Elevated Levels of Pro-Inflammatory Interleukin-6 in HIV Immunological Non-Responders Among the Indonesian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. CD4+ Analysis
2.3. Measurement of Viral Load
2.4. Determination of Cytokine Levels
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cytokine Measurement
3.3. Diagnostic Performance of IL-6 and IL-10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rb-Silva, R.; Goios, A.; Kelly, C.; Teixeira, P.; João, C.; Horta, A.; Correia-Neves, M. Definition of immunological nonresponse to antiretroviral therapy: A systematic review. J. Acquir. Immune Defic. Syndr. 2019, 82, 452–461. [Google Scholar] [CrossRef]
- Hernández-Walias, F.; Ruiz-de-León, M.J.; Rosado-Sánchez, I.; Vázquez, E.; Leal, M.; Moreno, S.; Vidal, F.; Blanco, J.; Pacheco, Y.M.; Vallejo, A. New signatures of poor CD4 cell recovery after suppressive antiretroviral therapy in HIV-1-infected individuals: Involvement of miR-192, IL-6, sCD14 and miR-144. Sci. Rep. 2020, 10, 2937. [Google Scholar] [CrossRef]
- Gómez-Mora, E.; Massanella, M.; García, E.; Giles, D.; Bernadó, M.; Urrea, V.; Carrillo, J.; Ouchi, D.; Puig, J.; Negredo, E.; et al. Elevated humoral response to cytomegalovirus in HIV-infected individuals with poor CD4+ T-cell immune recovery. PLoS ONE 2017, 12, e0184433. [Google Scholar]
- Nakanjako, D.; Kiragga, A.N.; Musick, B.S.; Yiannoutsos, C.T.; Wools-Kaloustian, K.; Diero, L.; Oyaro, P.; Lugina, E.; Ssali, J.C.; Kambugu, A.; et al. Frequency and impact of suboptimal immune recovery on first-line antiretroviral therapy within the International Epidemiologic Databases to Evaluate AIDS in East Africa. AIDS 2016, 30, 1913–1922. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Taramasso, L.; Labate, L.; Briano, F.; Brucci, G.; Mora, S.; Blanchi, S.; Giacomini, M.; Bassetti, M.; Di Biagio, A. CD4+ T lymphocyte recovery in the modern antiretroviral therapy era: Toward a new threshold for defining immunological non-responders. Front. Virol. 2023, 2, 822153. [Google Scholar]
- Pacheco, Y.M.; Jarrin, I.; Rosado, I.; Campins, A.A.; Berenguer, J.; Iribarren, J.A.; Rivero, M.; Muñoz-Medina, L.; Bernal-Morell, E.; Gutiérrez, F.; et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antivir. Res. 2015, 117, 69–74. [Google Scholar]
- Takuva, S.; Maskew, M.; Brennan, A.T.; Long, L.; Sanne, I.; Fox, M.P. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J. Int. AIDS Soc. 2014, 17, 18651. [Google Scholar] [CrossRef]
- Utay, N.S.; Hunt, P.W. Role of immune activation in progression to AIDS. Curr. Opin. HIV AIDS 2016, 11, 131–137. [Google Scholar] [CrossRef]
- Appay, V.; Kelleher, A.D. Immune activation and immune aging in HIV infection. Curr. Opin. HIV AIDS 2016, 11, 242–249. [Google Scholar]
- Bruzzesi, E.; Sereti, I. Residual immune activation and latency. Curr. Top. Microbiol. Immunol. 2018, 417, 157–180. [Google Scholar]
- Hileman, C.O.; Funderburg, N.T. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar]
- Burgos-Ramos, E.; Martos-Moreno, G.Á.; Argente, J.; Barrios, V. Multiplexed Bead Immunoassays: Advantages and Limitations in Pediatrics; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV persistence in gut-associated lymphoid tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retroviruses 2017, 33, 513–523. [Google Scholar]
- Maskew, M.; Brennan, A.T.; Westreich, D.; McNamara, L.; MacPhail, A.P.; Fox, M.P. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J. Womens Health 2013, 22, 113–120. [Google Scholar]
- Li, C.X.; Li, Y.Y.; He, L.P.; Kou, J.; Bai, J.S.; Liu, J.; Tian, B.; Cao, L.J.; Wang, K.H.; Kuang, Y.Q. The predictive role of CD4+ cell count and CD4/CD8 ratio in immune reconstitution outcome among HIV/AIDS patients receiving antiretroviral therapy: An eight-year observation in China. BMC Immunol. 2019, 20, 31. [Google Scholar] [CrossRef]
- Celerino da Silva, R.; Alves, N.M.P.; Gondim Silva, M.L.; Agrelli, A.; Coelho, A.V.C.; Guimarães, R.L.; Arraes, L.C.; Crovella, S.; Brandão, L.A.C. Brief report: Polymorphisms in TNF-α/TNFR1 pathway genes are associated with CD4+ T-cell recovery in HIV-1-infected individuals on antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2021, 88, 322–327. [Google Scholar]
- Noiman, A.; Esber, A.; Wang, X.; Bahemana, E.; Adamu, Y.; Iroezindu, M.; Kiweewa, F.; Maswai, J.; Owuoth, J.; Maganga, L.; et al. Clinical factors and outcomes associated with immune non-response among virally suppressed adults with HIV from Africa and the United States. Sci. Rep. 2022, 12, 1196. [Google Scholar]
- Keating, S.M.; Golub, E.T.; Nowicki, M.; Young, M.; Anastos, K.; Crystal, H.; Cohen, M.H.; Zhang, J.; Greenblatt, R.M.; Desai, S.; et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 2011, 25, 1823–1832. [Google Scholar]
- Lederman, M.M.; Calabrese, L.; Funderburg, N.T.; Clagett, B.; Medvik, K.; Bonilla, H.; Gripshover, B.; Salata, R.A.; Taege, A.; Lisgaris, M.; et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J. Infect. Dis. 2011, 204, 1217–1226. [Google Scholar]
- Bono, V.; Augello, M.; Tincati, C.; Marchetti, G. Failure of CD4+ T-cell recovery upon virally-effective cART: An enduring gap in the understanding of HIV+ immunological non-responders. N. Microbiol. 2022, 45, 155–172. [Google Scholar]
- Chen, L.; Bao, D.; Gu, L.; Gu, Y.; Zhou, L.; Gao, Z.; Huang, Y. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect. Dis. 2018, 18, 295. [Google Scholar]
- Rivera, M.M.; Soza, A.; Jazwinski, A.; Mi, L.; Kleiner, D.E.; Zhao, X.; Zuber, C.; Brust, D.; Hsu, E.; Simpson, J.; et al. HIV through the looking glass: Insights derived from Hepatitis B. J. Acquir. Immune Defic. Syndr. 2015, 68, 123–127. [Google Scholar]
- Hattab, S.; Guiguet, M.; Carcelain, G.; Fourati, S.; Guihot, A.; Autran, B.; Caby, F.; Marcelin, A.G.; Costagliola, D.; Katlama, C. Soluble biomarkers of immune activation and inflammation in HIV infection: Impact of 2 years of effective first-line combination antiretroviral therapy. HIV Med. 2015, 16, 553–562. [Google Scholar]
- Luo, Y.; Zheng, S.G. Hall of fame among pro-inflammatory cytokines: Interleukin-6 gene and its transcriptional regulation mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar]
- Shive, C.L.; Freeman, M.L.; Younes, S.A.; Kowal, C.M.; Canaday, D.H.; Rodriguez, B.; Lederman, M.M.; Anthony, D.D. Markers of T cell exhaustion and senescence and their relationship to plasma TGF-β levels in treated HIV+ immune non-responders. Front. Immunol. 2021, 12, 638010. [Google Scholar]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Dos Santos Guedes, M.C.; Carvalho-Silva, W.H.V.; Andrade-Santos, J.L.; Brelaz-de-Castro, M.C.A.; Souto, F.O.; Guimarães, R.L. Thymic exhaustion and increased immune activation are the main mechanisms involved in impaired immunological recovery of HIV-positive patients under ART. Viruses 2023, 15, 440. [Google Scholar] [CrossRef]
- Naidoo, S.J.; Naicker, T. The enigmatic interplay of interleukin-10 in the synergy of HIV infection comorbid with preeclampsia. Int. J. Mol. Sci. 2024, 25, 9434. [Google Scholar] [CrossRef]
- Indrati, A.R.; Sumarpo, A.; Atmadja, P.; Wisesa, R.R.; Ghozali, M.; Judistiani, R.T.D.; Setiabudiawan, B. Exploring alternative cytokines as potential biomarkers for latent tuberculosis infection in pregnant women. PLoS ONE 2022, 17, e0270552. [Google Scholar]
- Vos, W.A.J.W.; Navas, A.; Meeder, E.M.G.; Blaauw, M.J.T.; Groenendijk, A.L.; van Eekeren, L.E.; Otten, T.; Vadaq, N.; Matzaraki, V.; van Cranenbroek, B.; et al. HIV immunological non-responders are characterized by extensive immunosenescence and impaired lymphocyte cytokine production capacity. Front. Immunol. 2024, 15, 1350065. [Google Scholar] [CrossRef]
- Indrati, A.R.; Sumarpo, A.; Haryanto, J.; Rosmiati, N.M.D.; Munaya, S.; Turbawaty, D.K.; Wisaksana, R. Identification of cytokine signatures in HIV-infected individuals with and without Mycobacterium tuberculosis co-infection. Biomed. Rep. 2024, 21, 131. [Google Scholar]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar]
| Characteristic | HIV-INRs (n = 15) | HIV-IRs (n = 25) | p-Value |
|---|---|---|---|
| Age (years) | |||
| Median (min–max) | 35 (24–50) | 36 (24–51) | 0.695 |
| Gender | |||
| Male | 15 (100.0%) | 17 (68.0%) | 0.014 |
| Female | 0 (0.0%) | 8 (32.0%) | |
| Duration of ART (years) | |||
| Median (min–max) | 2 (1–10) | 5 (1–16) | 0.036 |
| CD4+ T cells (cell/µL) | |||
| Mean ± SD | 232.8 ± 62.5 | 570.2 ± 191.9 | <0.001 |
| Nadir CD4+ T cells (cell/µL) | |||
| Median (min–max) | 85 (19–224) | 232 (54–663) | <0.001 |
| Body Mass Index (kg/m2) | |||
| Mean ± SD | 21.4 ± 2.4 | 21.4 ± 1.8 | 0.968 |
| Coinfection | 12 (80%) | 13 (52%) | |
| Active Tuberculosis | 2 (18.2%) | 1 (5.9%) | 0.087 |
| Hepatitis B | 2 (18.2%) | 0 (0.0%) | |
| Hepatitis C | 0 (0.0%) | 4 (23.5%) | |
| Syphilis | 4 (36.4%) | 0 (0.0%) | |
| No coinfection | 3 (27.2%) | 12 (70.6%) |
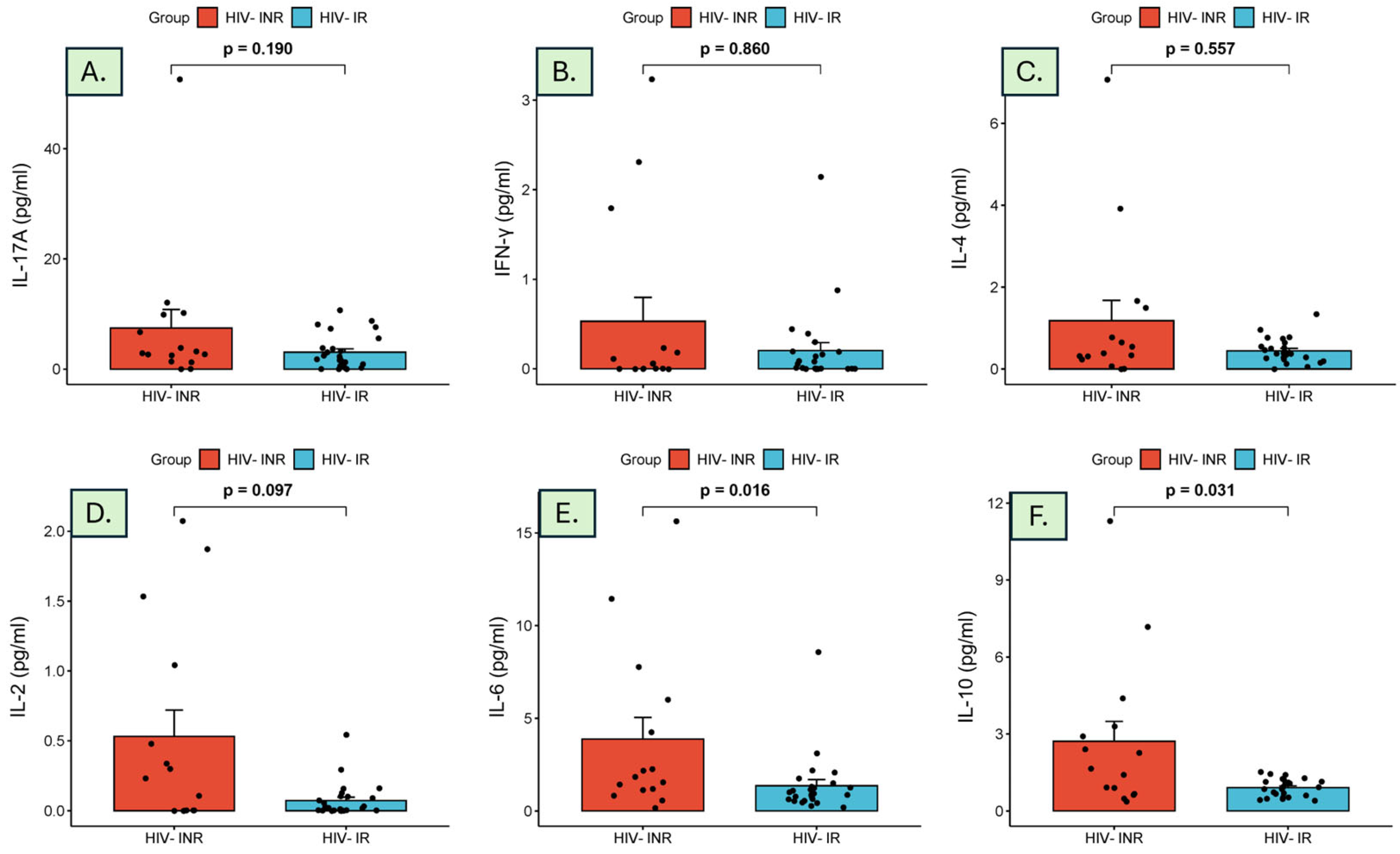
| Cytokine (pg/mL) | HIV-INRs (n = 15) | HIV-IRs (n = 25) | p-Value |
|---|---|---|---|
| IL-17A | 2.87 (0.00–52.52) | 1.74 (0.00–10.64) | 0.190 |
| IFN-γ | 0.06 (0.00–3.23) | 0.01 (0.00–2.14) | 0.860 |
| IL-4 | 0.39 (0.00–7.07) | 0.39 (0.00–1.34) | 0.557 |
| IL-2 | 0.23 (0.00–2.07) | 0.02 (0.00–0.54) | 0.097 |
| IL-6 | 1.84 (1.13–6.01) | 0.94 (0.58–1.40) | 0.016 |
| IL-10 | 1.65 (0.36–11.30) | 0.92 (0.40–1.51) | 0.031 |
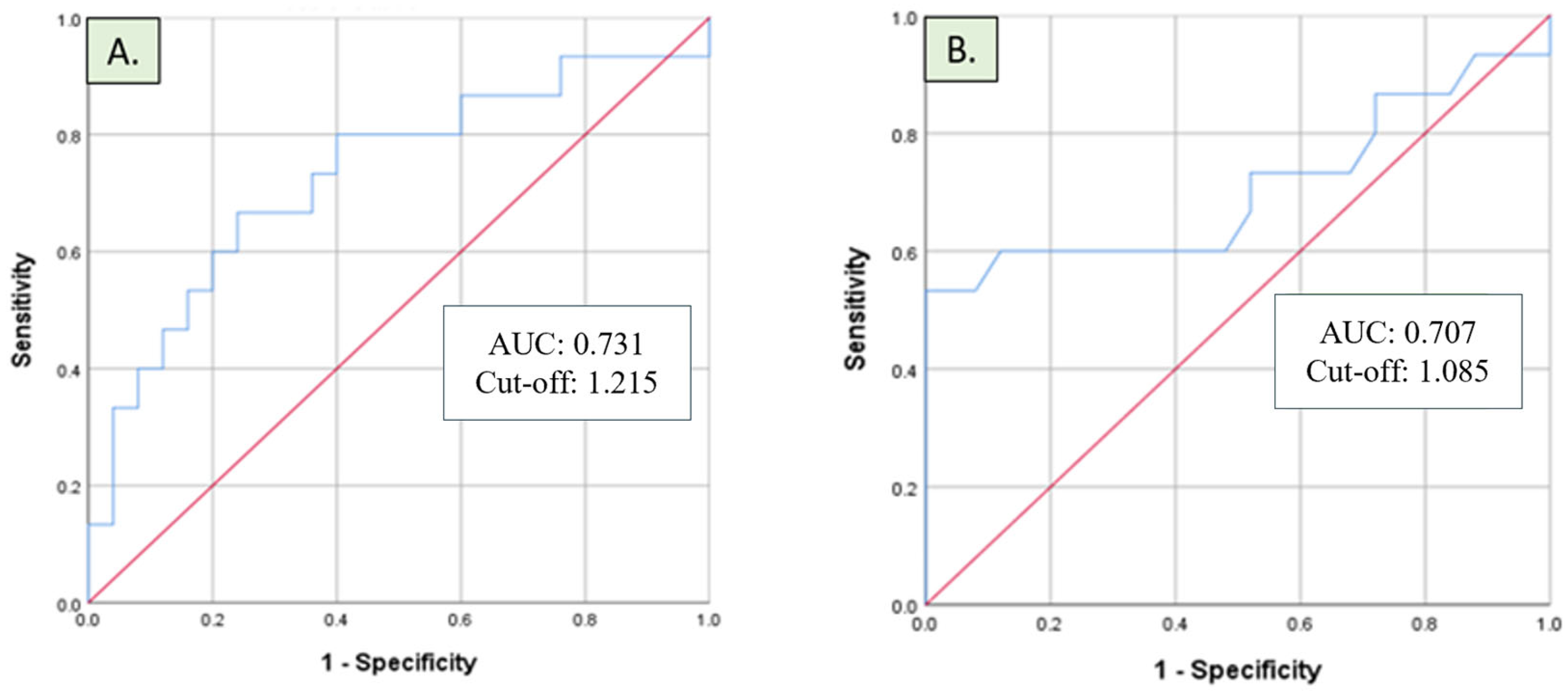
| Cytokine (pg/mL) | AUC | Cut-Off (pg/mL) | Sensitivity | Specificity |
|---|---|---|---|---|
| IL-6 | 0.731 | 1.215 | 0.667 | 0.360 |
| IL-10 | 0.707 | 1.085 | 0.600 | 0.360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indrati, A.R.; Kosasih, F.N.; Fadhilah, F.; Pratiwi, A.; Muthiah, U.; Logito, V.; Sumarpo, A.; Haryanto, J.; Munaya, S.; Rosmiati, N.M.D.; et al. Elevated Levels of Pro-Inflammatory Interleukin-6 in HIV Immunological Non-Responders Among the Indonesian Population. Diagnostics 2025, 15, 959. https://doi.org/10.3390/diagnostics15080959
Indrati AR, Kosasih FN, Fadhilah F, Pratiwi A, Muthiah U, Logito V, Sumarpo A, Haryanto J, Munaya S, Rosmiati NMD, et al. Elevated Levels of Pro-Inflammatory Interleukin-6 in HIV Immunological Non-Responders Among the Indonesian Population. Diagnostics. 2025; 15(8):959. https://doi.org/10.3390/diagnostics15080959
Chicago/Turabian StyleIndrati, Agnes Rengga, Felicia Nathania Kosasih, Fitri Fadhilah, Amelia Pratiwi, Ummi Muthiah, Verina Logito, Anton Sumarpo, Jane Haryanto, Shofa Munaya, Ni Made Dwi Rosmiati, and et al. 2025. "Elevated Levels of Pro-Inflammatory Interleukin-6 in HIV Immunological Non-Responders Among the Indonesian Population" Diagnostics 15, no. 8: 959. https://doi.org/10.3390/diagnostics15080959
APA StyleIndrati, A. R., Kosasih, F. N., Fadhilah, F., Pratiwi, A., Muthiah, U., Logito, V., Sumarpo, A., Haryanto, J., Munaya, S., Rosmiati, N. M. D., Turbawaty, D. K., & Wisaksana, R. (2025). Elevated Levels of Pro-Inflammatory Interleukin-6 in HIV Immunological Non-Responders Among the Indonesian Population. Diagnostics, 15(8), 959. https://doi.org/10.3390/diagnostics15080959






