Systematic Analysis of Multiple Imaging Modalities in Infants Diagnosed with Mucopolysaccharidosis by Newborn Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Imaging Studies
2.3. Skeletal Radiography
- Skull morphology and thickness;
- Vertebral body shape and alignment;
- Joint morphology focusing on hip dysplasia and genu valgum;
- Hand and wrist structures, including carpal and metacarpal bones;
- Bone density and cortical thickness;
- Presence of a J-shaped sella turcica.
2.4. Cardiac Ultrasound
- Cardiac valve morphology and function;
- Left ventricular dimensions and systolic function;
- Right ventricular size and function;
- Presence of valve regurgitation or stenosis;
- Wall thickness and myocardial texture;
- Presence of pericardial effusion.
2.5. Abdominal Ultrasound
- Liver size and parenchymal texture;
- Spleen size and echotexture;
- Presence of hepatosplenomegaly;
- Gallbladder and biliary tract assessment;
- Kidney size and echogenicity.
2.6. Biochemical Analyses
2.7. Statistical Analysis
3. Results
3.1. Patients Demographics and Baseline Characteristics
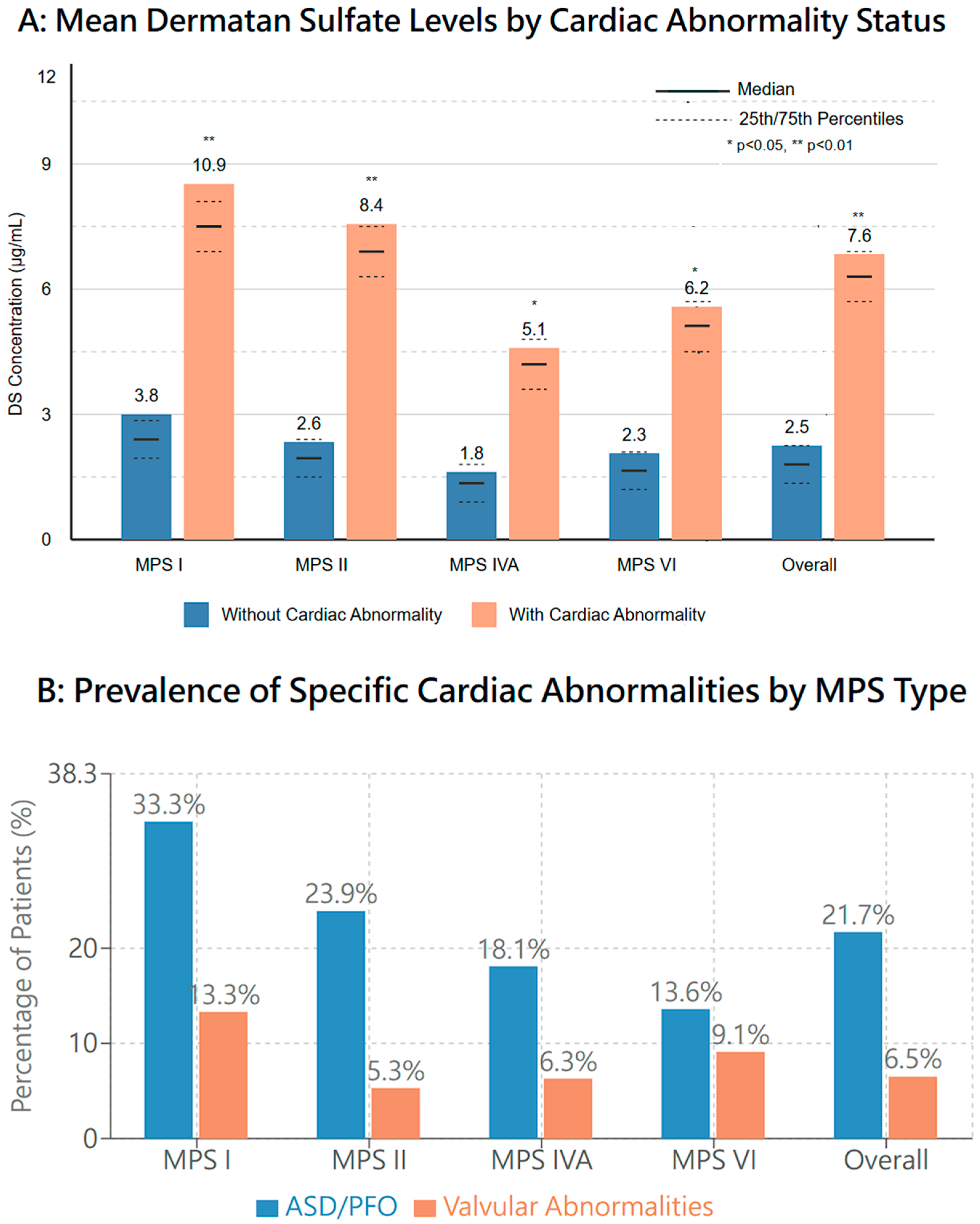
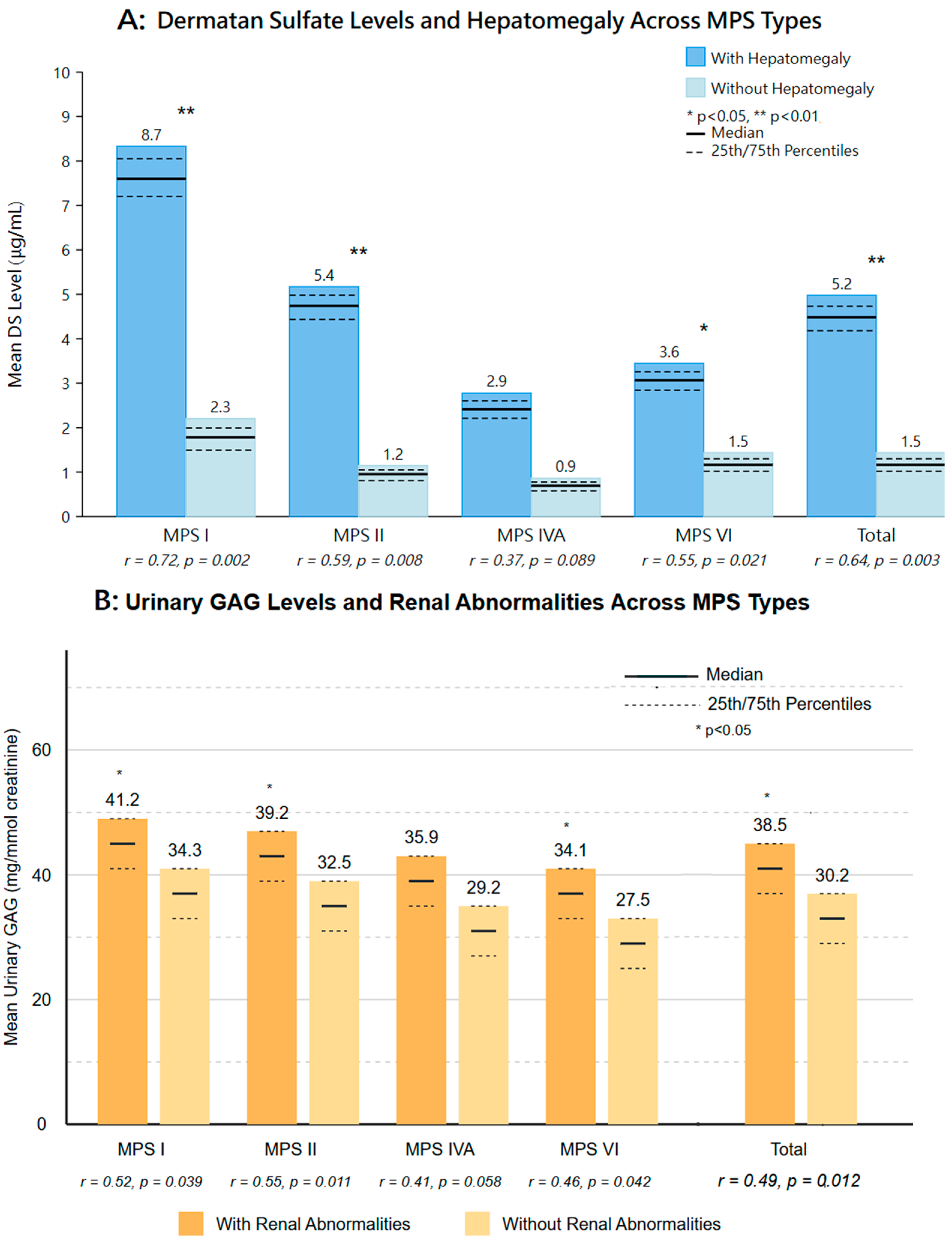
3.2. Prevalence of Imaging Abnormalities (Figure 1, Table 2)

| Radiographic Finding | MPS I (n = 15) | MPS II (n = 113) | MPS IVA (n = 127) | MPS VI (n = 22) |
|---|---|---|---|---|
| Skeletal X-ray | ||||
| Vertebral abnormalities | 1 (6.7%) | 18 (15.9%) | 21 (16.5%) | 2 (9.1%) |
| Hip dysplasia | 1 (6.7%) | 6 (5.3%) | 15 (11.8%) | 1 (4.5%) |
| Carpal/metacarpal changes | 2 (13.3%) | 8 (7.1%) | 9 (7.1%) | 1 (4.5%) |
| Cardiac Echo | ||||
| ASD/PFO | 5 (33.3%) | 27 (23.9%) | 23 (18.1%) | 3 (13.6%) |
| Valvular abnormalities | 2 (13.3%) | 6 (5.3%) | 8 (6.3%) | 2 (9.1%) |
| Abdominal Echo | ||||
| Hepatomegaly | 2 (13.3%) | 6 (5.3%) | 5 (3.9%) | 0 (0%) |
| Splenomegaly | 1 (6.7%) | 4 (3.5%) | 3 (2.4%) | 0 (0%) |
| Renal abnormalities | 1 (6.7%) | 13 (11.5%) | 12 (9.4%) | 1 (4.5%) |
| Gastric stasis | 0 (0%) | 2 (1.8%) | 4 (3.1%) | 3 (13.6%) |
| Ovarian cysts (in females) | 0 (0%) | 0 (0%) | 2 (5.5%) * | 0 (0%) |
3.3. Radiographic Findings
- MPS I: Proximal pointing of the metacarpal bones and bullet-shaped phalanges (13.3%);
- MPS II: Mild anterior vertebral beaking, particularly at the T12–L5 levels (15.9%);
- MPS IVA: Anterior vertebral beaking and posterior scalloping (16.5%);
- MPS VI: Vertebral body rounding (13.6%).
3.4. Cardiac Ultrasound Findings
- MPS I: Atrial septal defect/patent foramen ovale (ASD/PFO) (33.3%);
- MPS II: ASD/PFO (23.9%);
- MPS IVA: ASD/PFO (18.1%);
- MPS VI: ASD/PFO (13.6%).
3.5. Abdominal Ultrasound Findings
- MPS I: Hepatomegaly (13.3%);
- MPS II: Renal pelvic dilation (11.5%);
- MPS IVA: Renal pelvic dilation (9.4%) and ovarian cysts in females (5.5% of female patients);
- MPS VI: Gastric stasis (13.6%).
3.6. Disease Severity Classification and Imaging Correlation
- Predicted severe phenotype (n = 78): Patients with null mutations (no detectable protein), severe missense mutations previously associated with severe phenotypes, enzyme activity < 5% of normal reference range, high urinary GAG levels (>2× upper limit of normal), or other established predictors of severe disease.
- Predicted attenuated phenotype (n = 64): Patients with specific missense mutations previously documented to associate with attenuated disease, combinations of mutations known to result in milder phenotypes (including certain splice-site variants that retain partial activity), enzyme activity between 5–15% of normal reference range, or family history of attenuated disease.
- Unknown/indeterminate (n = 135): Patients with novel mutations of uncertain significance, contradictory predictive factors, or insufficient information for classification.
3.7. MPS Type-Specific Severity Distribution and Imaging Findings
- MPS I: Among 15 patients, 5 (33.3%) were classified as predicted severe, 4 (26.7%) as attenuated, and 6 as unknown. Imaging abnormalities were detected in 80% (4/5) of severe cases compared to 50% (2/4) of attenuated cases. Notably, cardiac abnormalities were present in both severe (100%) and attenuated (50%) phenotypes, while skeletal abnormalities were more frequent in the severe group (60% vs. 25%).
- MPS II: Of 113 patients, 35 (31.0%) were classified as predicted severe, 14 (12.4%) as attenuated, and 64 (56.6%) as unknown. Imaging abnormalities were observed in 48.6% (17/35) of severe cases and 35.7% (5/14) of attenuated cases. Among attenuated MPS II patients, cardiac valve abnormalities were the most common finding (35.7%), similar to reports in the literature of early cardiac manifestations in attenuated MPS II.
- MPS IVA: Among 127 patients, 26 (20.5%) were classified as predicted severe, 37 (29.1%) as attenuated, and 64 (50.4%) as unknown. Imaging abnormalities were detected in 53.8% (14/26) of severe cases and 24.3% (9/37) of attenuated cases. Vertebral changes were observed in both groups but were more pronounced in the severe phenotype (46.2% vs. 16.2%).
- MPS VI: Of 22 patients, 12 (54.5%) were classified as predicted severe, 9 (40.9%) as attenuated, and 1 (4.5%) as unknown. Imaging abnormalities were present in 41.7% (5/12) of severe cases and 11.1% (1/9) of attenuated cases. Abdominal abnormalities were predominantly observed in the severe group.
3.8. Biomarker Correlation with Severity and Imaging Findings (Table 3)
| MPS Type | Severity Category | N | Any Abnormality | Skeletal Abnormalities | Cardiac Abnormalities | Abdominal Abnormalities |
|---|---|---|---|---|---|---|
| I | Severe | 5 | 80% (4/5) | 60% (3/5) | 100% (5/5) | 40% (2/5) |
| Attenuated | 4 | 50% (2/4) | 25% (1/4) | 50% (2/4) | 0% (0/4) | |
| Unknown | 6 | 16.7% (1/6) | 0% (0/6) | 0% (0/6) | 16.7% (1/6) | |
| II | Severe | 35 | 48.6% (17/35) | 42.9% (15/35) | 37.1% (13/35) | 22.9% (8/35) |
| Attenuated | 14 | 35.7% (5/14) | 14.3% (2/14) | 35.7% (5/14) | 7.1% (1/14) | |
| Unknown | 64 | 25.0% (16/64) | 23.4% (15/64) | 23.4% (15/64) | 15.6% (10/64) | |
| IVA | Severe | 26 | 53.8% (14/26) | 46.2% (12/26) | 30.8% (8/26) | 19.2% (5/26) |
| Attenuated | 37 | 24.3% (9/37) | 16.2% (6/37) | 13.5% (5/37) | 8.1% (3/37) | |
| Unknown | 64 | 21.9% (14/64) | 18.8% (12/64) | 17.2% (11/64) | 10.9% (7/64) | |
| VI | Severe | 12 | 41.7% (5/12) | 25.0% (3/12) | 33.3% (4/12) | 25.0% (3/12) |
| Attenuated | 9 | 11.1% (1/9) | 11.1% (1/9) | 11.1% (1/9) | 0% (0/9) | |
| Unknown | 1 | 0% (0/1) | 0% (0/1) | 0% (0/1) | 0% (0/1) |
3.9. Comparison with Symptomatic MPS Patients and General Population
3.10. Association Between Imaging Findings and Clinical Parameters
3.11. Skeletal Manifestations
3.12. Cardiac Manifestations
3.13. Abdominal Manifestations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muenzer, J. The mucopolysaccharidoses: A heterogeneous group of disorders with variable pediatric presentations. J. Pediatr. 2004, 144, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.A. Mucopolysaccharidosis Type I. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993; pp. 1993–2025. [Google Scholar]
- Muenzer, J. Overview of the mucopolysaccharidoses. Rheumatology 2011, 50, v4–v12. [Google Scholar] [CrossRef]
- Chan, M.J.; Liao, H.C.; Gelb, M.H.; Chuang, C.K.; Liu, M.Y.; Chen, H.J.; Kao, S.M.; Lin, H.Y.; Huang, Y.H.; Kumar, A.B.; et al. Taiwan national newborn screening program by tandem mass spectrometry for mucopolysaccharidoses Types I, II, and VI. J. Pediatr. 2019, 205, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lee, C.L.; Tu, R.Y.; Lo, Y.T.; Sisca, F.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; et al. Nationwide Newborn Screening Program for Mucopolysaccharidoses in Taiwan And an update of the “gold standard” criteria required to make a confirmatory diagnosis. Diagnostics 2021, 11, 1583. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Tu, Y.R.; Lee, C.L.; Lo, Y.T.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; Wang, L.Y.; et al. Updated Confirmatory Diagnosis for Mucopolysaccharidoses in Taiwanese Infants and the Application of Gene Variants. Int. J. Mol. Sci. 2022, 23, 9979. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Huang, Y.H.; Chan, M.J.; Liao, H.C.; Lo, Y.T.; Wang, L.Y.; Tu, R.Y.; Fang, Y.Y.; et al. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 84. [Google Scholar] [CrossRef]
- Giugliani, R.; Lampe, C.; Guffon, N.; Ketteridge, D.; Leão-Teles, E.; Wraith, J.E.; Jones, S.A.; Piscia-Nichols, C.; Lin, P.; Quartel, A.; et al. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)—10-year follow-up of patients who previously participated in an MPS VI Survey Study. Am. J. Med. Genet. A 2014, 164, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Petty, R.E. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology 2011, 50, v19–v25. [Google Scholar] [CrossRef]
- Braunlin, E.A.; Harmatz, P.R.; Scarpa, M.; Furlanetto, B.; Kampmann, C.; Loehr, J.P.; Ponder, K.P.; Roberts, W.C.; Rosenfeld, H.M.; Giugliani, R. Cardiac disease in patients with mucopolysaccharidosis: Presentation, diagnosis and management. J. Inherit. Metab. Dis. 2011, 34, 1183–1197. [Google Scholar] [CrossRef]
- Jerves Serrano, T.; Gold, J.; Cooper, J.A.; Church, H.J.; Tylee, K.L.; Wu, H.Y.; Kim, S.Y.; Stepien, K.M. Hepatomegaly and splenomegaly: An approach to the diagnosis of lysosomal storage diseases. J. Clin. Med. 2024, 13, 1465. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Leung, W.T.; Wang, L. A basic understanding of mucopolysaccharidosis: Incidence, clinical features, diagnosis, and management. Intractable Rare Dis. Res. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lee, C.L.; Chang, Y.H.; Tu, Y.R.; Lo, Y.T.; Wu, J.Y.; Niu, D.M.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; et al. Implementation of Newborn Screening for Mucopolysaccharidosis Type IVA and Long-Term Monitoring in Taiwan. Genet. Med. 2024, 26, 101286. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chuang, C.K.; Chen, M.R.; Lin, S.-M.; Hung, C.-L.; Chang, C.-Y.; Chiu, P.C.; Tsai, W.-H.; Niu, D.-M.; Tsai, F.-J.; et al. Cardiac structure and function and effects of enzyme replacement therapy in patients with mucopolysaccharidoses I, II, IVA and VI. Mol. Genet. Metab. 2016, 117, 431–437. [Google Scholar] [CrossRef]
- Hendriksz, C.J.; Harmatz, P.; Beck, M.; Jones, S.; Wood, T.; Lachman, R.; Gravance, C.G.; Orii, T.; Tomatsu, S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013, 110, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Shorter, D.; Hong, T.; Osborn, D.A. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Syst. Rev. 2011, 9, CD004595. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, L.-Z.; Li, B.; Chung, H.-T.; Jiang, J.-X.; Chiang, J.Y.; Chiang, H.-J.; Yip, H.-K.; Sung, P.-H. Investigation of echocardiographic characteristics and predictors for persistent defects of patent foramen ovale or patent ductus arteriosus in Chinese newborns. Biomed. J. 2021, 44, 209–216. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Herndon, C.D.A.; Cooper, C.; Gatti, J.; Kirsch, A.; Kokorowski, P.; Lee, R.; Perez-Brayfield, M.; Metcalfe, P.; Yerkes, E.; et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J. Pediatr. Urol. 2010, 6, 212–231. [Google Scholar] [CrossRef]
- Jaremko, J.L.; Siminoski, K.; Firth, G.; Matzinger, M.A.; Shenouda, N.; Konji, V.N.; Roth, J.; Sbrocchi, A.M.; Reed, M.; O’Brien, K.; et al. Common normal variants of pediatric vertebral development that mimic fractures: A pictorial review from a national longitudinal bone health study. Pediatr. Radiol. 2015, 45, 593–605. [Google Scholar] [CrossRef]
- Oussoren, E.; Brands, M.M.; Ruijter, G.J.; der Ploeg, A.T.; Reuser, A.J. Bone, joint and tooth development in mucopolysaccharidoses: Relevance to therapeutic options. Biochim. Biophys. Acta 2011, 1812, 1542–1556. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Oikawa, H.; Smith, M.; Barrera, L.; Chinen, Y.; Thacker, M.M.; Mackenzie, W.G.; Suzuki, Y.; Orii, T.; et al. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [Google Scholar] [CrossRef]
- Lin, S.P.; Lin, H.Y.; Wang, T.J.; Chang, C.Y.; Lin, C.H.; Huang, S.F.; Tsai, C.C.; Liu, H.L.; Keutzer, J.; Chuang, C.K. A pilot newborn screening program for mucopolysaccharidosis type I in Taiwan. Orphanet J. Rare Dis. 2013, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.J.; Chen, Q.Q.; Dai, Y.L.; Dong, G.P.; Zou, C.C. The diagnosis and management of mucopolysaccharidosis type II. Ital. J. Pediatr. 2024, 50, 207. [Google Scholar] [CrossRef] [PubMed]
- Zafeiriou, D.I.; Batzios, S.P. Brain and Spinal MR Imaging Findings in Mucopolysaccharidoses: A Review. Am. J. Neuroradiol. 2013, 34, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Furujo, M.; Kosuga, M.; Okuyama, T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI: 10-Year follow up. Mol. Genet. Metab. Rep. 2017, 13, 69–75. [Google Scholar] [CrossRef]
- Gabrielli, O.; Clarke, L.A.; Bruni, S.; Coppa, G.V. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics 2010, 125, e183–e187. [Google Scholar] [CrossRef]
- Tajima, G.; Sakura, N.; Kosuga, M.; Okuyama, T.; Kobayashi, M. Effects of idursulfase enzyme replacement therapy for Mucopolysaccharidosis type II when started in early infancy: Comparison in two siblings. Mol. Genet. Metab. 2013, 108, 172–177. [Google Scholar] [CrossRef]
- Giugliani, R.; Federhen, A.; Rojas, M.V.; Vieira, T.; Artigalás, O.; Pinto, L.L.; Azevedo, A.C.; Acosta, A.; Bonfim, C.; Lourenço, C.M.; et al. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010, 33, 589–604. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chuang, C.K.; Lee, C.L.; Chen, M.R.; Sung, K.T.; Lin, S.M.; Hou, C.J.; Niu, D.M.; Chang, T.M.; Hung, C.L.; et al. Cardiac Evaluation using Two-dimensional Speckle-tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics 2020, 10, 62. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lee, C.L.; Chang, C.Y.; Chiu, P.C.; Chien, Y.H.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; Lin, S.J.; Lin, J.L.; et al. Survival and diagnostic age of 175 Taiwanese patients with mucopolysaccharidoses (1985–2019). Orphanet J. Rare Dis. 2020, 15, 314. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chang, Y.H.; Lee, C.L.; Tu, Y.R.; Lo, Y.T.; Hung, P.W.; Niu, D.M.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; et al. Newborn Screening Program for Mucopolysaccharidosis Type II and Long-Term Follow-up of the Screen-Positive Subjects in Taiwan. J. Pers. Med. 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | MPS I (n = 15) | MPS II (n = 113) | MPS IVA (n = 127) | MPS VI (n = 22) |
|---|---|---|---|---|
| Age at evaluation (months) * | 2.1 ± 0.8 (2.0, 1–5) | 1.8 ± 0.7 (2.0, 1–5) | 2.0 ± 0.9 (2.0, 1–5) | 1.9 ± 0.8 (2.0, 1–5) |
| Gender (Male/Female) | 8/7 | 113/0 | 89/38 | 14/8 |
| Enzyme activity (umol/4 h/mg protein) † | 1.2 ± 0.9 | 8.8 ± 7.2 | 2.9 ± 2.1 | 13.6 ± 8.4 |
| Urine GAG (mg/mmol creatinine) ‡ | 37.9 ± 15.8 | 32.4 ± 14.6 | 28.9 ± 13.2 | 26.8 ± 12.4 |
| Imaging Finding | MPS Type | Newborn Screening Cohort (This Study) | Symptomatic MPS Patients * | General Newborn Population ** |
|---|---|---|---|---|
| Skeletal Abnormalities | ||||
| Vertebral abnormalities | I | 6.7% | 70–90% | 0.05–0.1% |
| II | 15.9% | 80–95% | 0.05–0.1% | |
| IVA | 16.5% | 90–100% | 0.05–0.1% | |
| VI | 9.1% | 80–95% | 0.05–0.1% | |
| Hip dysplasia | I | 6.7% | 50–70% | 1–2% |
| II | 5.3% | 40–60% | 1–2% | |
| IVA | 11.8% | 60–80% | 1–2% | |
| VI | 4.5% | 40–60% | 1–2% | |
| Carpal/metacarpal changes | I | 13.3% | 60–80% | <0.01% |
| II | 7.1% | 50–70% | <0.01% | |
| IVA | 7.1% | 60–80% | <0.01% | |
| VI | 4.5% | 50–70% | <0.01% | |
| Cardiac Abnormalities | ||||
| ASD/PFO | I | 33.3% | 60–90% | 25–30% (mostly PFO) |
| II | 23.9% | 50–80% | 25–30% (mostly PFO) | |
| IVA | 18.1% | 40–60% | 25–30% (mostly PFO) | |
| VI | 13.6% | 60–85% | 25–30% (mostly PFO) | |
| Valvular abnormalities | I | 13.3% | 80–95% | 0.5–1% |
| II | 5.3% | 60–90% | 0.5–1% | |
| IVA | 6.3% | 40–60% | 0.5–1% | |
| VI | 9.1% | 75–95% | 0.5–1% | |
| Abdominal Abnormalities | ||||
| Hepatomegaly | I | 13.3% | 70–90% | <0.1% |
| II | 5.3% | 60–80% | <0.1% | |
| IVA | 3.9% | 30–50% | <0.1% | |
| VI | 0.0% | 50–80% | <0.1% | |
| Renal abnormalities | I | 6.7% | 30–50% | 1–2% |
| II | 11.5% | 40–60% | 1–2% | |
| IVA | 9.4% | 20–40% | 1–2% | |
| VI | 4.5% | 30–50% | 1–2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Chang, S.-W.; Fang, H.-H.; Chuang, C.-K.; Chiu, H.-C.; Chang, Y.-H.; Tu, Y.-R.; Lo, Y.-T.; Wu, J.-Y.; Lin, H.-Y.; et al. Systematic Analysis of Multiple Imaging Modalities in Infants Diagnosed with Mucopolysaccharidosis by Newborn Screening. Diagnostics 2025, 15, 980. https://doi.org/10.3390/diagnostics15080980
Lee C-L, Chang S-W, Fang H-H, Chuang C-K, Chiu H-C, Chang Y-H, Tu Y-R, Lo Y-T, Wu J-Y, Lin H-Y, et al. Systematic Analysis of Multiple Imaging Modalities in Infants Diagnosed with Mucopolysaccharidosis by Newborn Screening. Diagnostics. 2025; 15(8):980. https://doi.org/10.3390/diagnostics15080980
Chicago/Turabian StyleLee, Chung-Lin, Szu-Wen Chang, Hung-Hsiang Fang, Chih-Kuang Chuang, Huei-Ching Chiu, Ya-Hui Chang, Yuan-Rong Tu, Yun-Ting Lo, Jun-Yi Wu, Hsiang-Yu Lin, and et al. 2025. "Systematic Analysis of Multiple Imaging Modalities in Infants Diagnosed with Mucopolysaccharidosis by Newborn Screening" Diagnostics 15, no. 8: 980. https://doi.org/10.3390/diagnostics15080980
APA StyleLee, C.-L., Chang, S.-W., Fang, H.-H., Chuang, C.-K., Chiu, H.-C., Chang, Y.-H., Tu, Y.-R., Lo, Y.-T., Wu, J.-Y., Lin, H.-Y., & Lin, S.-P. (2025). Systematic Analysis of Multiple Imaging Modalities in Infants Diagnosed with Mucopolysaccharidosis by Newborn Screening. Diagnostics, 15(8), 980. https://doi.org/10.3390/diagnostics15080980








