Assessment of Spectral Computed Tomography Image Quality and Detection of Lesions in the Liver Based on Image Reconstruction Algorithms and Virtual Tube Voltage
Abstract
1. Introduction
2. Materials and Methods
Image Quality Assessment
3. Results
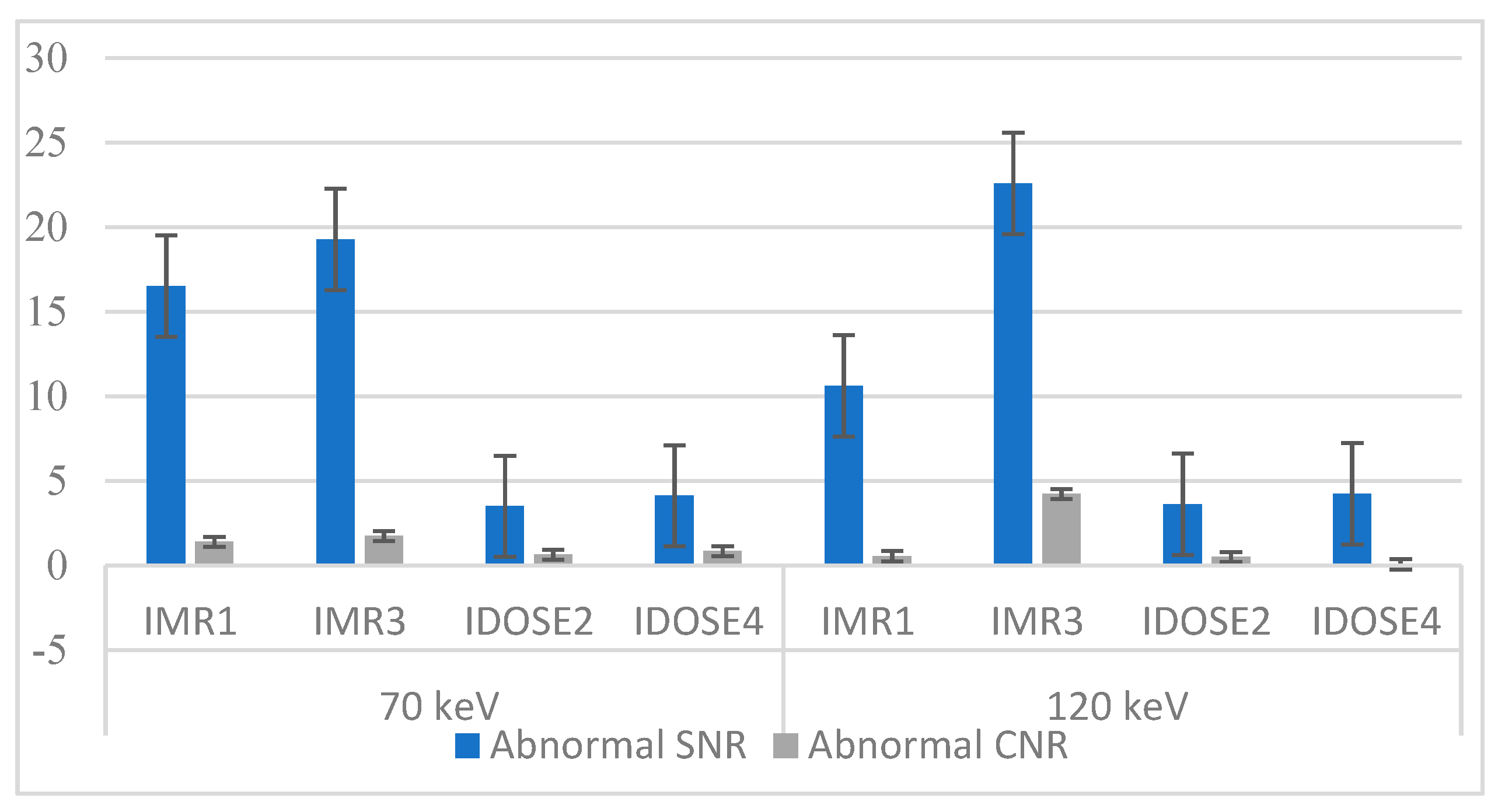
3.1. Imaging Assessment of Metastatic and Hemangioma Cases
3.2. Evaluation for Patients with Fatty Liver Disease
3.3. Image Quality Evaluation Based on Radiologist
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDCT | Spectral detector computed tomography |
| SNR | Signal-to-noise ratio |
| CNR | Contrast-to-noise ratio |
| IMR | Iterative model reconstruction |
| FBP | Filtered back projection |
| MDCT | Multidetector computed tomography |
References
- Cogley, J.R.; Miller, F.H. MR imaging of benign focal liver lesions. Radiol. Clin. N. Am. 2014, 52, 657–682. [Google Scholar] [PubMed]
- Nagayama, Y.; Inoue, T.; Oda, S.; Tanoue, S.; Nakaura, T.; Morinaga, J.; Ikeda, O.; Hirai, T. Unenhanced Dual-Layer Spectral-Detector CT for Characterizing Indeterminate Adrenal Lesions. Radiology 2021, 301, 369–378. [Google Scholar] [PubMed]
- Rajiah, P.; Abbara, S.; Halliburton, S.S. Spectral detector CT for cardiovascular applications. Diagn. Interv. Radiol. 2017, 23, 187. [Google Scholar]
- Hokamp, N.G.; Obmann, V.C.; Kessner, R.; Laukamp, K.; Persigehl, T.; Haneder, S.; Ramaiya, N. Improved visualization of hypodense liver lesions in virtual monoenergetic images from spectral detector CT: Proof of concept in a 3D-printed phantom and evaluation in 74 patients. Eur. J. Radiol. 2018, 109, 114–123. [Google Scholar]
- He, J.; Ma, X.; Wang, Q.; Fan, J.; Sun, Z. Spectral CT demonstration of the superior mesenteric artery: Comparison of monochromatic and polychromatic imaging. Acad. Radiol. 2014, 21, 364–368. [Google Scholar]
- Yin, X.-P.; Zuo, Z.-W.; Xu, Y.-J.; Wang, J.-N.; Liu, H.-J.; Liang, G.-L.; Gao, B.L. The optimal monochromatic spectral computed tomographic imaging plus adaptive statistical iterative reconstruction algorithm can improve the superior mesenteric vessel image quality. Eur. J. Radiol. 2017, 89, 47–53. [Google Scholar]
- Yin, X.-P.; Gao, B.-L.; Li, C.-Y.; Zhou, H.; Zhao, L.; Zheng, Y.-T.; Zhao, Y.X. Optimal monochromatic imaging of spectral computed tomography potentially improves the quality of hepatic vascular imaging. Korean J. Radiol. 2018, 19, 578. [Google Scholar]
- Zhao, L.-Q.; He, W.; Li, J.-Y.; Chen, J.-H.; Wang, K.-Y.; Tan, L. Improving image quality in portal venography with spectral CT imaging. Eur. J. Radiol. 2012, 81, 1677–1681. [Google Scholar]
- Doerner, J.; Wybranski, C.; Byrtus, J.; Houbois, C.; Hauger, M.; Heneweer, C.; Siedek, F.; Hickethier, T.; Hokamp, N.G.; Maintz, D.; et al. Intra-Individual comparison between abdominal virtual mono-energetic spectral and conventional images using a novel spectral detector CT. PLoS ONE 2017, 12, e0183759. [Google Scholar]
- Soesbe, T.C.; Lewis, M.A.; Xi, Y.; Browning, T.; Ananthakrishnan, L.; Fielding, J.R.; Lenkinski, R.E.; Leyendecker, J.R. A technique to identify isoattenuating gallstones with dual-layer spectral CT: An ex vivo phantom study. Radiology 2019, 292, 400–406. [Google Scholar]
- Yu, Y.; Guo, L.; Hu, C.; Chen, K. Spectral CT imaging in the differential diagnosis of necrotic hepatocellular carcinoma and hepatic abscess. Clin. Radiol. 2014, 69, e517–e524. [Google Scholar]
- Bak, S.; Kim, J.E.; Bae, K.; Cho, J.M.; Choi, H.C.; Park, M.J.; Choi, H.Y.; Shin, H.S.; Lee, S.M.; Kim, H.O. Quantification of liver extracellular volume using dual-energy CT: Utility for prediction of liver-related events in cirrhosis. Eur. Radiol. 2020, 30, 5317–5326. [Google Scholar] [PubMed]
- Hong, S.; Kim, J.E.; Cho, J.M.; Choi, H.C.; Won, J.H.; Na, J.B.; Choi, D.S.; Park, M.J.; Choi, H.Y.; Shin, H.S.; et al. Quantification of liver extracellular volume using dual-energy CT for ruling out high-risk varices in cirrhosis. Eur. J. Radiol. 2022, 148, 110151. [Google Scholar] [PubMed]
- Sofue, K.; Tsurusaki, M.; Mileto, A.; Hyodo, T.; Sasaki, K.; Nishii, T.; Chikugo, T.; Yada, N.; Kudo, M.; Sugimura, K.; et al. Dual-Energy computed tomography for non-invasive staging of liver fibrosis: Accuracy of iodine density measurements from contrast-enhanced data. Hepatol. Res. 2018, 48, 1008–1019. [Google Scholar]
- Lamb, P.; Sahani, D.V.; Fuentes-Orrego, J.M.; Patino, M.; Ghosh, A.; Mendonça, P.R. Stratification of patients with liver fibrosis using dual-energy CT. IEEE Trans. Med. Imaging 2014, 34, 807–815. [Google Scholar] [PubMed]
- Wang, N.; Ju, Y.; Wu, J.; Liu, A.; Chen, A.; Liu, J.; Liu, Y.; Li, J. Differentiation of liver abscess from liver metastasis using dual-energy spectral CT quantitative parameters. Eur. J. Radiol. 2019, 113, 204–208. [Google Scholar]
- Borges, A.P.; Antunes, C.; Caseiro-Alves, F. Spectral CT: Current Liver Applications. Diagnostics 2023, 13, 1673. [Google Scholar] [CrossRef]
- Große Hokamp, N.; Abdullayev, N.; Persigehl, T.; Schlaak, M.; Wybranski, C.; Holz, J.A.; Streichert, T.; Alkadhi, H.; Maintz, D.; Haneder, S. Precision and reliability of liver iodine quantification from spectral detector CT: Evidence from phantom and patient data. Eur. Radiol. 2019, 29, 2098–2106. [Google Scholar]
- Li, S.; Yuan, L.; Yue, M.; Xu, Y.; Liu, S.; Wang, F.; Liu, X.; Wang, F.; Deng, J.; Sun, Q.; et al. Early evaluation of liver metastasis using spectral CT to predict outcome in patients with colorectal cancer treated with FOLFOXIRI and bevacizumab. Cancer Imaging 2023, 23, 30. [Google Scholar]
- Singh, S.; Kalra, M.K.; Hsieh, J.; Licato, P.E.; Do, S.; Pien, H.H.; Blake, M.A. Abdominal CT: Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology 2010, 257, 373–383. [Google Scholar]
- Hosch, W.; Stiller, W.; Mueller, D.; Gitsioudis, G.; Welzel, J.; Dadrich, M.; Buss, S.J.; Giannitsis, E.; Kauczor, H.U.; Katus, H.A.; et al. Reduction of radiation exposure and improvement of image quality with BMI-adapted prospective cardiac computed tomography and iterative reconstruction. Eur. J. Radiol. 2012, 81, 3568–3576. [Google Scholar]
- Noël, P.B.; Fingerle, A.A.; Renger, B.; Münzel, D.; Rummeny, E.J.; Dobritz, M. Initial performance characterization of a clinical noise–suppressing reconstruction algorithm for MDCT. Am. J. Roentgenol. 2011, 197, 1404–1409. [Google Scholar]
- Laqmani, A.; Avanesov, M.; Butscheidt, S.; Kurfürst, M.; Sehner, S.; Schmidt-Holtz, J.; Derlin, T.; Behzadi, C.; Nagel, H.D.; Adam, G.; et al. Comparison of image quality and visibility of normal and abnormal findings at submillisievert chest CT using filtered back projection, iterative model reconstruction (IMR) and iDose4™. Eur. J. Radiol. 2016, 85, 1971–1979. [Google Scholar] [PubMed]
- Kulkarni, C.B.; Pullara, S.K.; Prabhu, N.K.; Patel, S.; Suresh, A.; Moorthy, S. Comparison of Knowledge-based Iterative Model Reconstruction (IMR) with Hybrid Iterative Reconstruction (iDose(4)) Techniques for Evaluation of Hepatocellular Carcinomas Using Computed Tomography. Acad. Radiol. 2021, 28 (Suppl. S1), S29–S36. [Google Scholar] [PubMed]
- El Kayal, N.; Lennartz, S.; Ekdawi, S.; Holz, J.; Slebocki, K.; Haneder, S.; Wybranski, C.; Mohallel, A.; Eid, M.; Grüll, H.; et al. Value of spectral detector computed tomography for assessment of pancreatic lesions. Eur. J. Radiol. 2019, 118, 215–222. [Google Scholar]
- Shapira, N.; Fokuhl, J.; Schultheiß, M.; Beck, S.; Kopp, F.K.; Pfeiffer, D.; Dangelmaier, J.; Pahn, G.; Sauter, A.P.; Renger, B.; et al. Liver lesion localisation and classification with convolutional neural networks: A comparison between conventional and spectral computed tomography. Biomed. Phys. Eng. Express 2020, 6, 015038. [Google Scholar]
- Yang, C.; Wang, W.; Cui, D.; Zhang, J.; Liu, L.; Wang, Y.; Li, W. Deep learning image reconstruction algorithms in low-dose radiation abdominal computed tomography: Assessment of image quality and lesion diagnostic confidence. Quant. Imaging Med. Surg. 2023, 13, 3161. [Google Scholar]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar]
- Iyama, Y.; Nakaura, T.; Iyama, A.; Kidoh, M.; Katahira, K.; Oda, S.; Utsunomiya, D.; Yamashita, Y. Feasibility of Iterative Model Reconstruction for Unenhanced Lumbar CT. Radiology 2017, 284, 153–160. [Google Scholar]
- Abdalqader, O.N.; Hjouj, M.; Aljamal, M.; Hjouj, F.; Abuzaid, M.; Mousa, M. Reduction of metal artefacts from bilateral hip prostheses during lower extremity computed tomography angiography: An experimental phantom study. J. Med. Radiat. Sci. 2024, 71, 421–431. [Google Scholar]
- Ozaki, K.; Higuchi, S.; Kimura, H.; Gabata, T. Liver metastases: Correlation between imaging features and pathomolecular environments. Radiographics 2022, 42, 1994–2013. [Google Scholar] [PubMed]
- Yu, H.; Chen, X.; Zhou, Y.; Chen, D.; Zhang, L. Impact of photoelectric effect on X-ray density logging and its correction. Appl. Radiat. Isot. 2020, 156, 108785. [Google Scholar] [PubMed]
- Anzidei, M.; Di Martino, M.; Sacconi, B.; Saba, L.; Boni, F.; Zaccagna, F.; Geiger, D.; Kirchin, M.A.; Napoli, A.; Bezzi, M.; et al. Evaluation of image quality, radiation dose and diagnostic performance of dual-energy CT datasets in patients with hepatocellular carcinoma. Clin. Radiol. 2015, 70, 966–973. [Google Scholar] [PubMed]
- Gallo, P.; D’Alessio, A.; Pascuzzo, R.; Gallo, S.; Fumagalli, M.L.; Ortenzia, O.; Tenconi, C.; Cavatorta, C.; Pignoli, E.; Ghetti, C.; et al. Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study. Appl. Sci. 2024, 15, 1724. [Google Scholar]
- Harun, H.H.; Karim, M.K.A.; Abbas, Z.; Sabarudin, A.; Muniandy, S.C.; Razak, H.R.A.; Ng, K.H. The influence of iterative reconstruction level on image quality and radiation dose in CT pulmonary angiography examinations. Radiat. Phys. Chem. 2021, 178, 108989. [Google Scholar]
- Laqmani, A.; Kurfürst, M.; Butscheidt, S.; Sehner, S.; Schmidt-Holtz, J.; Behzadi, C.; Nagel, H.D.; Adam, G.; Regier, M. CT pulmonary angiography at reduced radiation exposure and contrast material volume using iterative model reconstruction and iDose4 technique in comparison to FBP. PLoS ONE 2016, 11, e0162429. [Google Scholar]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.-S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar]
- Wellenberg, R.H.H.; van Osch, J.A.C.; Boelhouwers, H.J.; Edens, M.A.; Streekstra, G.J.; Ettema, H.B.; Boomsma, M. CT radiation dose reduction in patients with total hip arthroplasties using model-based iterative reconstruction and orthopaedic metal artefact reduction. Skelet. Radiol. 2019, 48, 1775–1785. [Google Scholar]
- Do, T.D.; Rheinheimer, S.; Kauczor, H.-U.; Stiller, W.; Weber, T.; Skornitzke, S. Image quality evaluation of dual-layer spectral CT in comparison to single-layer CT in a reduced-dose setting. Eur. Radiol. 2020, 30, 5709–5719. [Google Scholar]
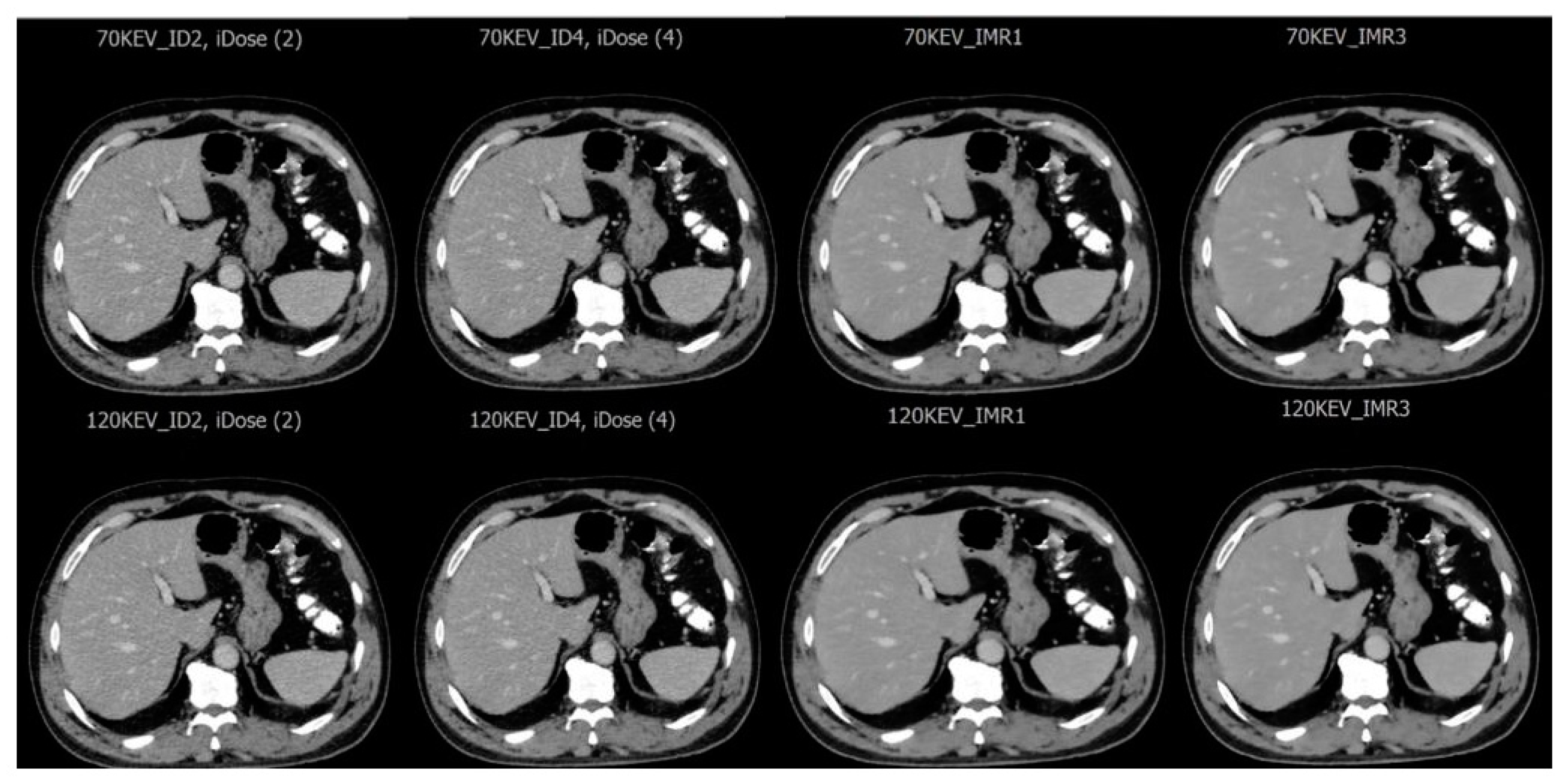
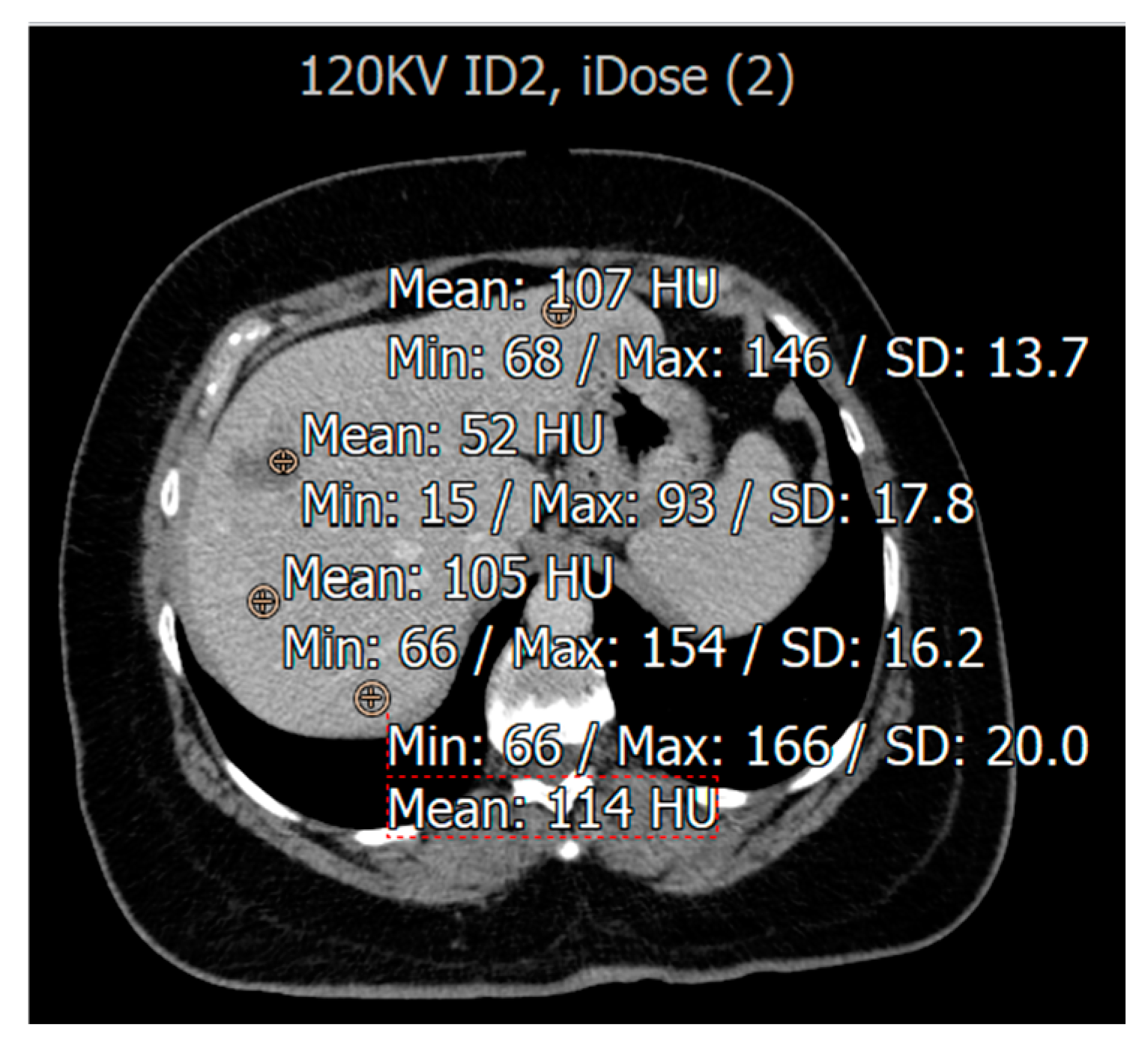
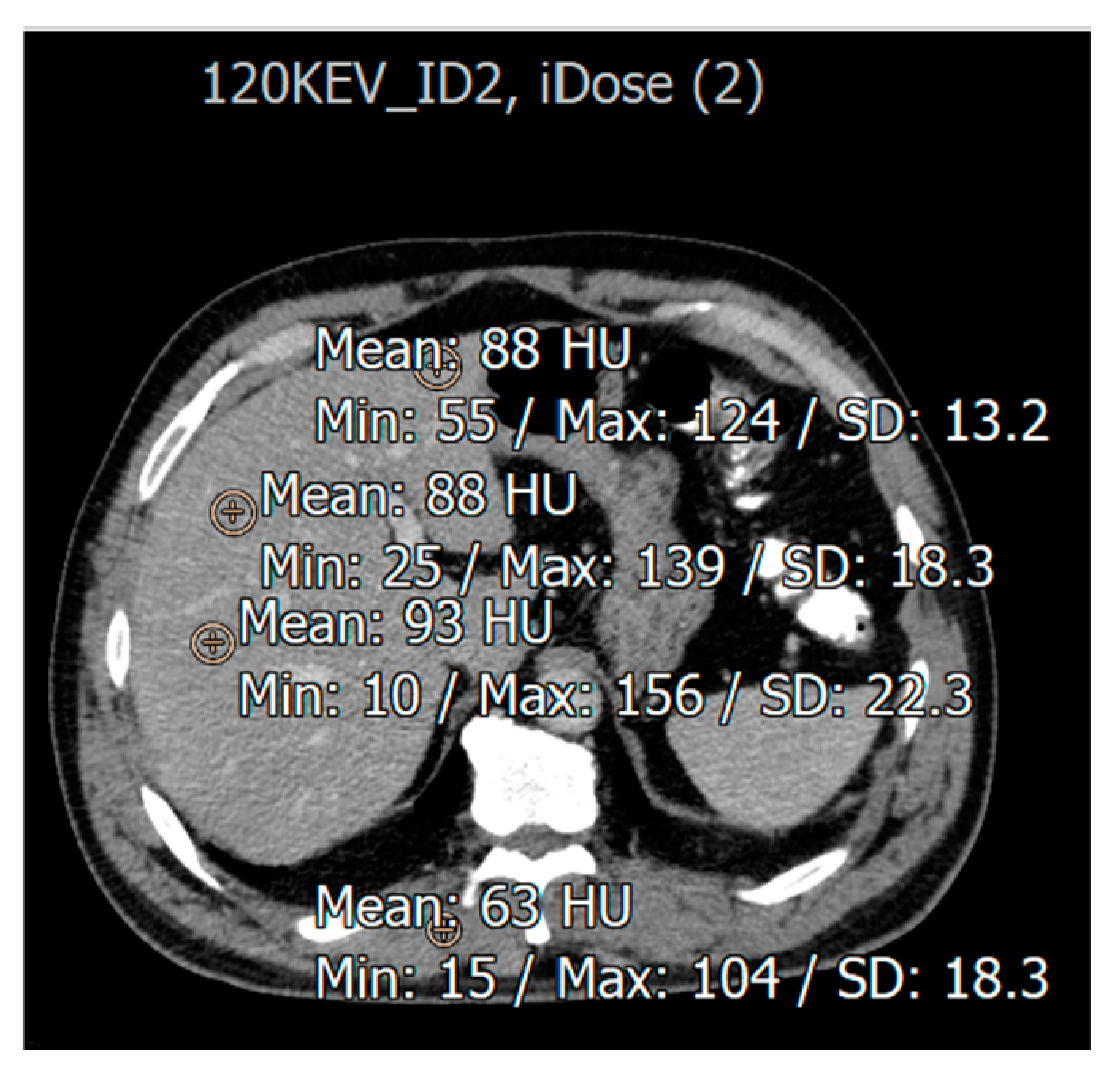
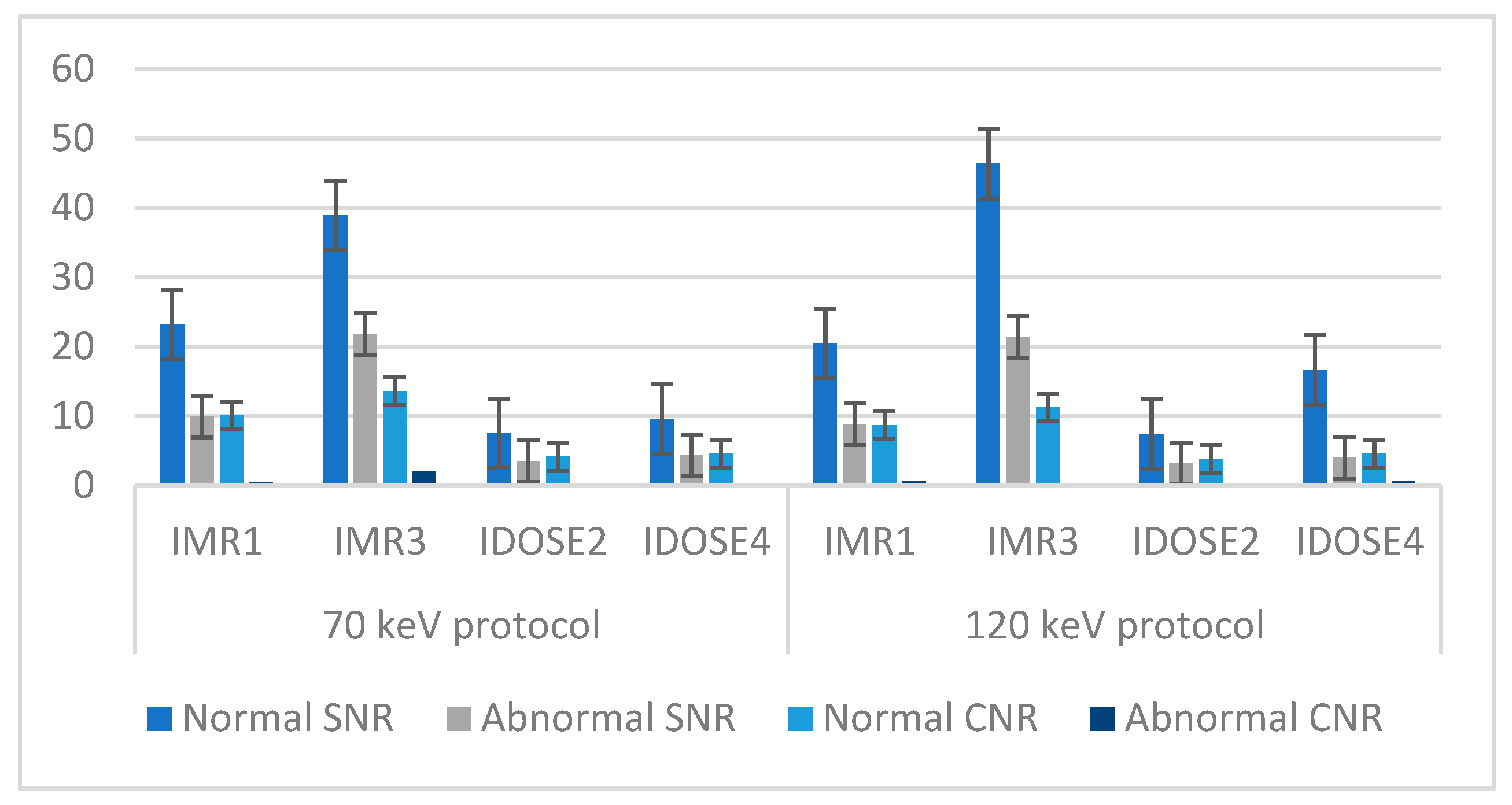

| Variable | % (n) |
|---|---|
| Age Group | |
| 18–29 | 6.3 (3) |
| 30–39 | 19.1 (9) |
| 40–59 | 38.3 (18) |
| 60–75 | 36.1 (17) |
| Sex | |
| Male | 59.6 (28) |
| Female | 40.4 (19) |
| Type of liver pathology | |
| Liver metastasis | 34 (16) |
| Hemangioma | 34 (16) |
| Fatty liver | 32 (15) |
| Reconstruction Techniques | iDose | IMR |
|---|---|---|
| Reconstructed image output | iDose2 70 keV | IMR1 70 keV |
| iDose2 120 keV | IMR1 120 keV | |
| iDose4 70 keV | IMR3 70 keV | |
| iDose4 120 keV | IMR3 120 keV |
| Reconstruction Techniques | Pearson Correlation | p-Value |
|---|---|---|
| IMR1 120 keV vs. IMR1 70 keV | 0.462 (**) | 0.001 |
| IMR3 120 keV vs. IMR3 70 keV | 0.789 (**) | 0.000 |
| iDose2 120 keV vs. iDose2 70 kev | 0.291 (*) | 0.045 |
| iDose4 120 keV vs. iDose4 70 keV | 0.390 (**) | 0.006 |
| Parameters | Mean | Std. Deviation |
|---|---|---|
| IMR1_70 kev | 3.58 | 0.986 |
| IMR3_70 kev | 2.83 | 1.534 |
| iDose2_70 kev | 2.94 | 1.295 |
| iDose4_70 kev | 2.88 | 0.815 |
| IMR1_120 kev | 3.46 | 0.988 |
| IMR3_120 kev | 2.65 | 1.391 |
| iDose2_120 kev | 2.75 | 1.391 |
| iDose4_120 kev | 2.85 | 0.945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamami, A.; Aljamal, M.; Almuqbil, N.; Al-Harbi, M.; Hamd, Z.Y. Assessment of Spectral Computed Tomography Image Quality and Detection of Lesions in the Liver Based on Image Reconstruction Algorithms and Virtual Tube Voltage. Diagnostics 2025, 15, 1043. https://doi.org/10.3390/diagnostics15081043
Hamami A, Aljamal M, Almuqbil N, Al-Harbi M, Hamd ZY. Assessment of Spectral Computed Tomography Image Quality and Detection of Lesions in the Liver Based on Image Reconstruction Algorithms and Virtual Tube Voltage. Diagnostics. 2025; 15(8):1043. https://doi.org/10.3390/diagnostics15081043
Chicago/Turabian StyleHamami, Areej, Mohammad Aljamal, Nora Almuqbil, Mohammad Al-Harbi, and Zuhal Y. Hamd. 2025. "Assessment of Spectral Computed Tomography Image Quality and Detection of Lesions in the Liver Based on Image Reconstruction Algorithms and Virtual Tube Voltage" Diagnostics 15, no. 8: 1043. https://doi.org/10.3390/diagnostics15081043
APA StyleHamami, A., Aljamal, M., Almuqbil, N., Al-Harbi, M., & Hamd, Z. Y. (2025). Assessment of Spectral Computed Tomography Image Quality and Detection of Lesions in the Liver Based on Image Reconstruction Algorithms and Virtual Tube Voltage. Diagnostics, 15(8), 1043. https://doi.org/10.3390/diagnostics15081043








