The Pericardium Cells Junctions Are a Target for Autoantibodies of Patients Affected by a Variant of Endemic Pemphigus Foliaceus in El Bagre and Surrounding Municipalities in Colombia, South America
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluation
2.3. Histopathology (H&E Stain), Direct Immunofluorescence (DIF), Indirect Immunofluorescence (IIF), Confocal Microscopy (CM) and Immunohistochemical (IHC) Studies
2.4. Statistical Analysis
3. Results
3.1. Clinical Symptoms of the El Bagre-EPF Patients
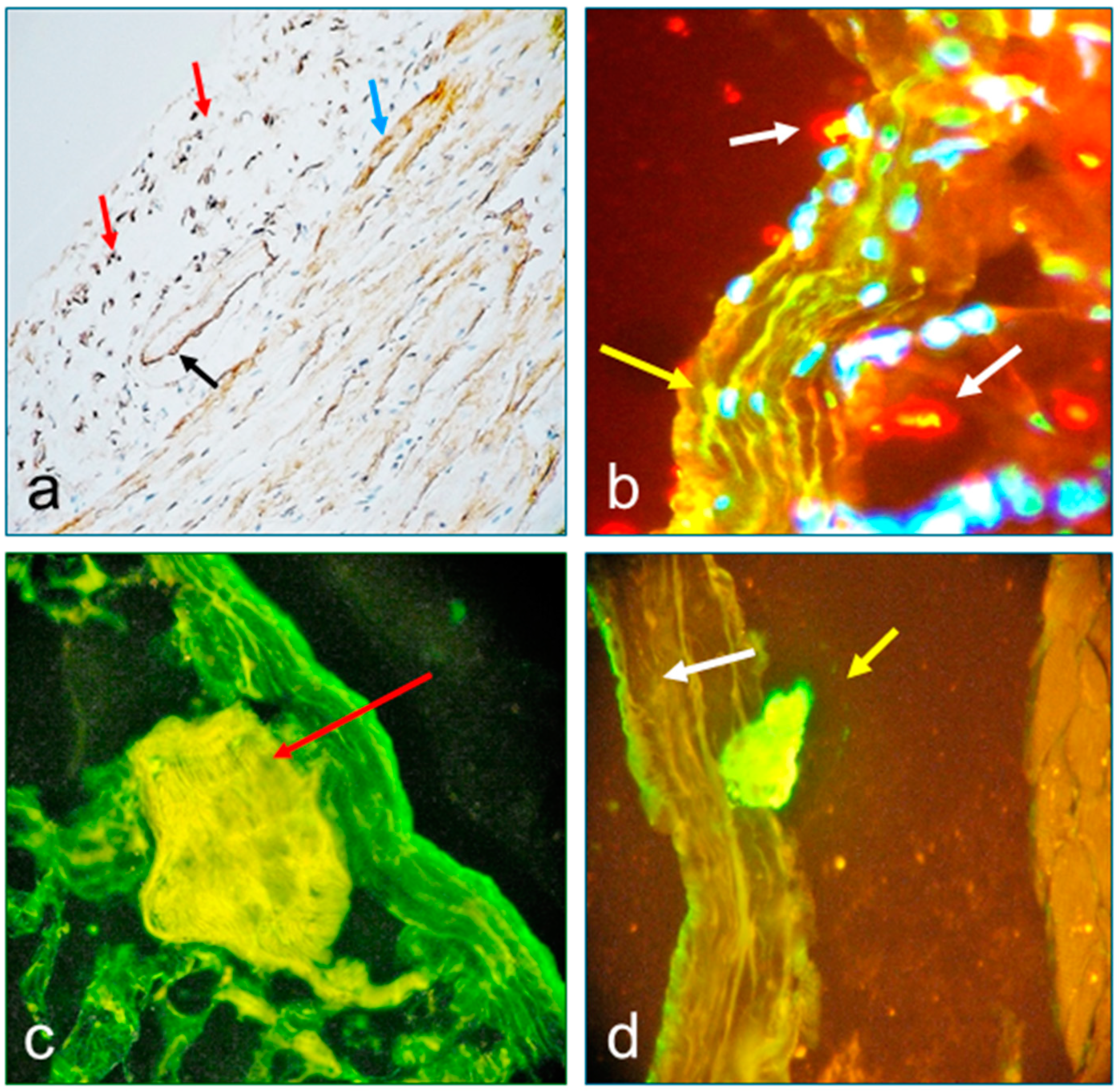
3.2. Results of Immunohistochemistry for Patient Tissues (IHC)
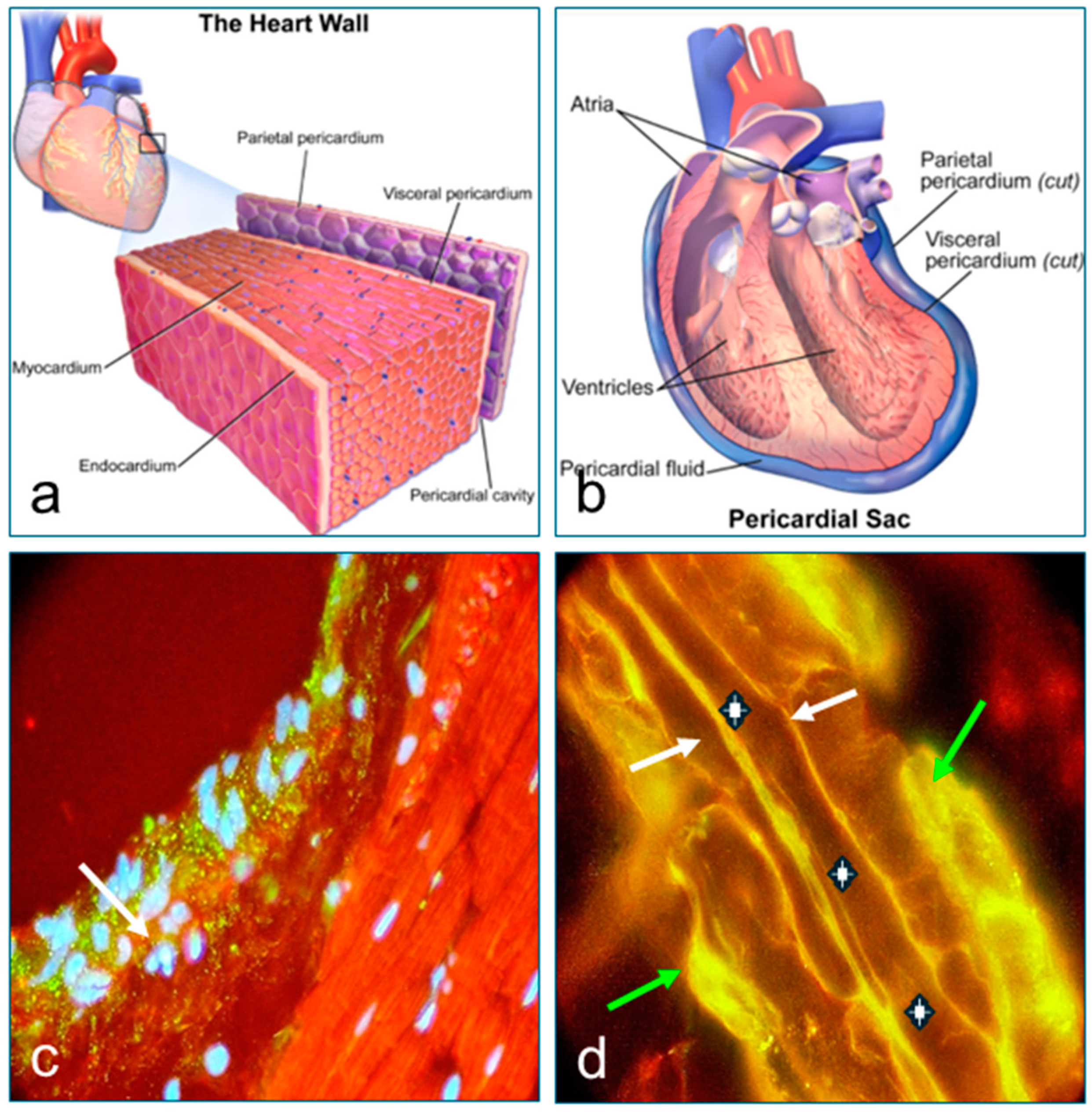
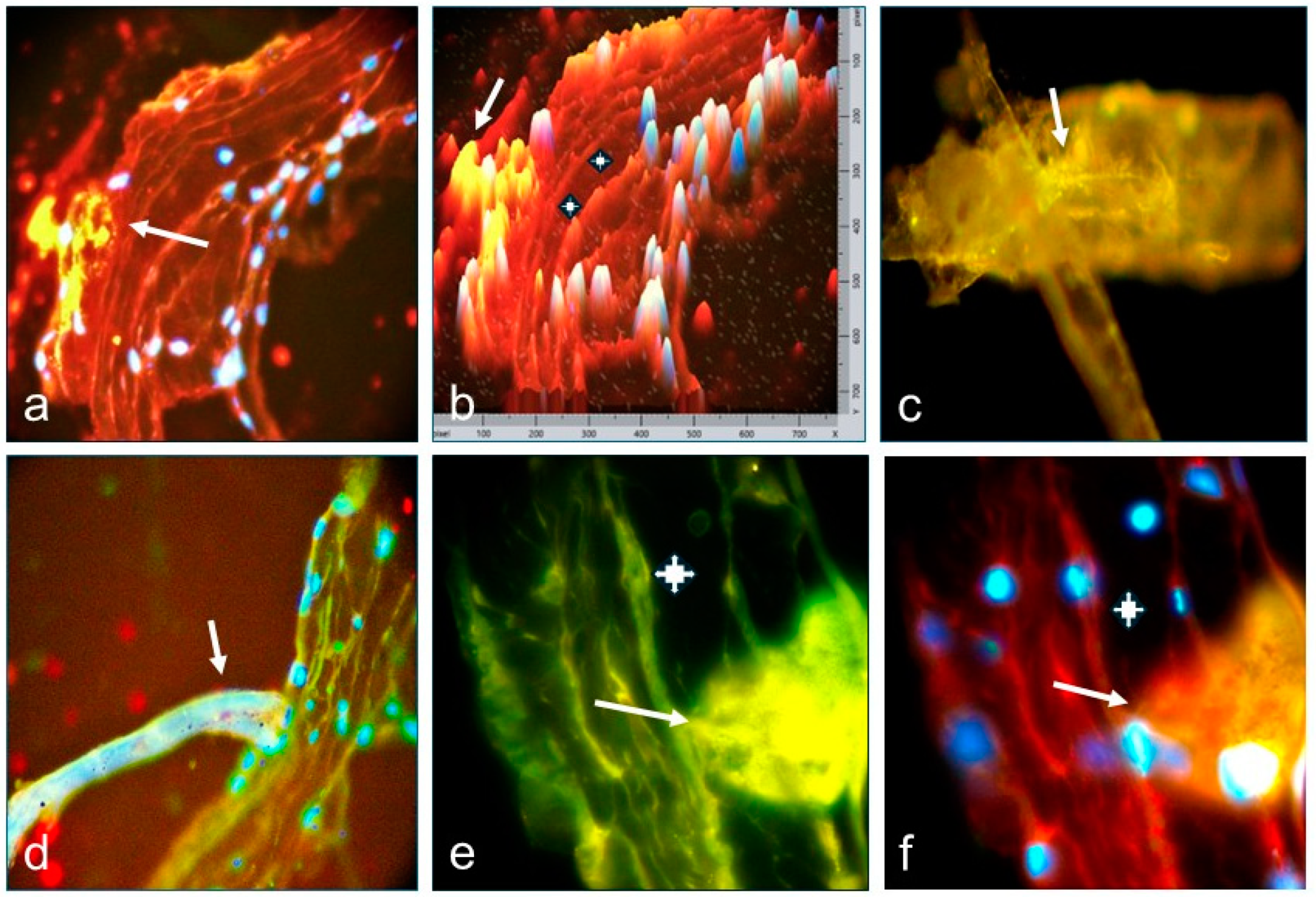
3.3. Results of Immunofluorescence Tests (DIF and IIF) and Confocal Microscopy (CM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPF | Endemic pemphigus foliaceus |
| El Bagre-EPF | Endemic pemphigus foliaceus in El Bagre |
| PF | Pemphigus foliaceus |
| FS | Fogo selvagem |
| H&E | Hematoxylin and eosin |
| DIF, IIF | Direct and indirect immunofluorescence |
| BMZ | Base membrane zone |
| ICS | Intercellular stain between keratinocytes |
| PAF | Paraformaldehyde |
| FITC | Fluorescein isothiocyanate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Dsg1 | Desmoglein 1 |
| CFM | Confocal microscopy |
| P0071 | Plakophilin 4 |
| ARVCF | Armadillo repeat gene deleted in Velo-Cardio-Facial syndrome |
| DP I-II | Desmoplakins I and II |
| MYZAP | Myocardium-enriched zonula occludens-1-associated protein |
| Abs | Antibodies |
| ARVCF | Arrhythmogenic right ventricular cardiomyopathy |
| ECG | Electrocardiogram |
References
- Aguiar Pupo, J. Aspectos originais do Pênfigo Foliáceo no Brasil. An. Bras. Dermatol. 1971, 46, 53–60. [Google Scholar]
- Aranha Campos, J. Pênfigo Foliáceo (Fogo Selvagem): Aspectos Clínicos e Epidemiológicos; Melhoramentos: São Paulo, Brazil, 1942; p. 126. [Google Scholar]
- Aranha Campos, J. Novas observações de casos familiares de pênfigo foliáceo. An. Paul. Med. Cirurg. 1945, 50, 73. [Google Scholar]
- Heimgartner, E.; de Heimgartner, V. Experiencias en enfermedades dermatológicas endémicas en la selva peruana: Leishmaniasis y pénfigo foliáceo endémico. Med. Cut. ILA 1976, 1, 1–6. [Google Scholar]
- Ramos, W.; Rojas, N.; Ortega-Loayza, A.G.; Tello, M.; Jiménez, G.; Cuba-Cáceres, N.; Ronceros, G.; De La Cruz-Vargas, J.A.; Vera-Ponce, V.J.; Guerrero, N.; et al. Ultrastructural skin alterations of healthy subjects with anti-desmoglein 1 antibodies in endemic areas to pemphigus foliaceus: A case series. J. Transl. Autoimmun. 2023, 7, 100208. [Google Scholar] [CrossRef] [PubMed]
- Morini, J.P.; Jomaa, B.; Gorgi, Y.; Saguem, M.H.; Nouira, R.; Roujeau, J.C.; Revuz, J. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch. Dermatol. 1993, 129, 69–73. [Google Scholar] [CrossRef]
- Jerbi, A.; Hachicha, H.; Bahloul, E.; Feki, S.; Sellami, K.; Abida, O.; Bouzid, A.; Turki, H.; Masmoudi, A.; Masmoudi, H. South Tunisian pemphigus patients beyond 60 years: Epidemiological profile and evolution. Int. J. Dermatol. 2019, 58, e219–e220. [Google Scholar] [CrossRef]
- Denguezli, M.; Ben Nejma, B.; Nouira, R.; Korbi, S.; Bardi, R.; Ayed, K. La dermatose bulleuse à IgA linéaire de l’enfant. Une série de 12 malades tunisiens [Iga linear bullous dermatosis in children. A series of 12 Tunisian patients]. Ann. Dermatol. Venereol. 1994, 121, 888–892. [Google Scholar]
- Zaraa, I.; Kerkeni, N.; Ishak, F.; Zribi, H.; El Euch, D.; Mokni, M.; Osman, A.B. Spectrum of autoimmune blistering dermatoses in Tunisia: An 11-year study and a review of the literature. Int. J. Dermatol. 2011, 50, 939–944. [Google Scholar] [CrossRef]
- Abreu-Velez, A.M.; Howard, M.S.; Jiao, Z.; Gao, W.; Yi, H.; Grossniklaus, H.E.; Duque-Ramírez, M.; Dudley, S.C. Cardiac autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia, South America. J. Clin. Immunol. 2011, 31, 985–997. [Google Scholar] [CrossRef]
- Abreu Velez, A.M.; Yi, H.; Warfvinge, G.; Howard, M.S. Autoantibodies to full body vascular cell junctions colocalize with MYZAP, ARVCF, desmoplakins I and II and p0071 in endemic pemphigus in Colombia, South America. Int. J. Dermatol. 2018, 57, 291–298. [Google Scholar] [CrossRef]
- Abreu Velez, A.M.; Howard, M.S.; Velazquez-Velez, J.E. Cardiac rhythm and pacemaking abnormalities in patients affected by endemic pemphigus in Colombia may be the result of deposition of autoantibodies, complement, fibrinogen, and other molecules. Heart Rhythm 2018, 15, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Velez, A.M.; Howard, M.S.; Yi, H.; Florez-Vargas, A.A. Patients affected by a new variant of endemic pemphigus foliaceus have autoantibodies colocalizing with MYZAP, p0071, desmoplakins 1–2 and ARVCF, causing renal damage. Clin. Exp. Dermatol. 2018, 43, 692–702. [Google Scholar] [CrossRef] [PubMed]
- de Balogh Valeria, T.; Pesantes, J.; Rojas, E. Pénfigo foliaceo var. brasilera (fuego salvaje) con glomerulonefritis focal y segmentaria: Presentación de un caso/Penphigus foliaceus var. brazil. (fogo selvagem) with focal and segmentary glomerulonefritis: Report of a case. Arch. Venez. Pueric. Pediatr. 1987, 50, 67–72. [Google Scholar]
- Brown, M.V. Fogo selvagem (pemphigus foliaceus). Review of the Brazilian literature. Arch. Dermatol. Syphilol. 1954, 69, 589–599. [Google Scholar] [CrossRef]
- Abreu-Velez, A.M.; Roselino, A.M.; Howard, M.S.; Messias-Reason, L.J. Endemic pemphigus foliaceus over a century: Part 2. N. Am. J. Med. Sci. 2010, 2, 114–125. [Google Scholar]
- McCarty, K.S., Jr.; Miller, L.S.; Cox, E.B.; Konrath, J.; McCarty, K.S., Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar]
- Kostreva, D.R.; Pontus, S.P. Pericardial mechanoreceptors with phrenic afferents. Am. J. Physiol. 1993, 264 Pt 2, H1836–H1846. [Google Scholar] [CrossRef]
- Sleight, P.; Widdicombe, J.G. Action potentials in afferent fibres from pericardial mechanoreceptors in the dog. J. Physiol. 1965, 181, 259–269. [Google Scholar] [CrossRef]
- Maliarenko, I.E. Kolichestvennaia kharakteristika pressornykh refleksov, vyzyvaemykh vozdeĭstviem molochnoĭ kisloty na refleksogennuiu zonu épikarda i perikarda [Quantitative characteristics of pressor reflexes caused by the action of lactic acid on the reflexogenic zone of the epicardium and pericardium]. Bull. Exp. Biol. Med. 1971, 71, 3–6. [Google Scholar]
- Kan, G.S. Ob izbiratel’nom vliianii nekotorykh khimicheskikh razdrazhitelei na refleksy s khemotseptorov. VIII. O vliianii streptomitsina na refleksy s khemotseptorov i mekhanotseptorov perikarda [Selective effect of certain chemical stimuli on reflexes from the chemoreceptors. VIII. Effect of streptomycin on reflexes from pericardial chemoreceptors and mechanoreceptors]. Bull. Exp. Biol. Med. 1958, 45, 53–57. [Google Scholar]
- Hoit, B.D. Anatomy and Physiology of the Pericardium. Cardiol. Clin. 2017, 35, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, R.; Sonani, B.; Dhamoon, A.S. Fibrinous Pericarditis. 8 August 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ishihara, T.; Ferrans, V.J.; Jones, M.; Boyce, S.W.; Kawanami, O.; Roberts, W.C. Histologic and ultrastructural features of normal human parietal pericardium. Am. J. Cardiol. 1980, 46, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Von Recklinghausen, F.V. Zur fettresorption. Arch. Pathol. Anat. 1863, 24, 172–208. [Google Scholar] [CrossRef]
- Valle, M.T.; Degl’Innocenti, M.L.; Bertelli, R.; Facchetti, P.; Perfumo, F.; Fenoglio, D.; Kunkl, A.; Gusmano, R.; Manca, F. Antigen-presenting function of human peritoneum mesothelial cells. Clin. Exp. Immunol. 1995, 101, 172–176. [Google Scholar] [CrossRef]
- Abreu-Velez, A.M.; Yi, H.; Howard, M.S. Cell junction protein armadillo repeat gene deleted in velo-cardio-facial syndrome is expressed in the skin and colocalizes with autoantibodies of patients affected by a new variant of endemic pemphigus foliaceus in Colombia. Dermatol. Pract. Concept. 2017, 7, 3–8. [Google Scholar] [CrossRef]
- Kluge, T.; Hovig, T. The ultrastructure of human and rat pericardium. I. Parietal and visceral mesothelium. Acta Pathol. Microbiol. Scand. 1967, 71, 529–546. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Roberts, W.C.; Spray, T.L. Pericardial heart disease. Curr. Probl. Cardiol. 1977, 2, 1–71. [Google Scholar] [CrossRef]
- Kong, H.; Rong, J.; Bain, C.; Zhang, X.; Parsons, S.; Chen, G.; Bassed, R. Pericardial Effusion Detection on Post-Mortem Computed Tomography Images Using Convolutional Neural Networks. Stud. Health Technol. Inform. 2024, 25, 745–749. [Google Scholar] [CrossRef]
- Cazenave, P.L. Lecons Sur Les Maladies de la Peau Professées à l’Ecole de Médecine de Paris; Ancinne Maison Béchet Jeune; Labe, Editeru. Libraire de la Faculte de Medicine de Paris, Place de l’école de Médicine: Paris, France, 1856; 233p. [Google Scholar]
- Gilibert, S. Monographie du Pemphigus, ou Traité de la Maladie Vésiculaire; Nabu Press: Paris, France, 1813; p. 378. [Google Scholar]
- Barnard, R.D. Marrow aplasia and estrapenia in pemphigus vulgaris; case report with autopsy findings and notes on relationship of erythroid marrow function to atopic-exudative skin lesions. Urol. Cutan. Rev. 1947, 51, 586–591. [Google Scholar]
- Wegmann, R.; Thewes, A. Spectrographie dans l’infra-rouge. II. Etude de la sérosité de bulles de la maladie de Brocq-Dühring et du pemphigus vulgaire. Presse Med. 1956, 64, 1158–1161. [Google Scholar]
- Piermaier, L.M.; Caspers, S.; Herold, C.; Wolf-Vollenbröker, M.; Brzoska, P.; Bechler, E.; Filler, T.J. Proprioceptors of the human pericardium. Basic Res. Cardiol. 2024, 119, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Kim, J.; Okajima, T.; Cho, N.J. Mechanical properties of paraformaldehyde-treated individual cells investigated by atomic force microscopy and scanning ion conductance microscopy. Nano Converg. 2017, 4, 5. [Google Scholar] [CrossRef]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids. 2017, 202, 28–37. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [CrossRef]
- Boutin, C.; Guerin, J.C.; Viallat, J.R.; Astoul, P.; Bejui-Thivolet, F. Exploration des maladies de la plèvre. Matériel utilisé et technique d’étude [Diseases of the pleura. Materials used and study technics]. Rev. Mal. Respir. 1992, 9, 81–97. (In French) [Google Scholar] [PubMed]
- Abrèu-Velez, A.M.; Beutner, E.H.; Montoya, F.; Bollag, W.B.; Hashimoto, T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J. Am. Acad. Dermatol. 2003, 49, 609–614. [Google Scholar] [CrossRef]
- Vijayashree, R.; Aruthra, P.; Ramesh Rao, K.A. Comparison of manual and automated methods of quantitation of oestrogen/progesterone receptor expression in breast carcinoma. J. Clin. Diagn. Res. 2015, 9, EC01–EC05. [Google Scholar] [CrossRef]
| Catalogue Number | IHC All the Products from Agilent-Dako | E/P1 P | SNF2 P | E/P C | SNF Cs |
|---|---|---|---|---|---|
| AR0423 | Polyclonal rabbit anti-human IgG (Range 1:250 to 1:500) | (+++) | (+++) | (−) | (−) |
| IR513 | Polyclonal rabbit anti-human IgM (Range 1:150 to 1:300). Flex ready to use. | (+++) | (+++) | (−) | (−) |
| IR510 | Polyclonal rabbit anti-human IgA (Range 1:100 to 1:200). Flex ready to use. High Ph antigen retrieval. | (−) | (−) | (−) | (−) |
| IR517 | Polyclonal rabbit anti-human IgD Flex ready to use. | (++) | (++) | (−) | (−) |
| A0094 | Polyclonal rabbit anti-human IgE. (Range 1:750 to 1:1500). | (−) | (−) | (−) | (−) |
| A 0062 | Polyclonal Rabbit anti-human C3C complement (Range 1:100 to 1:200). | (+++) | (+++) | (−) | (−) |
| M077 | Monoclonal mouse anti-human C5b-9 complement (Range 1:25 to 1:50). (MAC). | (+++) | (+++) | (−) | (−) |
| A0063 | Polyclonal rabbit anti-human C3d- complement (Range 1:400 to 1:800). | (+++) | (+++) | (−) | (−) |
| A0136 | Polyclonal rabbit C1-q Complement (range not provided). | (++) | (++) | (−) | (−) |
| A0001 | Polyclonal rabbit anti-human Albumin. (Range 1:2000 to 1:4000). | (++++) | (++++) | (−) | (−) |
| A0080 | Polyclonal rabbit anti-human fibrinogen (Range 1:200 to 1:400). | (++++) | (++++) | (−) | (−) |
| IR507 | Polyclonal rabbit anti-human Lambda. Flex ready to use. Heat induced epitope retrieval | (++++) | (++++) | (−) | (−) |
| IR506 | Polyclonal rabbit anti-human Kappa light chains Flex ready to use. Heat induced epitope retrieval | (++++) | (++++) | (−) | (−) |
| GA60066-2 | Negative control. Rabbit, FLEX RTU. Control reagent, Ready-to-use, Unconjugated for Immunohistochemistry, | (−) | (−) | (−) | (−) |
| Symptoms/Signs, EKG/Chest RX | Patients | Controls |
|---|---|---|
| Sharp piercing chest pain over the center or left side of the chest, which is generally more intense when breathing. | 33/45 | 30/45 |
| Continuous dyspnea. | 7/45 | 0/45 |
| Pericardial rub on auscultation. | 2/45 | 0/45 |
| Hiccups. | 7/45 | 2/45 |
| Dysphagia. | 10/45 | 3/45 |
| Hoarseness and syncope without respiratory infections. | 7/45 | 2/45 |
| Current smokers. | 2/45 | 345 |
| Past smokers. | 8/45 | 9/45 |
| Palpitations. | 38/45 | 12/45 |
| Fatigue. | 45/45 | 16/45 |
| Weakness. | 39/45 | 1/45 |
| Shortness of breath when reclining. | 7/45 | 0/45 |
| Previous history of viral infections previous the above symptoms. | 45/45 | 45/45 |
| Abdominal or leg swelling. | 7/45 | 1/45 |
| Cardiomegaly using chest X Ray. | 19/45 | 3/45 |
| New widespread ST-elevation or PR depression on ECG | 12/45 | 1/45 |
| Pleural infusion by X-ray | 0/45 | 0/45 |
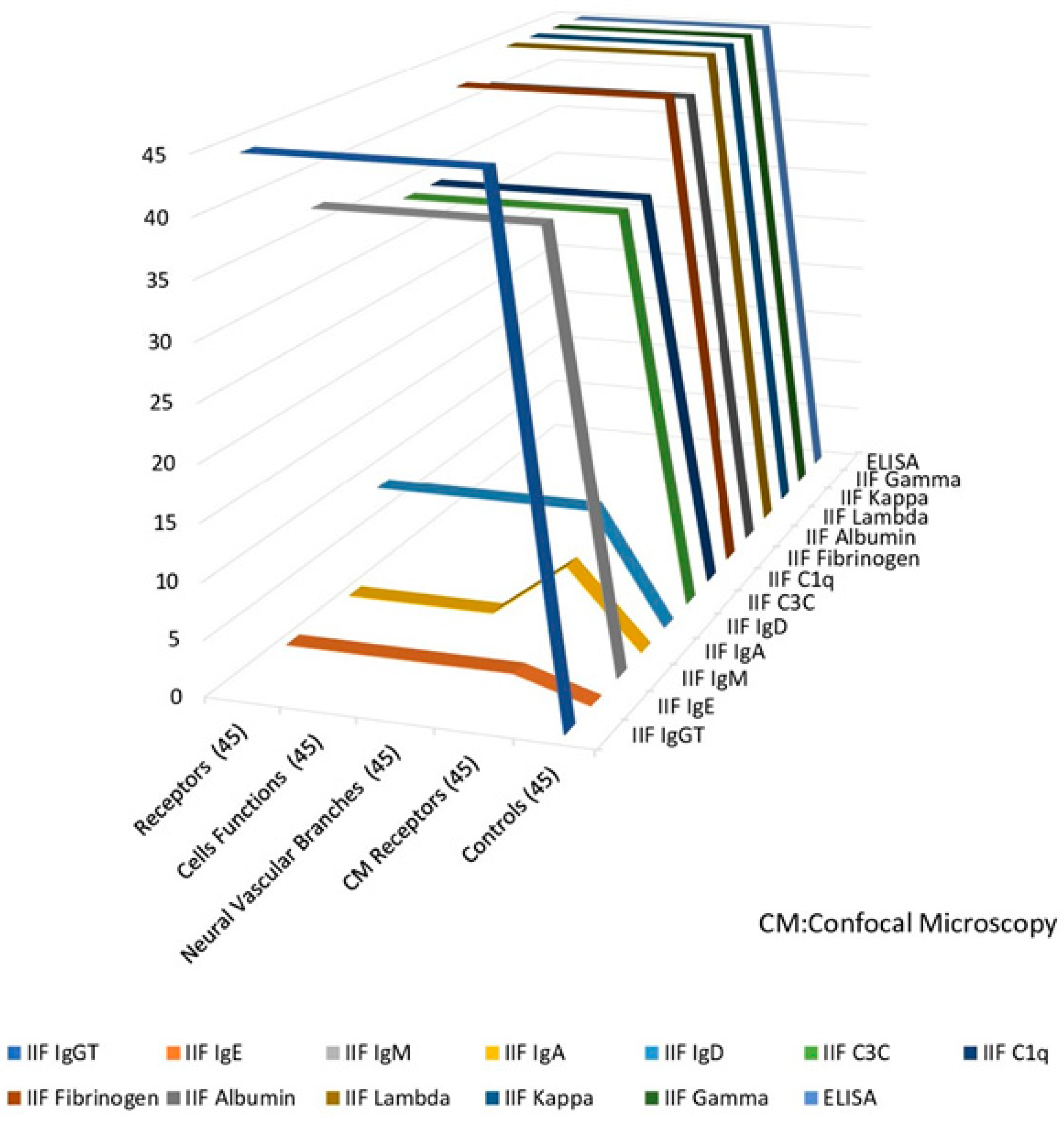
| TEST Antigen Source Cow | P-Ab Sensory Nerve Formations at E/P (45) | P-Ab Functions at E/P (45) | Patients-Ab to Neural Epidermal Branches at E/P (45) | CM P Sensory Nerve Formations at EP (45) | Controls (45) |
|---|---|---|---|---|---|
| IIF IgG total | 43 | 43 | 43 | 43 | 0 |
| IIF IgE | 1 | 1 | 1 | 1 | 0 |
| IIF IgM | 42 | 42 | 42 | 42 | 0 |
| IIF IgA | 0 | 0 | 0 | 0 | 0 |
| IIF IgD | 25 | 25 | 25 | 25 | 0 |
| IIF C3C | 38 | 38 | 38 | 38 | 0 |
| IIF C1q | 35 | 35 | 35 | 35 | 0 |
| IIF Fibrinogen | 45 | 45 | 45 | 45 | 0 |
| IIF Albumin | 42 | 42 | 42 | 42 | 0 |
| IIF Lambda | 45 | 45 | 45 | 45 | 0 |
| IIF Kappa | 45 | 45 | 45 | 45 | 0 |
| Confocal IIF IgG total | 43 | 43 | 43 | 43 | 0 |
| Confocal IIF IgE | 1 | 1 | 1 | 1 | 0 |
| Confocal IIF IgM | 42 | 42 | 42 | 42 | 0 |
| Confocal IIF IgA | 0 | 0 | 0 | 0 | 0 |
| Confocal IIF IgD | 25 | 25 | 25 | 25 | 0 |
| Confocal IIF C3C | 38 | 38 | 38 | 38 | 0 |
| Confocal IIF C1q | 35 | 35 | 35 | 35 | 0 |
| Confocal IIF Fibrinogen | 45 | 45 | 45 | 45 | 0 |
| Confocal IIF Albumin | 42 | 42 | 42 | 42 | 0 |
| Confocal IIF lambda | 45 | 45 | 45 | 45 | 0 |
| Confocal IIF Kappa | 45 | 45 | 45 | 45 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu Velez, A.M.; Hashimoto, T.; Upegui, Y.A.; Vélez Arango, J.M.; Olarte Aponte, A.M.; Vega, J.A.; Howard, M.S. The Pericardium Cells Junctions Are a Target for Autoantibodies of Patients Affected by a Variant of Endemic Pemphigus Foliaceus in El Bagre and Surrounding Municipalities in Colombia, South America. Diagnostics 2025, 15, 964. https://doi.org/10.3390/diagnostics15080964
Abreu Velez AM, Hashimoto T, Upegui YA, Vélez Arango JM, Olarte Aponte AM, Vega JA, Howard MS. The Pericardium Cells Junctions Are a Target for Autoantibodies of Patients Affected by a Variant of Endemic Pemphigus Foliaceus in El Bagre and Surrounding Municipalities in Colombia, South America. Diagnostics. 2025; 15(8):964. https://doi.org/10.3390/diagnostics15080964
Chicago/Turabian StyleAbreu Velez, Ana Maria, Takashi Hashimoto, Yulieth A. Upegui, Jorge Mario Vélez Arango, Adriana Milena Olarte Aponte, Jose A. Vega, and Michael S. Howard. 2025. "The Pericardium Cells Junctions Are a Target for Autoantibodies of Patients Affected by a Variant of Endemic Pemphigus Foliaceus in El Bagre and Surrounding Municipalities in Colombia, South America" Diagnostics 15, no. 8: 964. https://doi.org/10.3390/diagnostics15080964
APA StyleAbreu Velez, A. M., Hashimoto, T., Upegui, Y. A., Vélez Arango, J. M., Olarte Aponte, A. M., Vega, J. A., & Howard, M. S. (2025). The Pericardium Cells Junctions Are a Target for Autoantibodies of Patients Affected by a Variant of Endemic Pemphigus Foliaceus in El Bagre and Surrounding Municipalities in Colombia, South America. Diagnostics, 15(8), 964. https://doi.org/10.3390/diagnostics15080964








