * | * | - | * | - | * | - | - | 5/9 |
| Krell-Roesch, 2022 [58] | * | - | * | - | * | * | * | - | - | 5/9 |
| Manca, 2022 [59] | * | * | * | - | * | * | * | - | - | 6/9 |
| Miao, 2022 [60] | * | * | * | - | * | * | * | - | - | 6/9 |
| Waschkies, 2022 [61] | * | * | * | - | * | * | * | - | - | 6/9 |
| Aguzzoli, 2023 [62] | * | * | * | - | * | * | * | - | - | 6/9 |
| De Lucia, 2023 [63] | * | - | * | - | * | * | * | - | - | 5/9 |
| Greig Custo, 2023 [64] | * | * | * | - | * | * | * | - | - | 6/9 |
| Jiang, 2023 [65] | * | - | * | - | * | * | * | - | - | 5/9 |
| Kan, 2023 [66] | * | * | * | - | * | * | * | - | - | 6/9 |
| Kim, 2023 [67] | * | * | * | - | * | * | * | - | - | 6/9 |
| Krell-Roesch, 2023 [68] | * | * | * | - | * | * | * | - | - | 5/9 |
| Ozaki, 2023 [69] | - | * | * | - | * | * | * | - | - | 5/9 |
| Falgas, 2024 [70] | * | * | * | - | * | * | * | - | - | 6/9 |
| Frank, 2024 [71] | * | - | * | - | * | * | * | - | - | 5/9 |
| Huang, 2024 [72] | * | * | * | - | * | * | * | - | - | 5/9 |
| Hsu, 2024 [73] | * | * | * | - | * | * | * | - | - | 6/9 |
* = The study fulfills the criterion, - = The study does not fulfill the criterion.
References
- Ng, K.P.; Chiew, H.; Rosa-Neto, P.; Kandiah, N.; Ismail, Z.; Gauthier, S. Associations of AT (N) biomarkers with neuropsychiatric symptoms in preclinical Alzheimer’s disease and cognitively unimpaired individuals. Transl. Neurodegener. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.; Bennet, D.; Blennow, K.; Carillo, M.; Feldman, H.; Frisoni, G.; Hampel, H.; Jagust, W.; Johnson, K.; Knopman, D.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Jack, C.; Bennett, D.; Blennow, K.; Carrillo, M.; Dunn, B.; Haeberlein, S.; Holtzman, D.; Jagust, W.; Jenssen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Strategy and Action Plan on Ageing and Health; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- American Psychiatric Association. Washington American Psychiatric Association. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Petersen, R.C.; Nordberg, A.; Bäckman, L.; Albert, M.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Neurol. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; O’Connell, T.; Johnson, S.; Cline, S.; Merikle, E.; Martenyi, F.; Simpson, K. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr. Alzheimer Res. 2018, 15, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Schneider, L.S.; Gitlin, L.N.; Miller, D.S.; Smith, G.S.; Bell, J.; Evans, J.; Lee, M.; Porsteinsson, A.; Lanktot, K. Neuropsychiatric symptoms in Alzheimer’s disease: Past progress and anticipation of the future. Alzheimers Dement. 2013, 9, 602–608. [Google Scholar] [CrossRef]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. Am. Geriatr. Soc. 2014, 62, 762–769. [Google Scholar] [CrossRef]
- Cooper, C.; Sommerlad, A.; Lyketsos, C.G.; Livingston, G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry 2015, 172, 323–334. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Tascone, L.S.; Payne, M.E.; MacFall, J.; Azevedo, D.; de Castro, C.C.; Steffens, D.C.; Busatto, G.F.; Bottino, C. Cortical brain volume abnormalities associated with few or multiple neuropsychiatric symptoms in Alzheimer’s disease. PLoS ONE 2017, 12, e0177169. [Google Scholar] [CrossRef]
- Wise, E.A.; Rosenberg, P.B.; Lyketsos, C.G.; Leoutsakos, J.M. Time course of neuropsychiatric symptoms and cognitive diagnosis in National Alzheimer’s Coordinating Centers volunteers. Alzheimers Dement. Diagn. 2019, 11, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.X.; Mortby, M.E.; Pike, G.B.; Ballard, C.; Creese, B.; Corbett, A.; Pickering, E.; Hampshire, A.; Roach, P.; Smith, E.; et al. Linking cognitive and behavioral reserve: Evidence from the CAN-PROTECT study. Alzheimers Dement. 2024, 10, e12497. [Google Scholar] [CrossRef]
- Martin, E.; Velayudhan, L. Neuropsychiatric symptoms in mild cognitive impairment: A literature review. Dement. Geriatr. Cogn. Disord. 2020, 49, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Antonsdottir, I.M. Neuropsychiatric symptoms in dementia: A cause or consequence? Am. J. Geriatr. Psychiatry 2015, 172, 410–411. [Google Scholar] [CrossRef]
- Young, J.J.; Balachandran, S.; Garg, G.; Balasubramaniam, M.; Gupta, A.; Tampi, D.J.; Tampi, R.R. Personality and the risk factors for developing behavioral and psychological symptoms of dementia: A narrative review. Neurodegener. Dis. Manag. 2019, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, L.B.; Kales, H.C. Managing behavioral and psychological symptoms of dementia. Psychiatr. Clin. 2018, 41, 127–139. [Google Scholar] [CrossRef]
- Hodgson, N.A.; Gitlin, L.N.; Winter, L.; Czekanski, K. Undiagnosed illness and neuropsychiatric behaviors in community residing older adults with dementia. Alzheimer Dis. Assoc. Disord. 2011, 25, 109–115. [Google Scholar] [CrossRef]
- Gerlach, L.B.; Kales, H.C. Learning their language: The importance of detecting and managing pain in dementia. Am. J. Geriatr. Psychiatry 2017, 25, 155–157. [Google Scholar] [CrossRef]
- Khan, Z.; Da Silva, M.V.; Nunez, K.M.; Kalafatis, C.; Nowicki, S.; Walker, Z.; Testad, I.; Francis, P.; Ballard, C. Investigating the effects of impairment in non-verbal communication on neuropsychiatric symptoms and quality of life of people living with dementia. Alzheimers Dement. 2021, 7, e12172. [Google Scholar] [CrossRef]
- Miranda-Castillo, C.; Woods, B.; Galboda, K.; Oomman, S.; Olojugba, C.; Orrell, M. Unmet needs, quality of life and support networks of people with dementia living at home. Health Qual. Life Outcomes 2010, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Pierantozzi, M.; Chiaravalloti, A.; Sancesario, G.M.; Mercuri, N.B.; Franchini, F.; Sancesario, G.M.; Mercuri, N.; Franchini, F.; Schillaci, O.; et al. When cognitive decline and depression coexist in the elderly: CSF biomarkers analysis can differentiate Alzheimer’s disease from late-life depression. Front. Aging Neurosci. 2018, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Ruthirakuhan, M.; Herrmann, N.; Andreazza, A.C.; Verhoeff, N.P.; Gallagher, D.; Black, S.E.; Kiss, A.; Lanctôt, K.L. Agitation, oxidative stress, and cytokines in Alzheimer disease: Biomarker analyses from a clinical trial with nabilone for agitation. J. Geriatr. Psychiatry Neurol. 2019, 33, 175–184. [Google Scholar] [CrossRef]
- Banning, L.C.; Ramakers, I.H.; Köhler, S.; Bron, E.E.; Verhey, F.R.; De Deyn, P.P.; Claasen, J.; Koek, H.; Middelkoop, H.; van der Flier, W.; et al. The association between biomarkers and neuropsychiatric symptoms across the Alzheimer’s disease spectrum. Am. J. Geriatr. Psychiatry 2020, 28, 735–744. [Google Scholar] [CrossRef]
- Burhanullah, M.H.; Tschanz, J.T.; Peters, M.E.; Leoutsakos, J.M.; Matyi, J.; Lyketsos, C.G.; Nowrangi, M.; Rosenberg, P.B. Neuropsychiatric symptoms as risk factors for cognitive decline in clinically normal older adults: The cache county study. Am. J. Geriatr. Psychiatry 2020, 28, 64–71. [Google Scholar] [CrossRef]
- Huang, M.F.; Lee, W.J.; Yeh, Y.C.; Liao, Y.C.; Wang, S.J.; Yang, Y.H.; Chen, C.S.; Fuh, J.L. Genetics of neuropsychiatric symptoms in patients with Alzheimer’s disease: A 1-year follow-up study. Psychiatry Clin. Neurosci. 2020, 74, 645–651. [Google Scholar] [CrossRef]
- Almdahl, I.S.; Agartz, I.; Hugdahl, K.; Korsnes, M.S.; Alzheimer’s Disease Neuroimaging Initiative. Brain pathology and cognitive scores prior to onset of late-life depression. Int. J. Geriatr. Psychiatry 2022, 37, 1–15. [Google Scholar] [CrossRef]
- Binette, A.P.; Vachon-Presseau, É.; Morris, J.; Bateman, R.; Benzinger, T.; Collins, D.L.; Poirier, J.; Breitner, J.; Villeneuve, S. Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms, and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer’s disease. Biol. Psychiatry 2021, 89, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Babulal, G.M.; Chen, L.; Doherty, J.M.; Murphy, S.A.; Johnson, A.M.; Roe, C.M. Longitudinal changes in anger, anxiety, and fatigue are associated with cerebrospinal fluid biomarkers of Alzheimer’s disease. J. Alzheimers Dis. 2022, 87, 141–148. [Google Scholar] [CrossRef]
- Chan, C.K.; Pettigrew, C.; Soldan, A.; Zhu, Y.; Wang, M.C.; Albert, M.; Rosenberg, P.; BIOCARD Research Team. Association between late-life neuropsychiatric symptoms and cognitive decline in relation to white matter hyperintensities and amyloid burden. J. Alzheimers Dis. 2022, 86, 1415–1426. [Google Scholar] [CrossRef]
- Clark, C.; Richiardi, J.; Maréchal, B.; Bowman, G.L.; Dayon, L.; Popp, J. Systemic and central nervous system neuroinflammatory signatures of neuropsychiatric symptoms and related cognitive decline in older people. J. Neuroinflamm. 2022, 19, 127. [Google Scholar] [CrossRef]
- Johansson, M.; Stomrud, E.; Johansson, P.M.; Svenningsson, A.; Palmqvist, S.; Janelidze, S.; van Westen, D.; Mattsson-Carlgren, N.; Hansson, O. Development of apathy, anxiety, and depression in cognitively unimpaired older adults: Effects of Alzheimer’s disease pathology and cognitive decline. Biol. Psychiatry 2022, 92, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Levine, A.; Cohen, D.; Gehrman, P.; Zhu, X.; Devanand, D.P. The role of amyloid, tau, and APOE genotype on the relationship between informant-reported sleep disturbance and Alzheimer’s disease risks. J. Alzheimers Dis. 2022, 87, 1567–1580. [Google Scholar] [CrossRef]
- Babulal, G.M.; Chen, L.; Murphy, S.A.; Doherty, J.M.; Johnson, A.M.; Morris, J.C. Neuropsychiatric symptoms and Alzheimer disease biomarkers independently predict progression to incident cognitive impairment. Am. J. Geriatr. Psychiatry 2023, 31, 1190–1199. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Q.; Luo, X.; Qi, S.; Xu, X.; Fu, Z.; Hong, L.; Li, Z.; Fu, Y.; Chen, Y. Neuropsychiatric symptoms associated multimodal brain networks in Alzheimer’s disease. Hum. Brain Mapp. 2023, 44, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; García-Gutiérrez, F.; Orellana, A.; Montrreal, L.; de Rojas, I.; García-González, P.; Puerta, R.; Olive, C.; Cano, A.; Hernandez, I.; et al. The synergic effect of AT (N) profiles and depression on the risk of conversion to dementia in patients with mild cognitive impairment. Int. J. Mol. Sci. 2023, 24, 1371. [Google Scholar] [CrossRef] [PubMed]
- Pink, A.; Krell-Roesch, J.; Syrjanen, J.A.; Christenson, L.R.; Lowe, V.J.; Vemuri, P.; Fields, J.A.; Stokin, J.; Kremers, W.K.; Scharf, E.L.; et al. Interactions Between Neuropsychiatric Symptoms and Alzheimer’s Disease Neuroimaging Biomarkers in Predicting Longitudinal Cognitive Decline. Psychiatr. Res. Clin. Pract. 2023, 5, 4–15. [Google Scholar] [CrossRef]
- Burling, J.E.; Katz, Z.; Yuan, Z.; Munro, C.; Mimmack, K.; Ma, G.; Hanseeuw, B.J.; Papp, K.V.; Amariglio, R.E.; Vannini, P.; et al. Study partner report of apathy in older adults is associated with AD biomarkers: Findings from the Harvard aging brain study. Am. J. Geriatr. Psychiatry 2024, 32, 909–919. [Google Scholar] [CrossRef]
- Guan, D.X.; Rehman, T.; Nathan, S.; Durrani, R.; Potvin, O.; Duchesne, S.; Pike, G.B.; Smith, E.; Ismail, Z. Neuropsychiatric symptoms: Risk factor or disease marker? A study of structural imaging biomarkers of Alzheimer’s disease and incident cognitive decline. Hum. Brain Mapp. 2024, 45, e70016. [Google Scholar] [CrossRef]
- Ronat, L.; Hanganu, A.; Chylinski, D.; Van Egroo, M.; Narbutas, J.; Besson, G.; Muto, V.; Schmidt, C.; Bahri, M.A.; Phillips, C.; et al. Prediction of cognitive decline in healthy aging based on neuropsychiatric symptoms and PET-biomarkers of Alzheimer’s disease. J. Neurol. 2024, 271, 2067–2077. [Google Scholar] [CrossRef]
- Ronat, L.; Rönnlund, M.; Adolfsson, R.; Hanganu, A.; Pudas, S. Revised Temperament and Character Inventory factors predict neuropsychiatric symptoms and aging-related cognitive decline across 25 years. Front. Aging Neurosci. 2024, 16, 1335336. [Google Scholar] [CrossRef] [PubMed]
- Rabl, M.; Clark, C.; Dayon, L.; Bowman, G.L.; Popp, J. Blood plasma protein profiles of neuropsychiatric symptoms and related cognitive decline in older people. J. Neurochem. 2023, 164, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Leon, R.; Creese, B.; Ballard, C.; Robert, P.; Smith, E.E. Optimizing detection of Alzheimer’s disease in mild cognitive impairment: A 4-year biomarker study of mild behavioral impairment in ADNI and MEMENTO. Mol. Neurodegener. 2023, 18, 50. [Google Scholar] [CrossRef]
- Ghahremani, M.; Wang, M.; Chen, H.Y.; Zetterberg, H.; Smith, E.; Ismail, Z. Plasma phosphorylated tau at threonine 181 and neuropsychiatric symptoms in preclinical and prodromal Alzheimer disease. Neurology 2023, 100, 683–693. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, A.; Shi, H.; Jiang, S.; Li, W.; Jiang, T.; Wang, L.; Zhang, X.; Sun, M.; Zhao, M.; et al. Clinical and neuroimaging association between neuropsychiatric symptoms and nutritional status across the Alzheimer’s disease continuum: A longitudinal cohort study. J. Nutr. Health Aging 2024, 28, 100182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, P.; Mapstone, M.; Conwell, Y.; Porsteinsson, A.P.; Foxe, J.J.; Rajeev, R.; Feng, L.; Alzheimer’s Disease Neuroimaging Initiative. Identify a shared neural circuit linking multiple neuropsychiatric symptoms with Alzheimer’s pathology. Brain Imaging Behav. 2019, 13, 53–64. [Google Scholar] [CrossRef]
- Banning, L.C.; Ramakers, I.H.; Rosenberg, P.B.; Lyketsos, C.G.; Leoutsakos, J.M.; Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s disease biomarkers as predictors of trajectories of depression and apathy in cognitively normal individuals, mild cognitive impairment, and Alzheimer’s disease dementia. Int. J. Geriatr. Psychiatry 2020, 36, 224–234. [Google Scholar] [CrossRef]
- Sannemann, L.; Schild, A.K.; Altenstein, S.; Bartels, C.; Brosseron, F.; Buerger, K.; Cosma, N.C.; Freiesleben, S.D.; Glanz, W.; Heneka, M.T. Neuropsychiatric symptoms in at-risk groups for AD dementia and their association with worry and AD biomarkers—Results from the DELCODE study. Alzheimers Res. Ther. 2020, 12, 131. [Google Scholar] [CrossRef]
- Cotta Ramusino, M.; Perini, G.; Vaghi, G.; Dal Fabbro, B.; Capelli, M.; Picascia, M.; Franciotta, D.; Farina, L.; Ballante, E.; Costa, A. Correlation of frontal atrophy and CSF tau levels with neuropsychiatric symptoms in patients with cognitive impairment: A memory clinic experience. Front. Aging Neurosci. 2021, 13, 595758. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Miraldo, M.C.; de Castro-Neto, E.F.; de Almeida, S.S.; Matas, S.L.; Bertolucci, P.H.F.; Naffah-Mazzacoratti, M.D.G. Associations of neuropsychiatric features with cerebrospinal fluid biomarkers of amyloidogenesis and neurodegeneration in dementia with Lewy bodies compared with Alzheimer’s disease and cognitively healthy people. J. Alzheimers Dis. 2021, 81, 1295–1309. [Google Scholar] [CrossRef]
- Jacobs, H.I.; Riphagen, J.M.; Ramakers, I.H.; Verhey, F.R. Alzheimer’s disease pathology: Pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol. Psychiatry 2021, 26, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Siafarikas, N.; Kirsebom, B.E.; Srivastava, D.P.; Eriksson, C.M.; Auning, E.; Hessen, E.; Salbaek, G.; Blennow, K.; Aarsland, D.; Fladby, T. Cerebrospinal fluid markers for synaptic function and Alzheimer type changes in late life depression. Sci. Rep. 2021, 11, 20375. [Google Scholar] [CrossRef]
- Dang, M.; Chen, Q.; Zhao, X.; Chen, K.; Li, X.; Zhang, J.; Zhang, Z. Tau as a biomarker of cognitive impairment and neuropsychiatric symptoms in Alzheimer’s disease. Hum. Brain Mapp. 2023, 44, 327–340. [Google Scholar] [CrossRef]
- Henjum, K.; Watne, L.O.; Godang, K.; Halaas, N.B.; Eldholm, R.S.; Blennow, K.; Zetterberg, H.; Saltvedt, I.; Bollerslev, J.; Knapskog, A.B. Cerebrospinal fluid catecholamines in Alzheimer’s disease patients with and without biological disease. Transl. Psychiatry 2022, 12, 151. [Google Scholar] [CrossRef]
- Kan, C.N.; Xu, X.; Schmetterer, L.; Venketasubramanian, N.; Chen, C.; Tan, C.H. Interactions of comorbid neuropsychiatric subsyndromes with neurodegenerative and cerebrovascular pathologies on cognition. Neurobiol. Aging 2022, 109, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Krell-Roesch, J.; Zaniletti, I.; Syrjanen, J.A.; Kremers, W.K.; Algeciras-Schimnich, A.; Dage, J.L.; van Harten, A.; Fields, J.; Petersen, R.; Vassilaki, M.; et al. Plasma-derived biomarkers of Alzheimer’s disease and neuropsychiatric symptoms: A community-based study. Alzheimers Dement. 2022, 15, e12461. [Google Scholar] [CrossRef] [PubMed]
- Manca, R.; Jones, S.A.; Venneri, A. Macrostructural and microstructural white matter alterations are associated with apathy across the clinical Alzheimer’s disease spectrum. Brain Sci. 2022, 12, 1383. [Google Scholar] [CrossRef]
- Miao, R.; Chen, H.Y.; Gill, S.; Naude, J.; Smith, E.E.; Ismail, Z. Plasma β-amyloid in mild behavioural impairment–neuropsychiatric symptoms on the Alzheimer’s continuum. J. Geriatr. Psychiatry Neurol. 2022, 35, 434–441. [Google Scholar] [CrossRef]
- Waschkies, K.F.; Soch, J.; Darna, M.; Richter, A.; Altenstein, S.; Beyle, A.; Brosseron, F.; Buchholz, F.; Butryn, M.; Dobisch, L.; et al. Machine learning-based classification of Alzheimer’s disease and its at-risk states using personality traits, anxiety, and depression. Int. J. Geriatr. Psychiatry 2023, 38, e6007. [Google Scholar] [CrossRef]
- Aguzzoli, C.S.; Ferreira, P.C.; Povala, G.; Ferrari-Souza, J.P.; Bellaver, B.; Katz, C.S.; Zalzale, H.; Lussier, F.; Rohden, F.; Abbas, F.; et al. Neuropsychiatric symptoms and microglial activation in patients with Alzheimer disease. JAMA Netw. Open 2023, 6, 2345175. [Google Scholar] [CrossRef]
- De Lucia, N.; Carbone, G.; Muzii, B.; Ferrara, N.; Rengo, G.; Maldonato, N.M.; Femminella, G.D. Neuropsychiatric symptoms and their neural correlates in individuals with mild cognitive impairment. Int. Psychogeriatr. 2023, 35, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Greig Custo, M.T.; Lang, M.K.; Barker, W.W.; Gonzalez, J.; Vélez-Uribe, I.; Arruda, F.; Conniff, J.; Rodriguez, M.J.; Loewenstein, D.A.; Duara, R.; et al. The association of depression and apathy with Alzheimer’s disease biomarkers in a cross-cultural sample. Appl. Neuropsychol. Adult. 2024, 31, 849–865. [Google Scholar] [CrossRef]
- Jiang, J.; Hong, Y.; Li, W.; Wang, A.; Jiang, S.; Jiang, T.; Wang, W.; Yang, S.; Ren, Q.; Zou, X. Chain Mediation Analysis of the Effects of Nutrition and Cognition on the Association of Apolipoprotein E ɛ4 with neuropsychiatric symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 96, 669–681. [Google Scholar] [CrossRef]
- Kan, C.N.; Huang, X.; Zhang, L.; Hilal, S.; Reilhac, A.; Tanaka, T.; Venketasubramanian, N.; Chen, C.; Xu, X. Comorbid amyloid with cerebrovascular disease in domain-specific cognitive and neuropsychiatric disturbances: A cross-sectional memory clinic study. Neurobiol. Aging 2023, 132, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zhu, X.; Zhao, Y.; Bell, S.A.; Gehrman, P.R.; Cohen, D. Resting-state functional connectivity changes in older adults with sleep disturbance and the role of amyloid burden. Mol. Psychiatry 2023, 28, 4399–4406. [Google Scholar] [CrossRef]
- Krell-Roesch, J.; Rakusa, M.; Syrjanen, J.A.; van Harten, A.C.; Lowe, V.J.; Jack, C.R., Jr. Association between CSF biomarkers of Alzheimer’s disease and neuropsychiatric symptoms: Mayo Clinic Study of Aging. Alzheimers Dement. 2023, 19, 4498–4506. [Google Scholar] [CrossRef]
- Ozaki, T.; Hashimoto, N.; Udo, N.; Narita, H.; Nakagawa, S.; Kusumi, I. Neurobiological correlation between phosphorylated tau and mood symptoms in memory clinic patients. Psychogeriatrics 2023, 23, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Walsh, M.; Hurley, L.; Groh, J.; Blennow, K.; Zetterberg, H.; Tripodis, Y.; Budson, A.E.; Maureen, K.O.; Martin, B. Cognition Mediates the Association Between Cerebrospinal Fluid Biomarkers of Amyloid and P-Tau and Neuropsychiatric Symptoms. J. Alzheimers Dis. 2024, 100, 1055–1073. [Google Scholar] [CrossRef]
- Falgàs, N.; Peña-González, M.; Val-Guardiola, A.; Pérez-Millan, A.; Guillén, N.; Sarto, J.; Esteller, D.; Bosch, B.; Fernández-Villullas, G.; Tort-Merino, A.; et al. Locus coeruleus integrity and neuropsychiatric symptoms in a cohort of early-and late-onset Alzheimer’s disease. Alzheimers Dement. 2024, 20, 6351–6364. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Q.; Xie, F.; Guo, Q. Neuropsychiatric symptoms in Alzheimer’s continuum and their association with plasma biomarkers. J. Affect Disord. 2024, 348, 200–206. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wang, S.I.; Lin, H.C.; Lin, E.S.; Yang, F.P.; Chang, C.M.; Wei, J.C.C. Difference of Cerebrospinal Fluid Biomarkers and Neuropsychiatric Symptoms Profiles among Normal Cognition, Mild Cognitive Impairment, and Dementia Patient. Int. J. Mol. Sci. 2024, 25, 3919. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cummings, J. The neuropsychiatric inventory: Development and applications. J. Geriatr. Psychiatry Neurol. 2020, 33, 73–84. [Google Scholar] [CrossRef]
- Körner, A.; Czajkowska, Z.; Albani, C.; Drapeau, M.; Geyer, M.; Braehler, E. Efficient and valid assessment of personality traits: Population norms of a brief version of the NEO Five-Factor Inventory (NEO-FFI). Arch. Psychiatry Psychother. 2015, 17, 21–32. [Google Scholar] [CrossRef]
- Showraki, A.; Murari, G.; Ismail, Z.; Barfett, J.J.; Fornazzari, L.; Munoz, D.G.; Schweizer, T.; Fischer, C.E. Cerebrospinal fluid correlates of neuropsychiatric symptoms in patients with Alzheimer’s disease/mild cognitive impairment: A systematic review. J. Alzheimers Dis. 2019, 71, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett Jr, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).