A Possible Role of Clinical Factors in Choosing the Best Treatment Modality in Cesarean Scar Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection
2.3. Imaging Acquisition
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSP | cesarean scar pregnancy |
| hCG | human chorionic gonadotropin |
| D&C | dilatation and curettage |
| csec | cesarean section |
| FHB | fetal heartbeat |
| MTX | methotrexate |
| RMT | residual myometrial thickness |
References
- Society for Maternal-Fetal Medicine (SMFM); Miller, R.; Gyamfi-Bannerman, C.; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy. Am. J. Obstet. Gynecol. 2022, 227, B9–B20. [Google Scholar]
- Morlando, M.; Conte, A.; Schiattarella, A. Reproductive outcome after cesarean scar pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 91, 102362. [Google Scholar] [CrossRef]
- Seow, K.M.; Huang, L.W.; Lin, Y.H.; Lin, M.Y.; Tsai, Y.L.; Hwang, J.L. Cesarean scar pregnancy: Issues in management. Ultrasound Obstet. Gynecol. 2004, 23, 247–253. [Google Scholar] [CrossRef]
- Calì, G.; Timor-Tritsch, I.E.; Palacios-Jaraquemada, J.; Monteaugudo, A.; Buca, D.; Forlani, F.; Familiari, A.; Scambia, G.; Acharya, G.; D’Antonio, F. Outcome of Cesarean scar pregnancy managed expectantly: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Viana Pinto, P.; Costa, M.A. Cesarean Scar Pregnancy: A systematic review on expectant management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 288, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Jordans, I.P.M.; Verberkt, C.; De Leeuw, R.A.; Bilardo, C.M.; Bosch, T.V.D.; Bourne, T.; Brölmann, H.A.M.; Dueholm, M.; Hehenkamp, W.J.K.; Jastrow, N.; et al. Definition and sonographic reporting system for Cesarean scar pregnancy in early gestation: Modified Delphi method. Ultrasound Obstet. Gynecol. 2022, 59, 437–449. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.; Buca, D.; Di Mascio, D.; Cali, G.; D’amico, A.; Monteagudo, A.; Tinari, S.; Morlando, M.; Nappi, L.; Greco, P.; et al. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 53–59. [Google Scholar] [CrossRef]
- Cali, G.; Forlani, F.; Timor-Tritsch, I.E.; Palacios-Jaraquemada, J.; Minneci, G.; D’Antonio, F. Natural history of Cesarean scar pregnancy on prenatal ultrasound: The crossover sign. Ultrasound Obstet. Gynecol. 2017, 50, 100–104. [Google Scholar] [CrossRef]
- Kaelin Agten, A.; Cali, G.; Monteagudo, A.; Oviedo, J.; Ramos, J.; Timor-Tritsch, I. The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche”. Am. J. Obstet. Gynecol. 2017, 216, 510.e1–510.e6. [Google Scholar] [CrossRef]
- Gonzalez, N.; Tulandi, T. Cesarean Scar Pregnancy: A Systematic Review. J. Minim. Invasive Gynecol. 2017, 24, 731–738. [Google Scholar] [CrossRef]
- Glassman, D.; Mohamed-Nady, N.; Sauer, M.V. Treatment of cesarean scar ectopic pregnancy by gravid total abdominal hysterectomy. Am. J. Obstet. Gynecol. 2023, 228, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Kathopoulis, N.; Chatzipapas, I.; Samartzis, K.; Theodora, M.; Lardou, I.; Protopapas, A. Laparoscopic management of cesarean scar pregnancy: Report of two cases with video-presentation of different operative techniques and literature review. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102066. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ohba, T.; Katabuchi, H. Safety and Efficacy of a Single Local Methotrexate Injection for Cesarean Scar Pregnancy. J. Minim. Invasive Gynecol. 2022, 29, 416–423. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, S.; Wang, F.; Wang, Y.; Shi, Z.; Zhou, X.; Zhou, J.; Huang, J. Cesarean scar pregnancy: Noninvasive and effective treatment with high-intensity focused ultrasound. Am. J. Obstet. Gynecol. 2014, 211, 356.e1–356.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; He, J.; Bai, J.; Zhang, L. The impact of gestational sac size on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment for caesarean scar pregnancy. Int. J. Hyperth. 2018, 35, 291–297. [Google Scholar] [CrossRef]
- Pickett, C.M.; Minalt, N.; Higgins, O.M.; Bernard, C.; Kasper, K.M. A laparoscopic approach to cesarean scar ectopic pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 417–419. [Google Scholar] [CrossRef]
- Vervoort, A.J.; Uittenbogaard, L.B.; Hehenkamp, W.J.; Brolmann, H.A.; Mol, B.W.; Huirne, J.A. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum. Reprod. 2015, 30, 2695–2702. [Google Scholar] [CrossRef]
- Jordans, I.P.M.; de Leeuw, R.A.; Stegwee, S.I.; Amso, N.N.; Barri-Soldevila, P.N.; van den Bosch, T.; Bourne, T.; Brölmann, H.A.M.; Donnez, O.; Dueholm, M.; et al. Sonographic examination of uterine niche in non-pregnant women: A modified Delphi procedure. Ultrasound Obstet. Gynecol. 2019, 53, 107–115. [Google Scholar] [CrossRef]
- Wong, W.S.F.; Fung, W.T. Magnetic Resonance Imaging in the Evaluation of Cesarean Scar Defect. Gynecol. Minim. Invasive Ther. 2018, 7, 104–107. [Google Scholar] [CrossRef]
- Wang, J.; Pang, Q.; Wei, W.; Cheng, L.; Huang, F.; Cao, Y.; Hu, M.; Yan, S.; He, Y.; Wei, Z. Definition of large niche after Cesarean section based on prediction of postmenstrual spotting: Chinese cohort study in non-pregnant women. Ultrasound Obstet. Gynecol. 2022, 59, 450–456. [Google Scholar] [CrossRef]
- McLeish, S.F.; Murchison, A.B.; Smith, D.M.; Ghahremani, T.; Johnson, I.M.; Magann, E.F. Predicting Uterine Rupture Risk Using Lower Uterine Segment Measurement During Pregnancy with Cesarean History: How Reliable Is It? A Review. Obstet. Gynecol. Surv. 2023, 78, 302–308. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Monteagudo, A.; Cali, G.; Kaelin Agten, A.; Palacios-Jaraquemada, J.M.; D’Antonio, F. Hidden in plain sight: Role of residual myometrial thickness to predict outcome of Cesarean scar pregnancy. Ultrasound Obstet. Gynecol. 2023, 62, 624–632. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K. Births in the United States, 2022; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2023; pp. 1–8.
- Kim, H.Y.; Lee, D.; Kim, J.; Noh, E.; Ahn, K.-H.; Hong, S.-C.; Kim, H.-J.; Oh, M.-J.; Cho, G.J. Secular trends in cesarean sections and risk factors in South Korea (2006–2015). Obstet. Gynecol. Sci. 2020, 63, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Spong, C.Y.; Yule, C.S.; Fleming, E.T.; Lafferty, A.K.; McIntire, D.D.; Twickler, D.M. The Cesarean Scar of Pregnancy: Ultrasound Findings and Expectant Management Outcomes. Am. J. Perinatol. 2024, 41, e1445–e1450. [Google Scholar] [CrossRef]
- Ban, Y.; Shen, J.; Wang, X.; Zhang, T.; Lu, X.; Qu, W.; Hao, Y.; Mao, Z.; Li, S.; Tao, G.; et al. Cesarean Scar Ectopic Pregnancy Clinical Classification System with Recommended Surgical Strategy. Obstet. Gynecol. 2023, 141, 927–936. [Google Scholar] [CrossRef]
- Jurkovic, D.; Knez, J.; Appiah, A.; Farahani, L.; Mavrelos, D.; Ross, J.A. Surgical treatment of Cesarean scar ectopic pregnancy: Efficacy and safety of ultrasound-guided suction curettage. Ultrasound Obstet. Gynecol. 2016, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, A.; Sayour, I.; Sleiman, Z.; Centini, G.; Lazzeri, L.; Giorgi, M.; Zupi, E.; Habib, N. “In Situ” Methotrexate Injection Followed by Hysteroscopic Resection for Caesarean Scar Pregnancy: A Single-Center Experience. J. Clin. Med. 2023, 12, 2304. [Google Scholar] [CrossRef] [PubMed]
- Timor-Tritsch, I.E.; Monteagudo, A. Unforeseen consequences of the increasing rate of cesarean deliveries: Early placenta accreta and cesarean scar pregnancy. A review. Am. J. Obstet. Gynecol. 2012, 207, 14–29. [Google Scholar] [CrossRef]
- Paquette, K.; Markey, S.; Roberge, S.; Girard, M.; Bujold, E.; Semers, S. First and third trimester uterine scar thickness in women after previous caesarean: A prospective comparative study. J. Obstet. Gynecol. Can. 2019, 41, 59–63. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, J.; Xie, J.; Li, Q. Diagnostic value and efficacy evaluation value of transvaginal color doppler ultrasound parameters for uterine scar pregnancy and sub-type after cesarean section. BMC Med. Imaging 2024, 24, 239. [Google Scholar] [CrossRef]
- Jauniaux, E.; Zosmer, N.; De Braud, L.V.; Ashoor, G.; Ross, J.; Jurkovic, D. Development of the utero-placental circulation in cesarean scar pregnancies: A case-control study. Am. J. Obstet. Gynecol. 2022, 226, 399.e1–399.e10. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Debbink, M.; Griffith, A.; Kaiser, J.; Woodward, P. Cesarean Scar Ectopic Pregnancy: A Do-Not-Miss Diagnosis. Radiographics 2024, 44, e230199. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.-H.; Guo, X.; Li, M.; Liao, Y.; Gaudet, L.; Walker, M.; Lei, H.; Wen, S.W. Risk factors and consequences of undiagnosed cesarean scar pregnancy: A cohort study in China. BMC Pregnancy Childbirth 2019, 19, 383. [Google Scholar] [CrossRef]
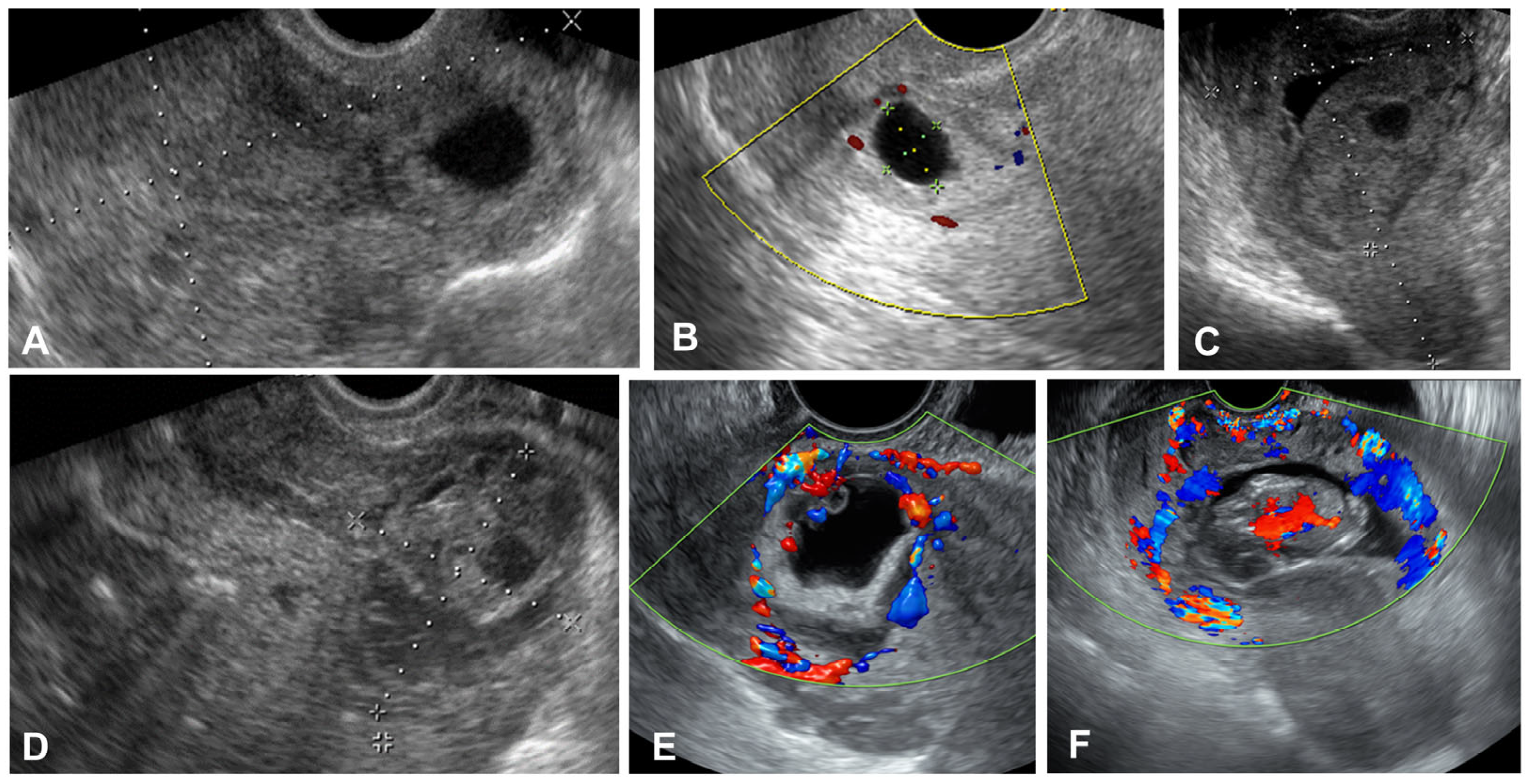
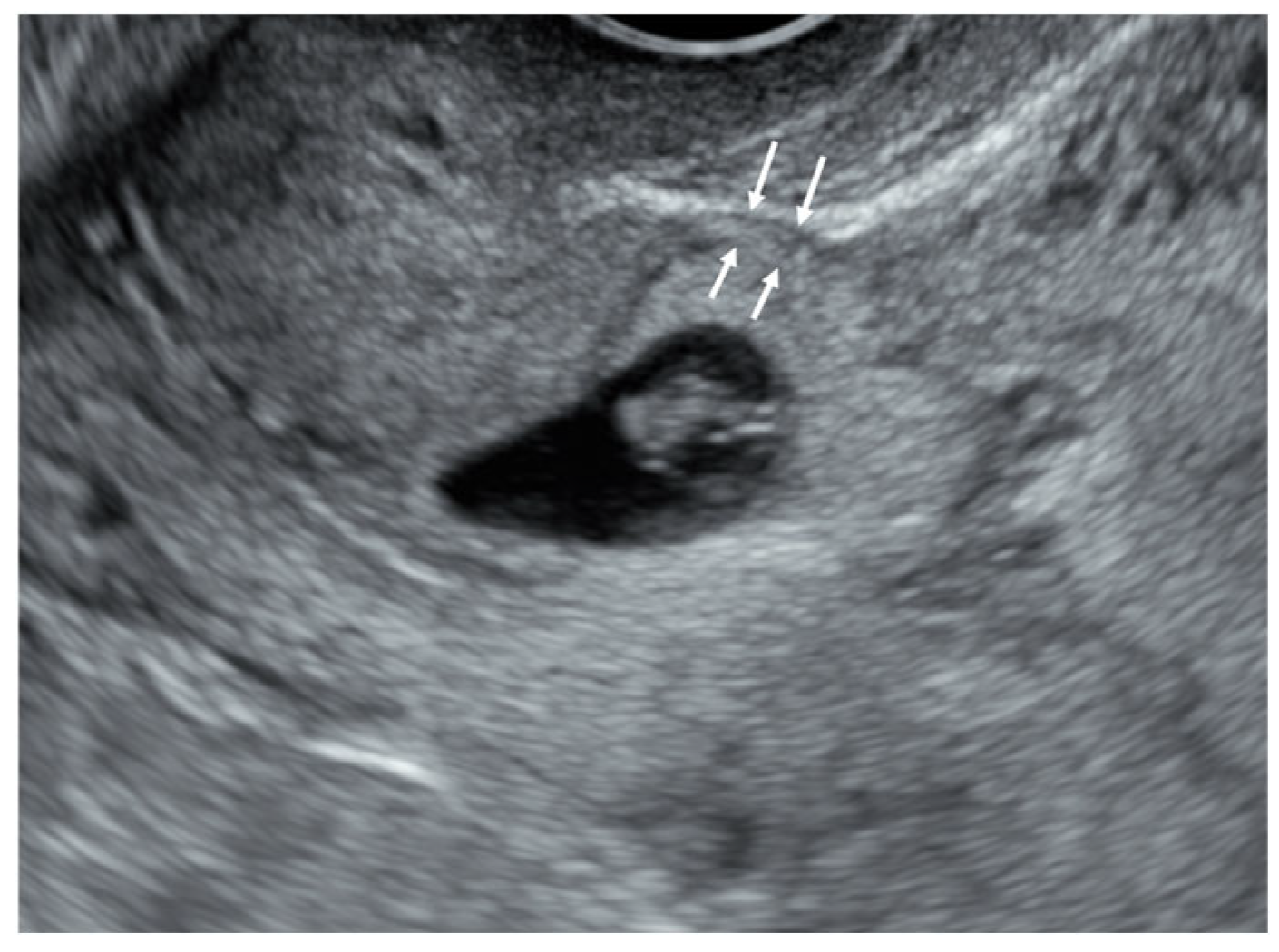

| D&C (n = 84) | D&C + MTX (n = 12) | D&C + Foley Ballooning (n = 21) | Invasive Treatment a (n = 11) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 34.9 ± 4.9 | 37.8 ± 4.7 | 35.1 ± 4.7 | 35.4 ± 5.0 | 0.333 |
| Gestational age (weeks) | 6.1 ± 1.0 | 6.9 ± 1.3 | 7.0 ± 1.4 | 8.1 ± 2.3 | <0.001 |
| Beta hCG | 28,940 ± 116,770 | 18,476 ± 17,864 | 43,868 ± 38,287 | 32,754 ± 31,978 | 0.944 |
| Mean gestational sac diameter (cm) | 1.6 ± 0.8 | 2.4 ± 1.3 | 3.2 ± 1.1 | 4.7 ± 2.6 | <0.001 |
| CRL (cm) | 0.4 ± 0.3 | 1.1 ± 1.2 | 1.1 ± 0.8 | 2.6 ± 2.2 | <0.001 |
| RMT (cm) | 0.3 ± 0.1 | 0.38 ± 0.17 | 0.26 ± 0.11 | 0.21 ± 0.11 | 0.033 |
| Number of csec | 1.6 ± 0.6 | 1.8 ± 0.7 | 1.8 ± 0.5 | 1.6 ± 0.7 | 0.746 |
| FHB (%) | 30.7 | 36.4 | 52.9 | 62.5 | 0.146 |
| Transfusion (%) | 14.5 | 25 | 47.6 | 72.7 | <0.001 |
| Hypervascularity (%) | 14 | 37.5 | 75 | 100 | <0.001 |
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Gestational age | 1.311 | 0.962–1.804 | 0.086 | 1.490 | 0.930–2.557 | 0.114 |
| RMT | 0.051 | 0.001–1.284 | 0.089 | 0.064 | 0.001–7.494 | 0.287 |
| Number of csec | 0.917 | 0.471–1.756 | 0.794 | 1.068 | 0.366–3.016 | 0.900 |
| FHB | 1.344 | 0.539–3.286 | 0.517 | 1.973 | 0.519–7.621 | 0.314 |
| Hypervascularity | 3.152 | 1.171–8.743 | 0.024 | 1.298 | 0.249–6.218 | 0.747 |
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Gestational age | 2.115 | 1.345–3.583 | <0.001 | 3.524 | 1.740–10.14 | 0.003 |
| FHB | 5.156 | 1.05–37.37 | 0.058 | 11.39 | 1.13–360.6 | 0.076 |
| Number of csec | 0.936 | 0.320–2.556 | 0.899 | 1.249 | 0.186–8.083 | 0.807 |
| Age | 1.008 | 0.888–1.151 | 0.899 | 1.296 | 0.978–1.885 | 0.113 |
| OR | 95%CI | p-Value | aOR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Gestational age | 0.495 | 0.324–0.706 | <0.001 | 0.388 | 0.158–0.774 | 0.018 |
| RMT | 5.057 | 0.366–84.26 | 0.238 | 0.037 | 0.001–3.925 | 0.168 |
| Number of csec | 0.817 | 0.456–1.455 | 0.494 | 0.472 | 0.154–1.303 | 0.159 |
| Hypervascularity | 0.125 | 0.039–0.349 | <0.001 | 0.156 | 0.029–0.692 | 0.048 |
| FHB | 0.563 | 0.256–1.227 | 0.149 | 0.400 | 0.095–1.629 | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.-H.; Kim, H.-Y.; Ouh, Y.-T.; Min, K.-J.; Yi, K.-W.; Lee, N.-W. A Possible Role of Clinical Factors in Choosing the Best Treatment Modality in Cesarean Scar Pregnancy. Diagnostics 2025, 15, 965. https://doi.org/10.3390/diagnostics15080965
Song K-H, Kim H-Y, Ouh Y-T, Min K-J, Yi K-W, Lee N-W. A Possible Role of Clinical Factors in Choosing the Best Treatment Modality in Cesarean Scar Pregnancy. Diagnostics. 2025; 15(8):965. https://doi.org/10.3390/diagnostics15080965
Chicago/Turabian StyleSong, Kwan-Heup, Ho-Yeon Kim, Yung-Taek Ouh, Kyung-Jin Min, Kyong-Wook Yi, and Nak-Woo Lee. 2025. "A Possible Role of Clinical Factors in Choosing the Best Treatment Modality in Cesarean Scar Pregnancy" Diagnostics 15, no. 8: 965. https://doi.org/10.3390/diagnostics15080965
APA StyleSong, K.-H., Kim, H.-Y., Ouh, Y.-T., Min, K.-J., Yi, K.-W., & Lee, N.-W. (2025). A Possible Role of Clinical Factors in Choosing the Best Treatment Modality in Cesarean Scar Pregnancy. Diagnostics, 15(8), 965. https://doi.org/10.3390/diagnostics15080965







