A Rare Case of Xeroderma Pigmentosum: Nivolumab Treatment for Three Cutaneous Malignancies with Clinical and Metabolic Imaging Correlation
Abstract
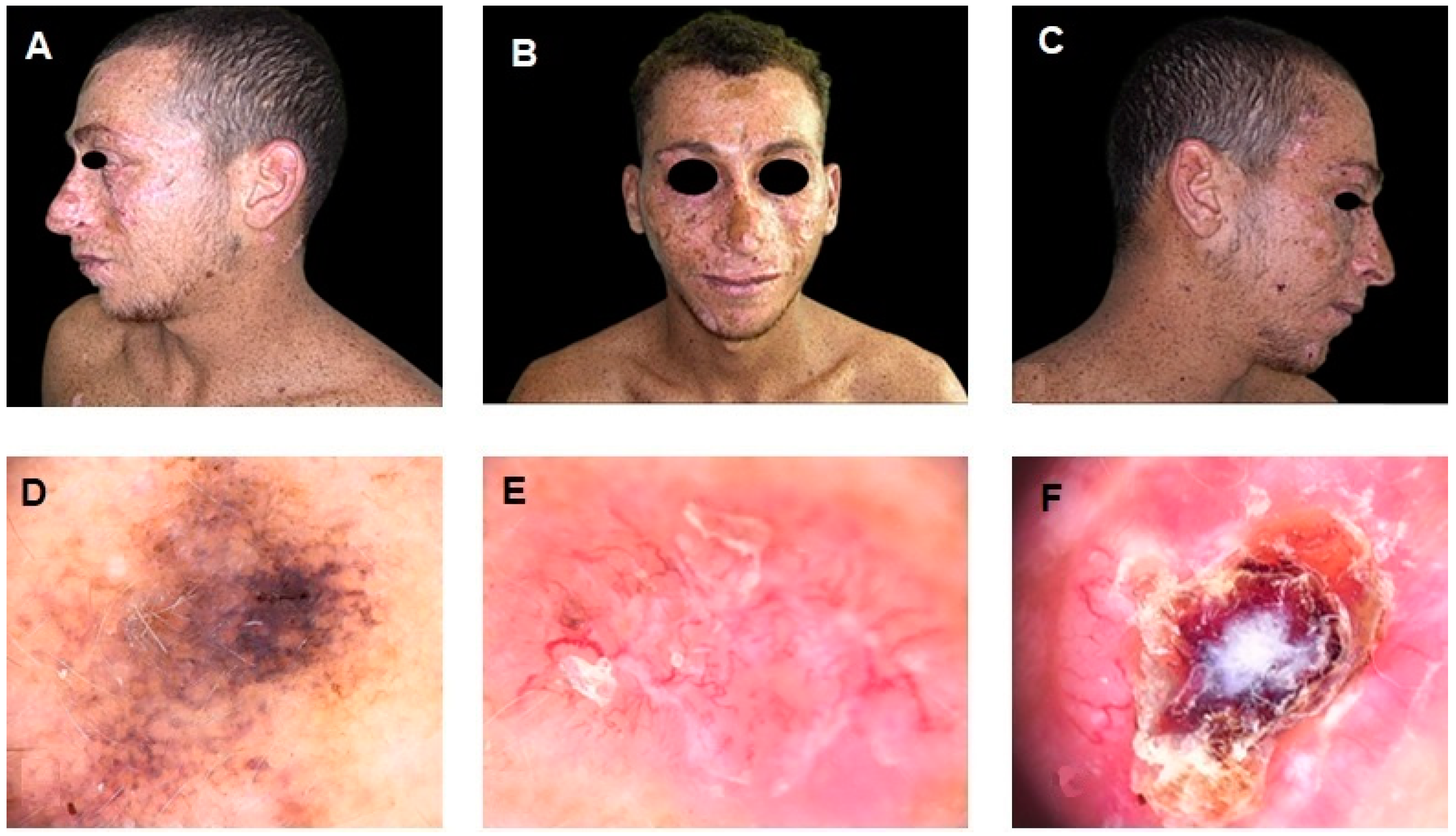
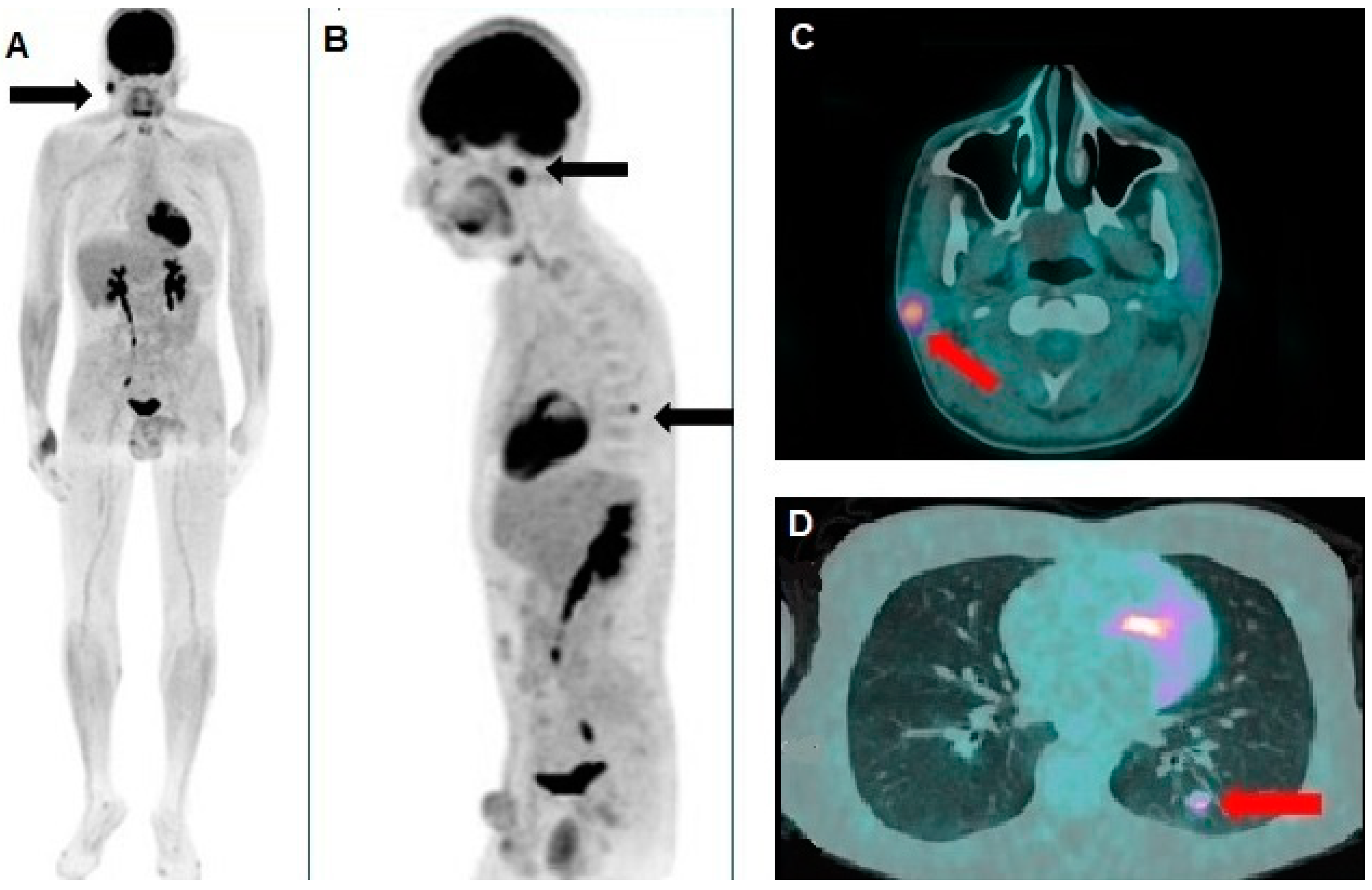
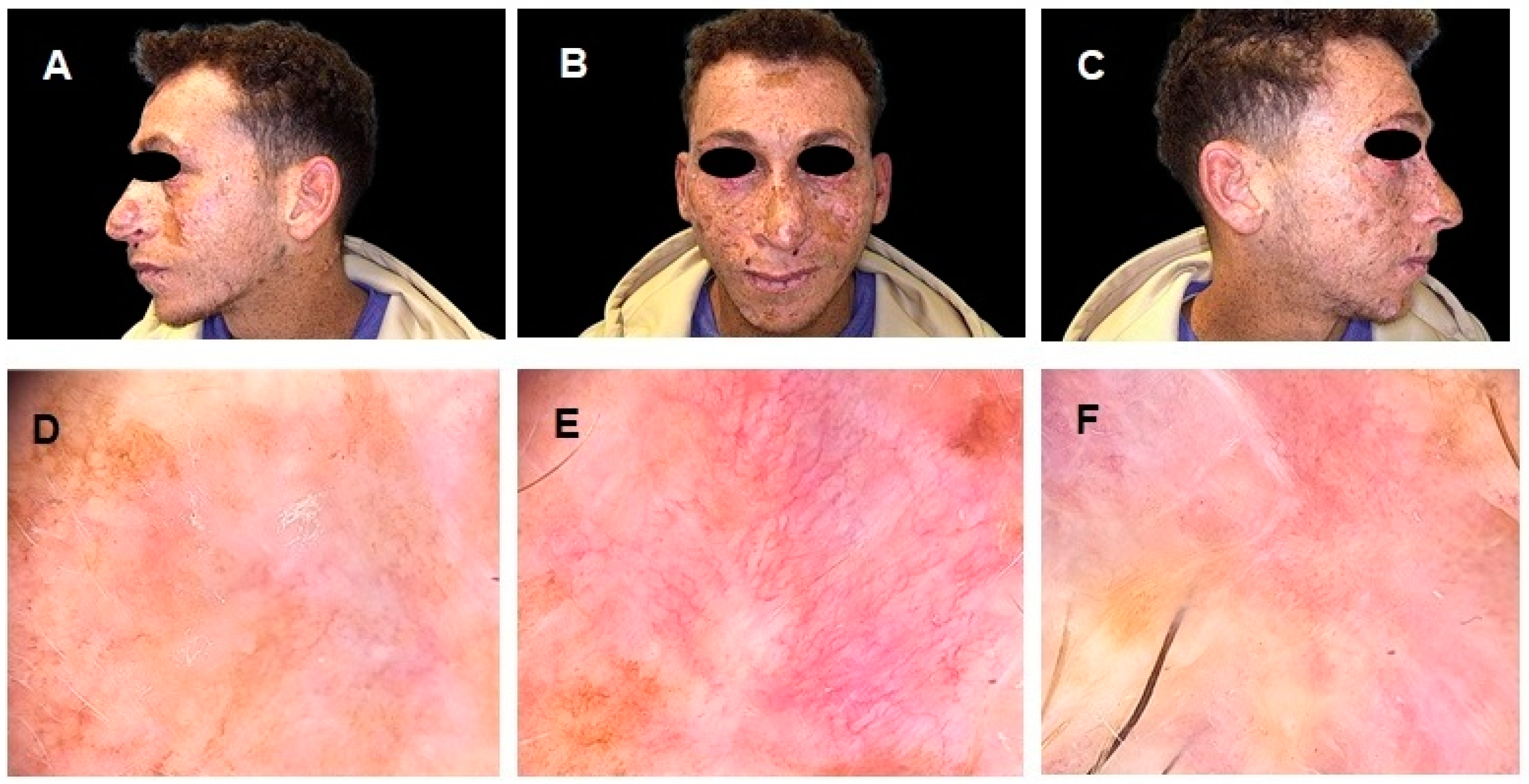
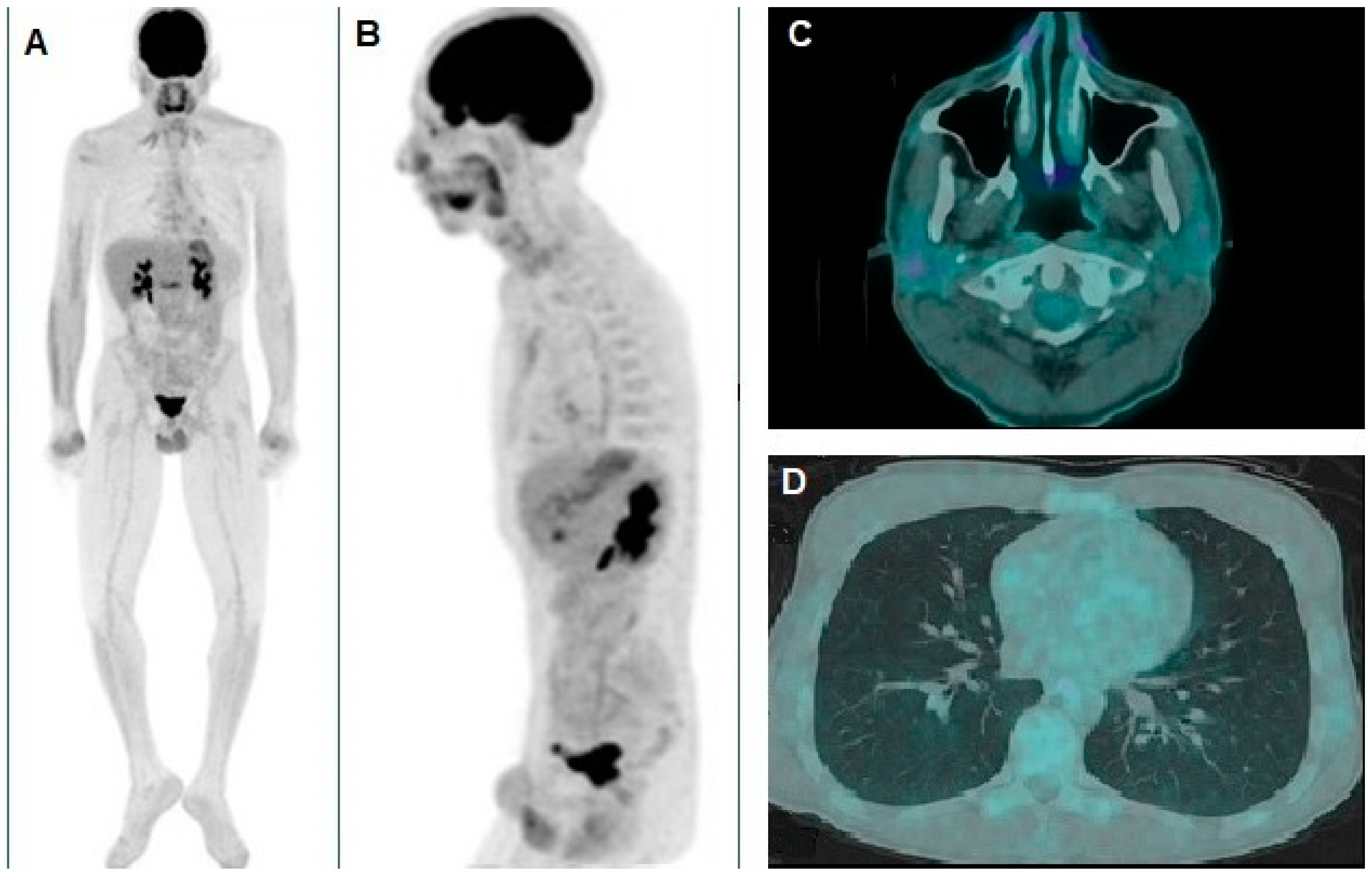
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 1. Epidemiology, Diagnostics and Prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Baumann, B.C.; Bordeaux, J.; Chen, P.-L.; Chin, R.; Contreras, C.M.; et al. NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. J. Natl. Compr. Cancer Netw. 2021, 19, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Black, J.O. Xeroderma Pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kleijer, W.J.; Laugel, V.; Berneburg, M.; Nardo, T.; Fawcett, H.; Gratchev, A.; Jaspers, N.G.J.; Sarasin, A.; Stefanini, M.; Lehmann, A.R. Incidence of DNA Repair Deficiency Disorders in Western Europe: Xeroderma Pigmentosum, Cockayne Syndrome and Trichothiodystrophy. DNA Repair 2008, 7, 744–750. [Google Scholar] [CrossRef]
- Cleaver, J.E. Defective Repair Replication of DNA in Xeroderma Pigmentosum. Nature 1968, 218, 652–656. [Google Scholar] [CrossRef]
- Setlow, R.B.; Regan, J.D.; German, J.; Carrier, W.L. Evidence That Xeroderma Pigmentosum Cells Do Not Perform the First Step in the Repair of Ultraviolet Damage to Their DNA. Proc. Natl. Acad. Sci. USA 1969, 64, 1035–1041. [Google Scholar] [CrossRef]
- Epstein, J.H.; Fukuyama, K.; Reed, W.B.; Epstein, W.L. Defect in DNA Synthesis in Skin of Patients with Xeroderma Pigmentosum Demonstrated In Vivo. Science 1970, 168, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Paszkowska-Szczur, K.; Scott, R.J.; Serrano-Fernandez, P.; Mirecka, A.; Gapska, P.; Górski, B.; Cybulski, C.; Maleszka, R.; Sulikowski, M.; Nagay, L.; et al. Xeroderma pigmentosum Genes and Melanoma Risk. Int. J. Cancer 2013, 133, 1094–1100. [Google Scholar] [CrossRef]
- Wade, M.H.; Plotnick, H. Xeroderma Pigmentosum and Squamous Cell Carcinoma of the Tongue. J. Am. Acad. Dermatol. 1985, 12, 515–521. [Google Scholar] [CrossRef]
- Chambon, F.; Osdoit, S.; Bagny, K.; Moro, A.; Nguyen, J.; Réguerre, Y. Dramatic Response to Nivolumab in Xeroderma Pigmentosum Skin Tumor. Pediatr. Blood Cancer 2018, 65, e26837. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W.; Wang, E.; Marincola, F.M.; Rammensee, H.-G.; Restifo, N.P.; for the Organizing Committee of the 2013 SITC Workshop on Personalized Immunotherapy. Mining the Mutanome: Developing Highly Personalized Immunotherapies Based on Mutational Analysis of Tumors. J. ImmunoTherapy Cancer 2013, 1, 11. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Geukes Foppen, M.H.; Goldinger, S.M.; et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Bardelli, A.; Chan, T.A.; Turajlic, S. The Status of Tumor Mutational Burden and Immunotherapy. Nat. Cancer 2022, 3, 652–656. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Zalaquett, N.G.; Kreidieh, L.; Youssef, B.; Mourad, M.; Kreidieh, F. Case Report: Neoadjuvant-Intent Pembrolizumab Resulted in Complete Response in a Xeroderma Pigmentosum Patient with Locally Advanced Resectable Cutaneous Squamous Cell Carcinoma of the Nose. Front. Med. 2024, 11, 1488400. [Google Scholar] [CrossRef]
- Kaste, S.C. Imaging of Pediatric Cutaneous Melanoma. Pediatr. Radiol. 2019, 49, 1476–1487. [Google Scholar] [CrossRef]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The Role and Potential of 18F-FDG PET/CT in Malignant Melanoma: Prognostication, Monitoring Response to Targeted and Immunotherapy, and Radiomics. Diagnostics 2022, 12, 929. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Weru, V.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The Prognostic Value of [18F]FDG PET/CT Based Response Monitoring in Metastatic Melanoma Patients Undergoing Immunotherapy: Comparison of Different Metabolic Criteria. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2699–2714. [Google Scholar] [CrossRef]
- Anderson, T.M.; Chang, B.H.; Huang, A.C.; Xu, X.; Yoon, D.; Shang, C.G.; Mick, R.; Schubert, E.; McGettigan, S.; Kreider, K.; et al. FDG PET/CT Imaging 1 Week after a Single Dose of Pembrolizumab Predicts Treatment Response in Patients with Advanced Melanoma. Clin. Cancer Res. 2024, 30, 1758–1767. [Google Scholar] [CrossRef]
- Iravani, A.; Wallace, R.; Lo, S.N.; Galligan, A.; Weppler, A.M.; Hicks, R.J.; Sandhu, S. FDG PET/CT Prognostic Markers in Patients with Advanced Melanoma Treated with Ipilimumab and Nivolumab. Radiology 2023, 307, e221180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proietti, I.; Pirisino, R.; Azzella, G.; Coppolelli, V.; Greco, M.E.; Casciani, E.; Potenza, C.; Filippi, L. A Rare Case of Xeroderma Pigmentosum: Nivolumab Treatment for Three Cutaneous Malignancies with Clinical and Metabolic Imaging Correlation. Diagnostics 2025, 15, 979. https://doi.org/10.3390/diagnostics15080979
Proietti I, Pirisino R, Azzella G, Coppolelli V, Greco ME, Casciani E, Potenza C, Filippi L. A Rare Case of Xeroderma Pigmentosum: Nivolumab Treatment for Three Cutaneous Malignancies with Clinical and Metabolic Imaging Correlation. Diagnostics. 2025; 15(8):979. https://doi.org/10.3390/diagnostics15080979
Chicago/Turabian StyleProietti, Ilaria, Riccardo Pirisino, Giulia Azzella, Vincenzo Coppolelli, Maria Elisabetta Greco, Emanuele Casciani, Concetta Potenza, and Luca Filippi. 2025. "A Rare Case of Xeroderma Pigmentosum: Nivolumab Treatment for Three Cutaneous Malignancies with Clinical and Metabolic Imaging Correlation" Diagnostics 15, no. 8: 979. https://doi.org/10.3390/diagnostics15080979
APA StyleProietti, I., Pirisino, R., Azzella, G., Coppolelli, V., Greco, M. E., Casciani, E., Potenza, C., & Filippi, L. (2025). A Rare Case of Xeroderma Pigmentosum: Nivolumab Treatment for Three Cutaneous Malignancies with Clinical and Metabolic Imaging Correlation. Diagnostics, 15(8), 979. https://doi.org/10.3390/diagnostics15080979






