Impact of Nerve-Sparing Techniques on Prostate-Specific Antigen Persistence Following Robot-Assisted Radical Prostatectomy: A Multivariable Analysis of Clinical and Pathological Predictors
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Variables Considered
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Univariate Analysis of Clinical and Surgical Factors and Postoperative PSA Levels
3.3. Multivariable Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirone, V.; Imbimbo, C.; Arcaniolo, D.; Franco, M.; La Rocca, R.; Venturino, L.; Spirito, L.; Creta, M.; Verze, P. Knowledge, attitudes, and practices towards prostate cancer screening amongst men living in the southern Italian peninsula: The Prevention and Research in Oncology (PRO) non-profit Foundation experience. World J. Urol. 2017, 35, 1857–1862. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.-P.; van der Kwast, T.; Wiegel, T.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zincke, H.; Bergstralh, E.J.; Blute, M.L.; Myers, R.P.; Barrett, D.M.; Lieber, M.M.; Martin, S.K.; Oesterling, J.E. Radical prostatectomy for clinically localized prostate cancer: Long-term results of 1,143 patients from a single institution. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1994, 12, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, H.; Lin, S.; Chen, H.; Xie, L.; Zheng, X. Analysis of risk factors for persistent PSA after radical prostatectomy: Results from a high-volume center in Southeast China. BMC Urol. 2024, 24, 185. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Barone, B.; Amicuzi, U.; Massanova, M.; Napolitano, L.; Reccia, P.; Mirto, B.F.; Balsamo, R.; Giudice, F.D.; Ferro, M.; Busetto, G.M.; et al. The Correlation Between Body Mass Index and Prostate Volume: A Retrospective Analysis of Pre and Postoperative Measurements in Prostate Cancer Patients. Prostate 2024, 85, 433–442. [Google Scholar] [CrossRef]
- Mikel Hubanks, J.; Boorjian, S.A.; Frank, I.; Gettman, M.T.; Houston Thompson, R.; Rangel, L.J.; Bergstralh, E.J.; Jeffrey Karnes, R. The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol. Oncol. 2014, 32, 26.e1–26.e7. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Rosenkrantz, A.B.; Taneja, S.S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for prostate cancer diagnosis: Current status and future directions. Nat. Rev. Urol. 2020, 17, 41–61. [Google Scholar] [CrossRef]
- Massanova, M.; Barone, B.; Caputo, V.; Napolitano, L.; Ponsiglione, A.; Del Giudice, F.; Ferro, M.; Lucarelli, G.; Lasorsa, F.; Busetto, G.; et al. The detection rate for prostate cancer in systematic and targeted prostate biopsy in biopsy-naive patients, according to the localization of the lesion at the mpMRI: A single-center retrospective observational study. Prostate 2024, 84, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Napolitano, L.; Crocetto, F.; Sciorio, C.; Sio, M.D.; Miranda, A.; Romano, M.; Priadko, K. Prostate and gut: Any relationship? A narrative review on the available evidence and putative mechanisms. Prostate 2024, 84, 513–524. [Google Scholar] [CrossRef]
- Komori, T.; Matsumoto, K.; Kosaka, T.; Takeda, T.; Kamitani, R.; Yasumizu, Y.; Tanaka, N.; Morita, S.; Mizuno, R.; Asanuma, H.; et al. Long-Term Prognosis and Treatment Strategy of Persistent PSA After Radical Prostatectomy. Ann. Surg. Oncol. 2023, 30, 6936–6942. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Venclovas, Z.; Sasnauskas, G.; Ruzgas, T. The Significance of Prostate Specific Antigen Persistence in Prostate Cancer Risk Groups on Long-Term Oncological Outcomes. Cancers 2021, 13, 2453. [Google Scholar] [CrossRef]
- Soeterik, T.F.W.; van Melick, H.H.E.; Dijksman, L.M.; Stomps, S.; Witjes, J.A.; van Basten, J.P.A. Nerve Sparing during Robot-Assisted Radical Prostatectomy Increases the Risk of Ipsilateral Positive Surgical Margins. J. Urol. 2020, 204, 91–95. [Google Scholar] [CrossRef]
- Catalona, W.J.; Bigg, S.W. Nerve-sparing radical prostatectomy: Evaluation of results after 250 patients. J. Urol. 1990, 143, 538–543; discussion 544. [Google Scholar] [CrossRef]
- Kyriazis, I.; Spinos, T.; Tsaturyan, A.; Kallidonis, P.; Stolzenburg, J.U.; Liatsikos, E. Different Nerve-Sparing Techniques during Radical Prostatectomy and Their Impact on Functional Outcomes. Cancers 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Michl, U.; Tennstedt, P.; Feldmeier, L.; Mandel, P.; Oh, S.J.; Ahyai, S.; Budäus, L.; Chun, F.K.H.; Haese, A.; Heinzer, H.; et al. Nerve-sparing Surgery Technique, Not the Preservation of the Neurovascular Bundles, Leads to Improved Long-term Continence Rates After Radical Prostatectomy. Eur. Urol. 2016, 69, 584–589. [Google Scholar] [CrossRef]
- Ghadjar, P.; Aebersold, D.M.; Albrecht, C.; Böhmer, D.; Flentje, M.; Ganswindt, U.; Höcht, S.; Hölscher, T.; Sedlmayer, F.; Wenz, F.; et al. Use of androgen deprivation and salvage radiation therapy for patients with prostate cancer and biochemical recurrence after prostatectomy. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al 2018, 194, 619–626. [Google Scholar] [CrossRef]
- Potter, S.R.; I Epstein, J.; Partin, A.W. Seminal vesicle invasion by prostate cancer: Prognostic significance and therapeutic implications. Rev. Urol. 2000, 2, 190–195. [Google Scholar]
- Spirito, L.; Marra, A.; Mirone, V.; Manfredi, C.; Fusco, F.; Napolitano, L.; Servillo, G.; Logrieco, N.; Buonanno, P. Role of spinal anesthesia in robot-assisted radical prostatectomy: Gamble or opportunity? Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. Nefrol. 2023, 95, 11311. [Google Scholar] [CrossRef]
- Gatti, L.; Antonelli, A.; Gritti, A.; Finamanti, M.; Peroni, A.; Simeone, C. Short and medium term oncological results after robot-assisted prostatectomy: A comparative prospective non randomized study. Urologia 2013, 80, 135–139. [Google Scholar] [CrossRef]
- Spirito, L.; Chessa, F.; Hagman, A.; Lantz, A.; Celentano, G.; Sanchez-Salas, R.; La Rocca, R.; Olsson, M.; Akre, O.; Mirone, V.; et al. Long-Term Oncological Outcomes after Nerve-Sparing Robot-Assisted Radical Prostatectomy for High-Risk Localized Prostate Cancer: A Single-Center, Two-Arm Prospective Study. Diagnostics 2024, 14, 803. [Google Scholar] [CrossRef]
- Grossi, F.S.; Utano, E.; Minafra, P.; Prontera, P.P.; Schiralli, F.; De Cillis, A.; Martinelli, E.; Lattarulo, M.; Luka, M.; Carrieri, A.; et al. Oncological and functional outcomes of extraperitoneal laparoscopic radical prostatectomy: An 18-years, single-center experience. Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. Nefrol. 2021, 93, 268–273. [Google Scholar] [CrossRef]
- Anguas-Gracia, A.; Antón-Solanas, I.; Echániz-Serrano, E.; Subirón-Valera, A.B.; Rodríguez-Roca, B.; Juárez-Vela, R.; Satustegui-Dordá, P.J.; Fernández-Rodríguez, M.T.; Gea-Caballero, V.; Tejada-Garrido, C.I.; et al. Quality of Life after Radical Prostatectomy: A Longitudinal Study. Nurs. Rep. Pavia Italy 2023, 13, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Bianco, F.J.; Serio, A.M.; Eastham, J.A.; Kattan, M.W.; Pontes, J.E.; Vickers, A.J.; Scardino, P.T. Surgeon Experience is Strongly Associated with Biochemical Recurrence after Radical Prostatectomy for all Preoperative Risk Categories. J. Urol. 2008, 179, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Gandaglia, G.; Ploussard, G.; Marra, G.; Valerio, M.; Campi, R.; Mari, A.; Minervini, A.; Serni, S.; Moschini, M.; et al. Risk Stratification of Patients Candidate to Radical Prostatectomy Based on Clinical and Multiparametric Magnetic Resonance Imaging Parameters: Development and External Validation of Novel Risk Groups. Eur. Urol. 2022, 81, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Moris, L.; Gandaglia, G.; Vilaseca, A.; Van den Broeck, T.; Briers, E.; De Santis, M.; Gillessen, S.; Grivas, N.; O’Hanlon, S.; Henry, A.; et al. Evaluation of Oncological Outcomes and Data Quality in Studies Assessing Nerve-sparing Versus Non-Nerve-sparing Radical Prostatectomy in Nonmetastatic Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2022, 8, 690–700. [Google Scholar] [CrossRef]
- Stewart, S.B.; Moul, J.W.; Polascik, T.J.; Koontz, B.F.; Robertson, C.N.; Freedland, S.J.; George, D.J.; Lee, W.R.; Armstrong, A.J.; Bañez, L.L. Does the multidisciplinary approach improve oncological outcomes in men undergoing surgical treatment for prostate cancer? Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2014, 21, 1215–1219. [Google Scholar] [CrossRef]
- Hendriks, M.P.; Jager, A.; Ebben, K.C.W.J.; van Til, J.A.; Siesling, S. Clinical decision support systems for multidisciplinary team decision-making in patients with solid cancer: Composition of an implementation model based on a scoping review. Crit. Rev. Oncol. Hematol. 2024, 195, 104267. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, S.; Lawhn-Heath, C.; Behr, S. PSMA PET imaging in the diagnosis and management of prostate cancer. Abdom. Radiol. N. Y. 2023, 48, 3610–3623. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Barone, B.; Caputo, V.F.; Fontana, M.; de Cobelli, O.; Ferro, M. BRCA Germline Mutations in Prostate Cancer: The Future Is Tailored. Diagnostics 2021, 11, 908. [Google Scholar] [CrossRef]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Cennamo, M.; Bruzzese, D.; Crocetto, F.; Velotta, R.; Terracciano, D. A Combinatorial Neural Network Analysis Reveals a Synergistic Behaviour of Multiparametric Magnetic Resonance and Prostate Health Index in the Identification of Clinically Significant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, e406–e410. [Google Scholar] [CrossRef]
- Gravina, M.; Spirito, L.; Celentano, G.; Capece, M.; Creta, M.; Califano, G.; Collà Ruvolo, C.; Morra, S.; Imbriaco, M.; Di Bello, F.; et al. Machine Learning and Clinical-Radiological Characteristics for the Classification of Prostate Cancer in PI-RADS 3 Lesions. Diagnostics 2022, 12, 1565. [Google Scholar] [CrossRef]
- Gentile, F.; La Civita, E.; Ventura, B.D.; Ferro, M.; Bruzzese, D.; Crocetto, F.; Tennstedt, P.; Steuber, T.; Velotta, R.; Terracciano, D. A Neural Network Model Combining [-2]proPSA, freePSA, Total PSA, Cathepsin D, and Thrombospondin-1 Showed Increased Accuracy in the Identification of Clinically Significant Prostate Cancer. Cancers 2023, 15, 1355. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Buonerba, L.; Ingenito, C.; Crocetto, F.; Buonerba, C.; Libroia, A.; Sciarra, A.; Ragone, G.; Sanseverino, R.; Iaccarino, S.; et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected with COVID-19 in South Italy. Oncology 2020, 98, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Buonerba, L.; Scafuri, L.; Caputo, V.; Barone, B.; Sciarra, A.; Verde, A.; Calogero, A.; Buonerba, C.; Di Lorenzo, G. COVID-19 and prostate cancer: A complex scenario with multiple facets. Future Sci. OA 2021, 8, FSO760. [Google Scholar] [CrossRef]
- Barone, B.; Napolitano, L.; Calace, F.P.; Del Biondo, D.; Napodano, G.; Grillo, M.; Reccia, P.; De Luca, L.; Prezioso, D.; Muto, M.; et al. Reliability of Multiparametric Magnetic Resonance Imaging in Patients with a Previous Negative Biopsy: Comparison with Biopsy-Naïve Patients in the Detection of Clinically Significant Prostate Cancer. Diagnostics 2023, 13, 1939. [Google Scholar] [CrossRef]
- Ponsiglione, A.; Stanzione, A.; Minieri, A.; Musella, R.; D’Elia, A.C.; Negroni, D.; Sacco, M.; Brancaccio, D.; Sicignano, E.; Muto, F.; et al. Impact of software-assisted structured reporting on radiology residents approaching prostate MRI. Eur. J. Radiol. 2024, 183, 111889. [Google Scholar] [CrossRef]
- Keller, E.X.; Bachofner, J.; Britschgi, A.J.; Saba, K.; Mortezavi, A.; Kaufmann, B.; Fankhauser, C.D.; Wild, P.; Sulser, T.; Hermanns, T.; et al. Prognostic value of unifocal and multifocal positive surgical margins in a large series of robot-assisted radical prostatectomy for prostate cancer. World J. Urol. 2019, 37, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Borchers, H.; Brehmer, B.; Kirschner-Hermanns, R.; Reineke, T.; Tietze, L.; Jakse, G. Erectile function after non-nerve-sparing radical prostatectomy: Fact or fiction? Urol. Int. 2006, 76, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Katz, D.; Nelson, C.J.; Mulhall, J.P. Erectile function recovery in patients after non-nerve sparing radical prostatectomy. Andrology 2014, 2, 951–954. [Google Scholar] [CrossRef]
- Sivathasan, S.; Patel, K.M.; Smart, S.; Nathan, A.; Warren, A.; Shah, N.; Lamb, B.W. Incremental modification of robotic prostatectomy technique can lead to aggregated marginal gains to significantly improve functional outcomes without compromising oncological control. J. Robot. Surg. 2022, 16, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, H.; Edmunds, K.; Fairbairn, D.; Roberts, M.J.; Chambers, S.; Smith, D.P.; Horvath, L.; Arora, S.; Scuffham, P. Guidelines for genetic testing in prostate cancer: A scoping review. Prostate Cancer Prostatic Dis. 2024, 27, 594–603. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E. Smoking and prostate cancer survival and recurrence. JAMA 2011, 305, 2548–2555. [Google Scholar] [CrossRef]
| Variable | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Preoperative PSA Level | 11.85 | 7.63 | 2.00 | 34.00 |
| Age | 63.46 | 6.48 | 43.00 | 85.00 |
| Preoperative Gleason Score | 7.26 | 1.08 | 4.00 | 10.00 |
| Tumor Length (mm) | 17.47 | 21.43 | 0.00 | 205.00 |
| Postoperative Gleason Score | 7.42 | 0.91 | 5.00 | 10.00 |
| Prostate Weight (g) | 42.17 | 22.98 | 15.00 | 214.00 |
| Total Lymph Nodes Examined | 15.68 | 8.24 | 1.00 | 45.00 |
| Postoperative PSA Level | 0.70 | 4.42 | 0.01 | 79.00 |
| % | ||||
| Nerve-Sparing Surgery (Yes) | 55% | - | - | - |
| Unilateral Nerve-Sparing (%) | 51% | - | - | - |
| Variable | Correlation Coefficient | p-Value | Comments |
|---|---|---|---|
| Preoperative PSA Level | −0.0487 | 0.1886 | No significant correlation with postoperative PSA. |
| Age | −0.0086 | 0.8163 | Minimal impact of age on postoperative PSA levels. |
| Tumor Length | 0.1285 | <0.001 | Positive correlation; longer tumors linked to higher postoperative PSA. |
| Prostate Weight | −0.0024 | 0.9679 | Negligible influence on postoperative PSA. |
| Variable | Test Statistic | p-Value | Comments |
|---|---|---|---|
| Preoperative Gleason Score | 24.2127 | 4.77 × 10−4 | Statistically significant; higher Gleason scores correlate with higher PSA. |
| Postoperative Gleason Score | 24.7385 | 1.57 × 10−4 | Strong association; aggressive tumors reflect elevated PSA levels. |
| Pathological Tumor Stage | 45.1013 | 3.79 × 10−9 | Highly significant; advanced stages (e.g., pT3b) associate with higher PSA. |
| Nerve-Sparing Surgery | 3.8608 | 0.0494 | Significant; differences exist between nerve-sparing and non-nerve-sparing. |
| Side of Nerve-Sparing | 6.5022 | 0.0387 | Statistically significant; unilateral vs. bilateral sparing impacts PSA. |
| Subgroup | Mean PSA (ng/mL) | Median PSA (ng/mL) | Standard Deviation (ng/mL) | Comments |
|---|---|---|---|---|
| Preoperative Gleason Score | ||||
| Gleason ≤ 6 | 0.12 | 0.05 | 0.10 | Lower PSA reflects less aggressive disease. |
| Gleason = 7 | 0.32 | 0.10 | 0.80 | Moderate elevation, typical of intermediate-risk disease. |
| Gleason > 7 | 4.50 | 0.70 | 18.20 | Significant variability; suggests aggressive biology. |
| Pathological Tumor Stage | ||||
| pT2 | 0.09 | 0.05 | 0.06 | PSA remains low for organ-confined disease. |
| pT3a | 0.22 | 0.05 | 0.45 | Moderate increase linked to extraprostatic extension. |
| pT3b | 2.14 | 0.20 | 8.50 | PSA significantly elevated, indicating higher disease burden. |
| Nerve-Sparing Surgery | ||||
| Yes | 0.20 | 0.05 | 0.40 | Lower PSA values reflect successful nerve preservation. |
| No | 0.65 | 0.10 | 2.30 | Higher variability suggests residual disease in some cases. |
| Side of Nerve-Sparing | ||||
| Unilateral | 0.30 | 0.05 | 0.50 | Comparable outcomes to bilateral sparing. |
| Bilateral | 0.35 | 0.10 | 0.60 | Slightly elevated PSA but not clinically significant. |
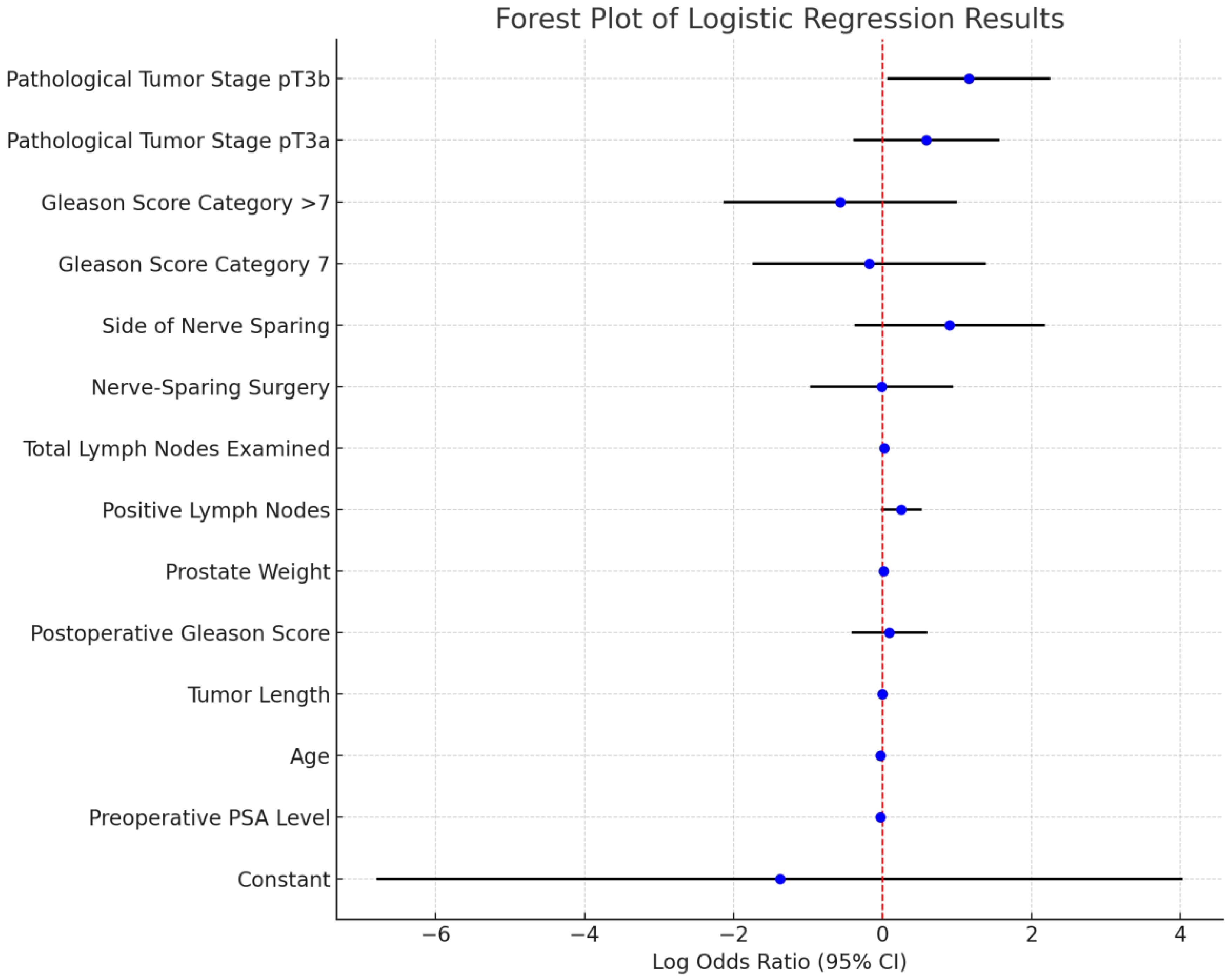
| Variable | Coefficient | Standard Error | z-Score | p-Value |
|---|---|---|---|---|
| Constant | −1.38 | 2.76 | −0.50 | 0.62 |
| Preoperative PSA Level | −0.03 | 0.03 | −0.98 | 0.33 |
| Age | −0.03 | 0.03 | −0.92 | 0.36 |
| Tumor Length | 0.00 | 0.01 | 0.14 | 0.89 |
| Postoperative Gleason Score | 0.09 | 0.26 | 0.34 | 0.74 |
| Prostate Weight | 0.01 | 0.01 | 1.39 | 0.17 |
| Positive Lymph Nodes | 0.25 | 0.14 | 1.77 | 0.08 |
| Total Lymph Nodes Examined | 0.02 | 0.03 | 0.93 | 0.35 |
| Nerve-Sparing Surgery | −0.01 | 0.49 | −0.01 | 0.99 |
| Side of Nerve-Sparing | 0.90 | 0.65 | 1.40 | 0.16 |
| Gleason Score Category = 7 | −0.18 | 0.80 | −0.23 | 0.82 |
| Gleason Score Category > 7 | −0.57 | 0.80 | −0.72 | 0.47 |
| Pathological Tumor Stage pT3a | 0.59 | 0.50 | 1.19 | 0.23 |
| Pathological Tumor Stage pT3b | 1.16 | 0.56 | 2.08 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spirito, L.; Sciorio, C.; Romano, L.; Di Girolamo, A.; Ruffo, A.; Romeo, G.; Crocetto, F.; Napolitano, L.; Stizzo, M.; Bottone, F.; et al. Impact of Nerve-Sparing Techniques on Prostate-Specific Antigen Persistence Following Robot-Assisted Radical Prostatectomy: A Multivariable Analysis of Clinical and Pathological Predictors. Diagnostics 2025, 15, 987. https://doi.org/10.3390/diagnostics15080987
Spirito L, Sciorio C, Romano L, Di Girolamo A, Ruffo A, Romeo G, Crocetto F, Napolitano L, Stizzo M, Bottone F, et al. Impact of Nerve-Sparing Techniques on Prostate-Specific Antigen Persistence Following Robot-Assisted Radical Prostatectomy: A Multivariable Analysis of Clinical and Pathological Predictors. Diagnostics. 2025; 15(8):987. https://doi.org/10.3390/diagnostics15080987
Chicago/Turabian StyleSpirito, Lorenzo, Carmine Sciorio, Lorenzo Romano, Antonio Di Girolamo, Antonio Ruffo, Giuseppe Romeo, Felice Crocetto, Luigi Napolitano, Marco Stizzo, Francesco Bottone, and et al. 2025. "Impact of Nerve-Sparing Techniques on Prostate-Specific Antigen Persistence Following Robot-Assisted Radical Prostatectomy: A Multivariable Analysis of Clinical and Pathological Predictors" Diagnostics 15, no. 8: 987. https://doi.org/10.3390/diagnostics15080987
APA StyleSpirito, L., Sciorio, C., Romano, L., Di Girolamo, A., Ruffo, A., Romeo, G., Crocetto, F., Napolitano, L., Stizzo, M., Bottone, F., Quattrone, C., & Imperatore, V. (2025). Impact of Nerve-Sparing Techniques on Prostate-Specific Antigen Persistence Following Robot-Assisted Radical Prostatectomy: A Multivariable Analysis of Clinical and Pathological Predictors. Diagnostics, 15(8), 987. https://doi.org/10.3390/diagnostics15080987






