Mesopancreas—Anatomical Insights and Its Implications for Diagnosis and Clinical and Surgical Practice
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Pancreas Development
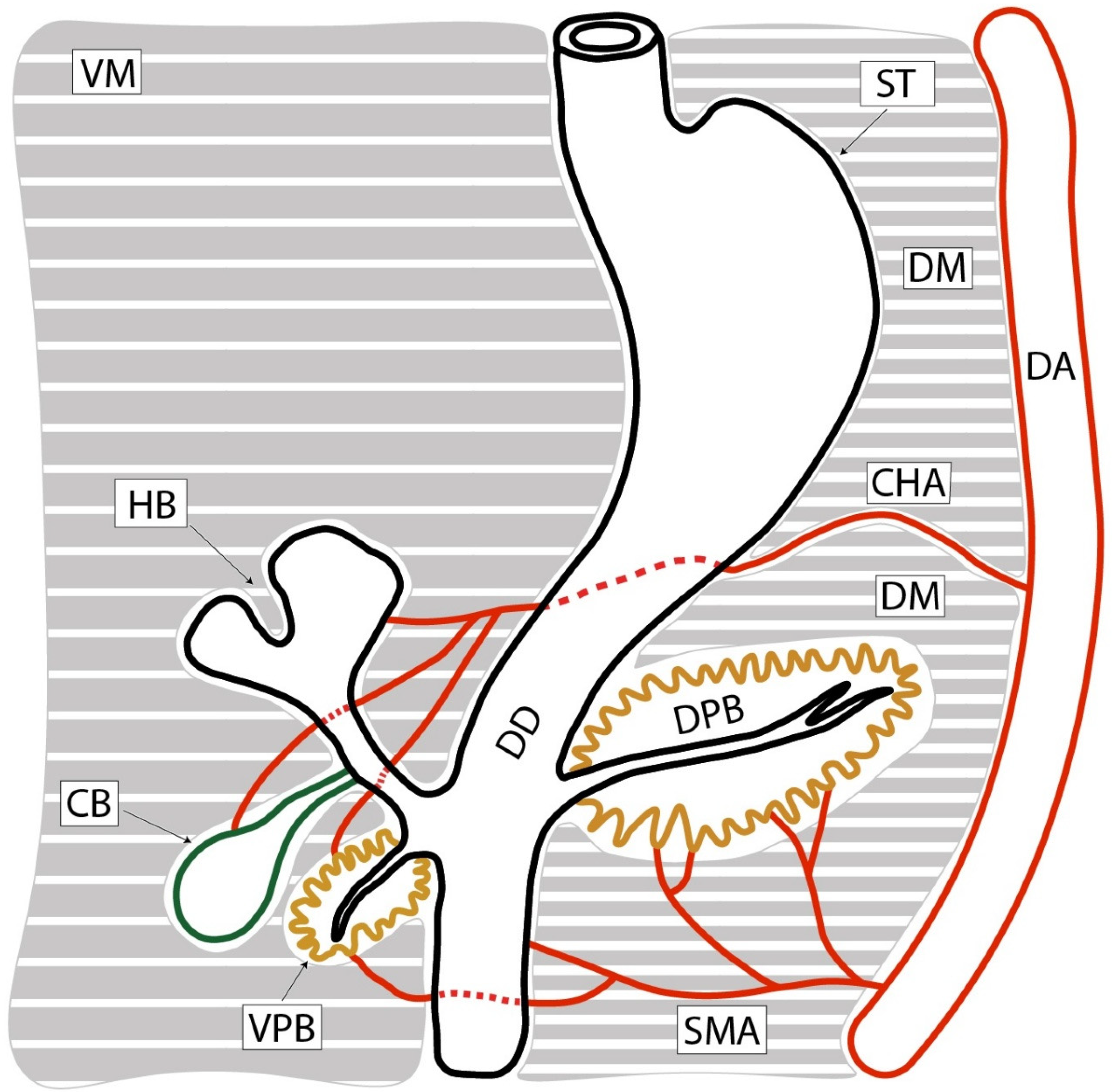
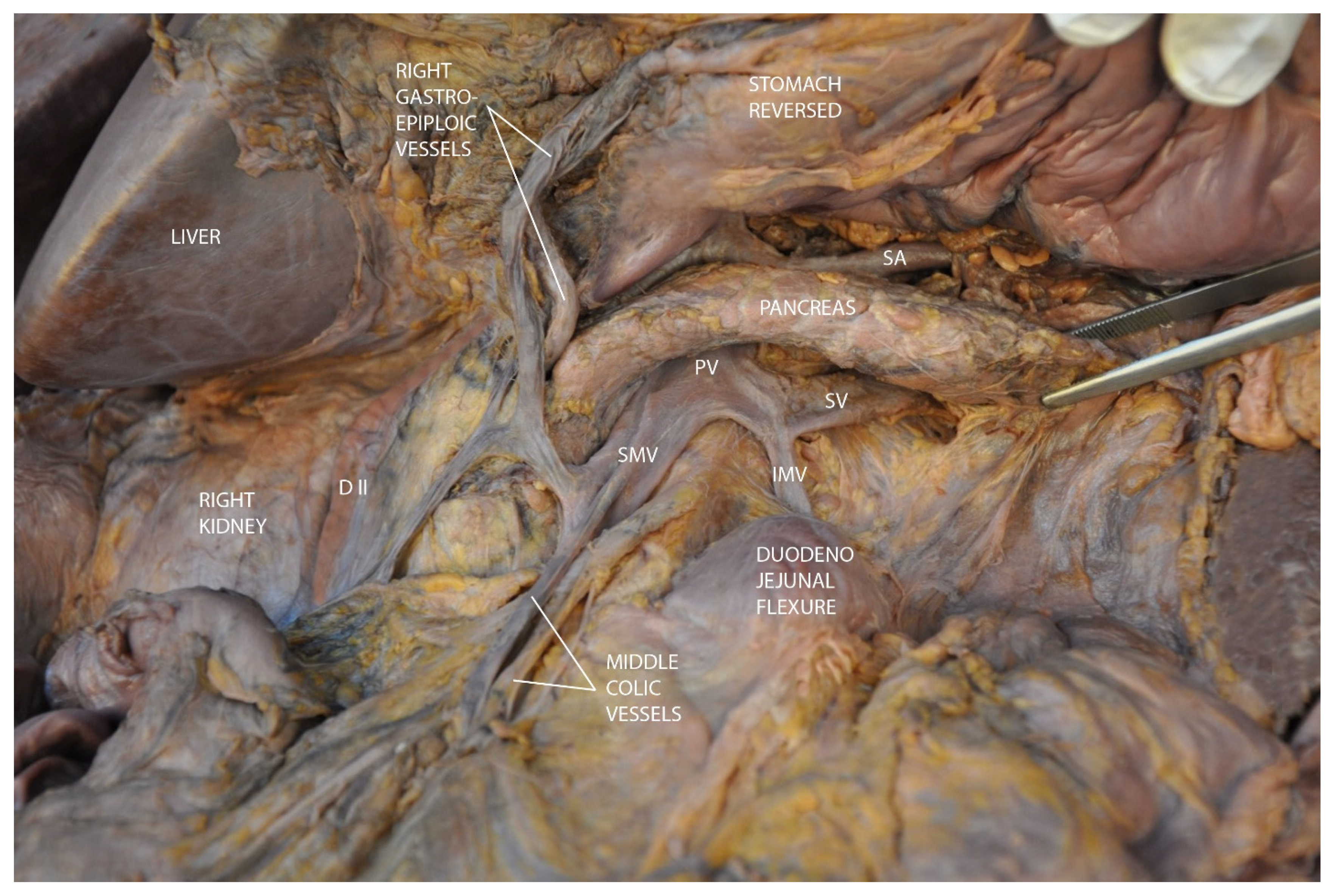
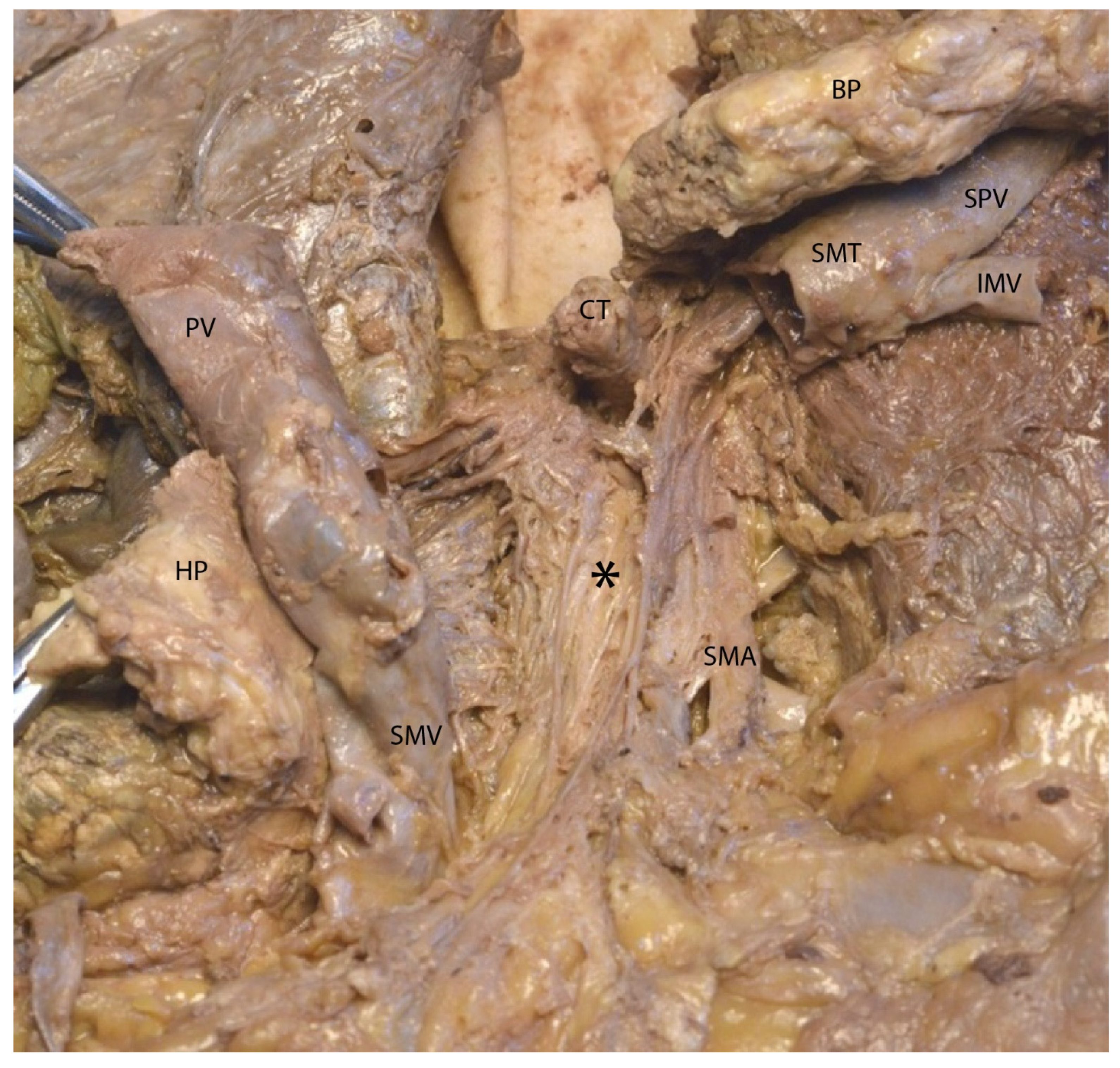
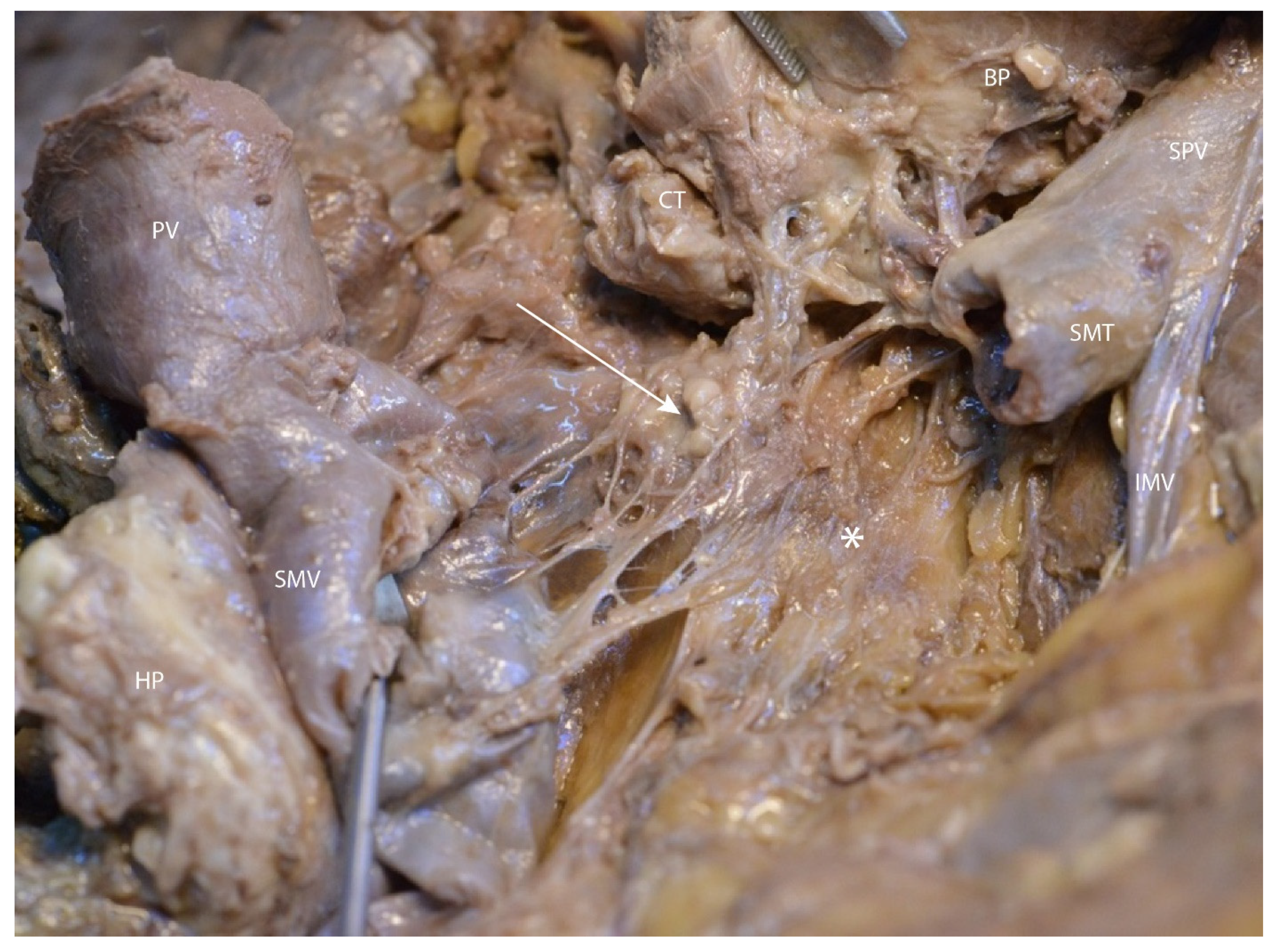
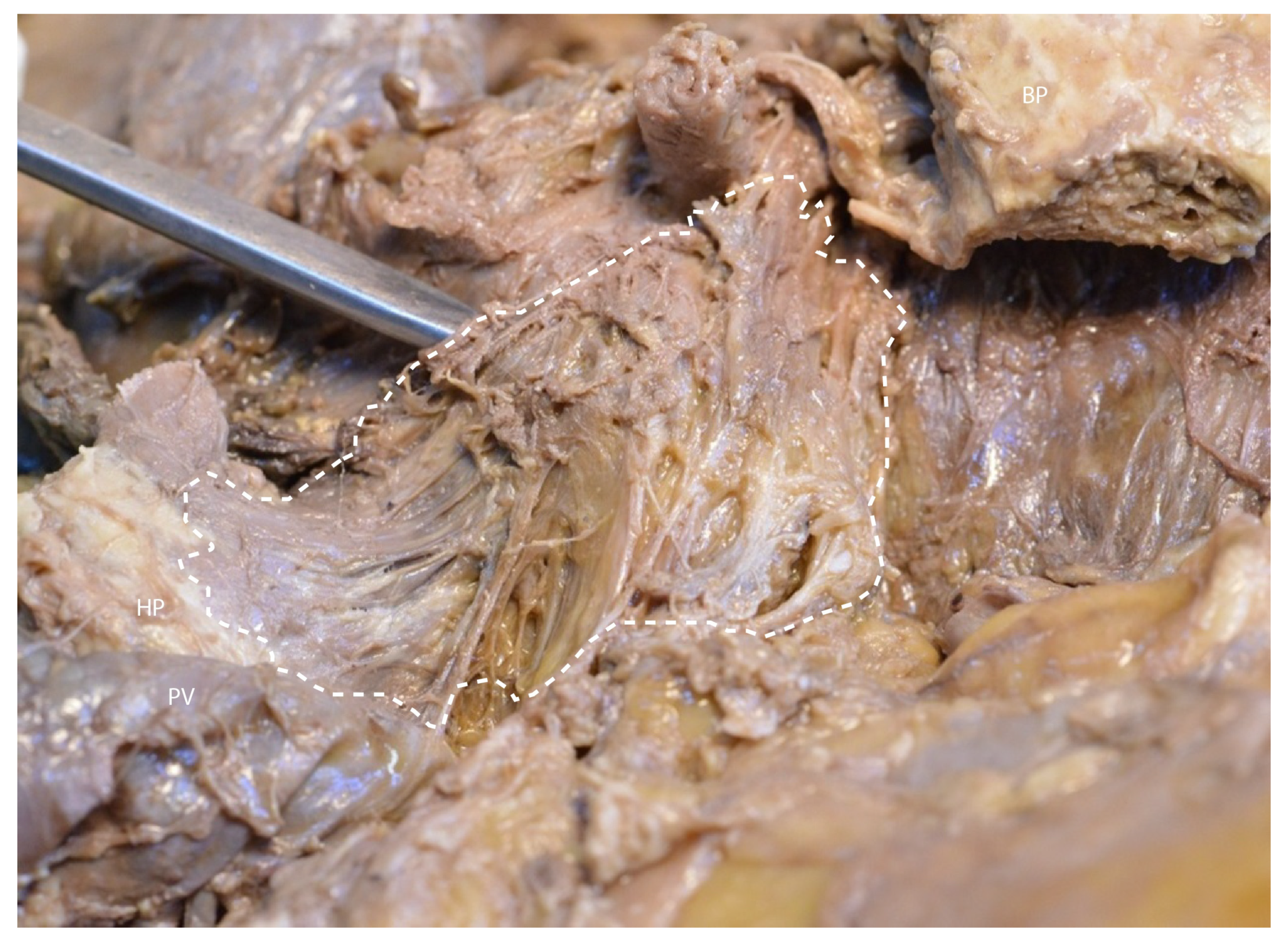
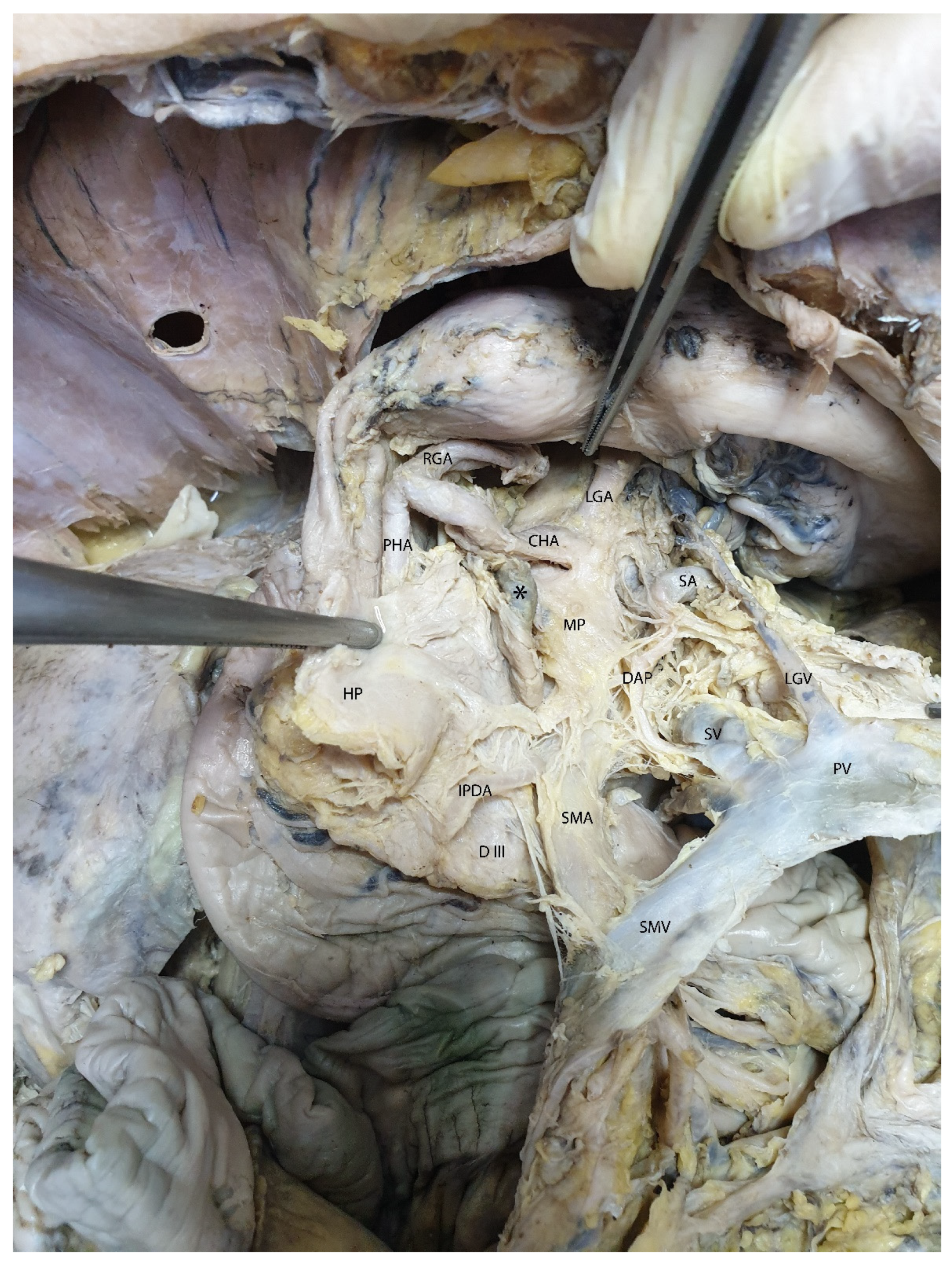
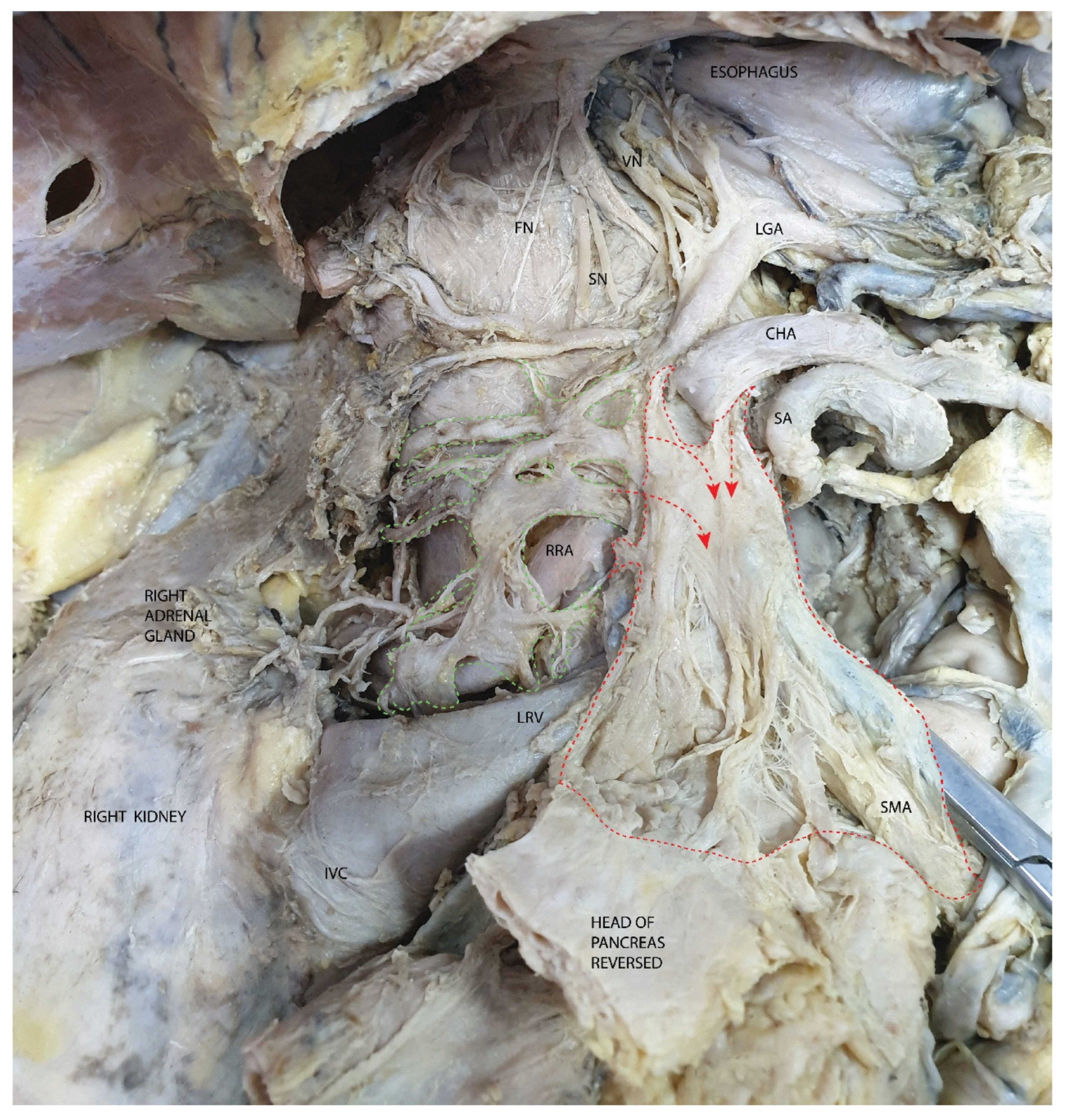
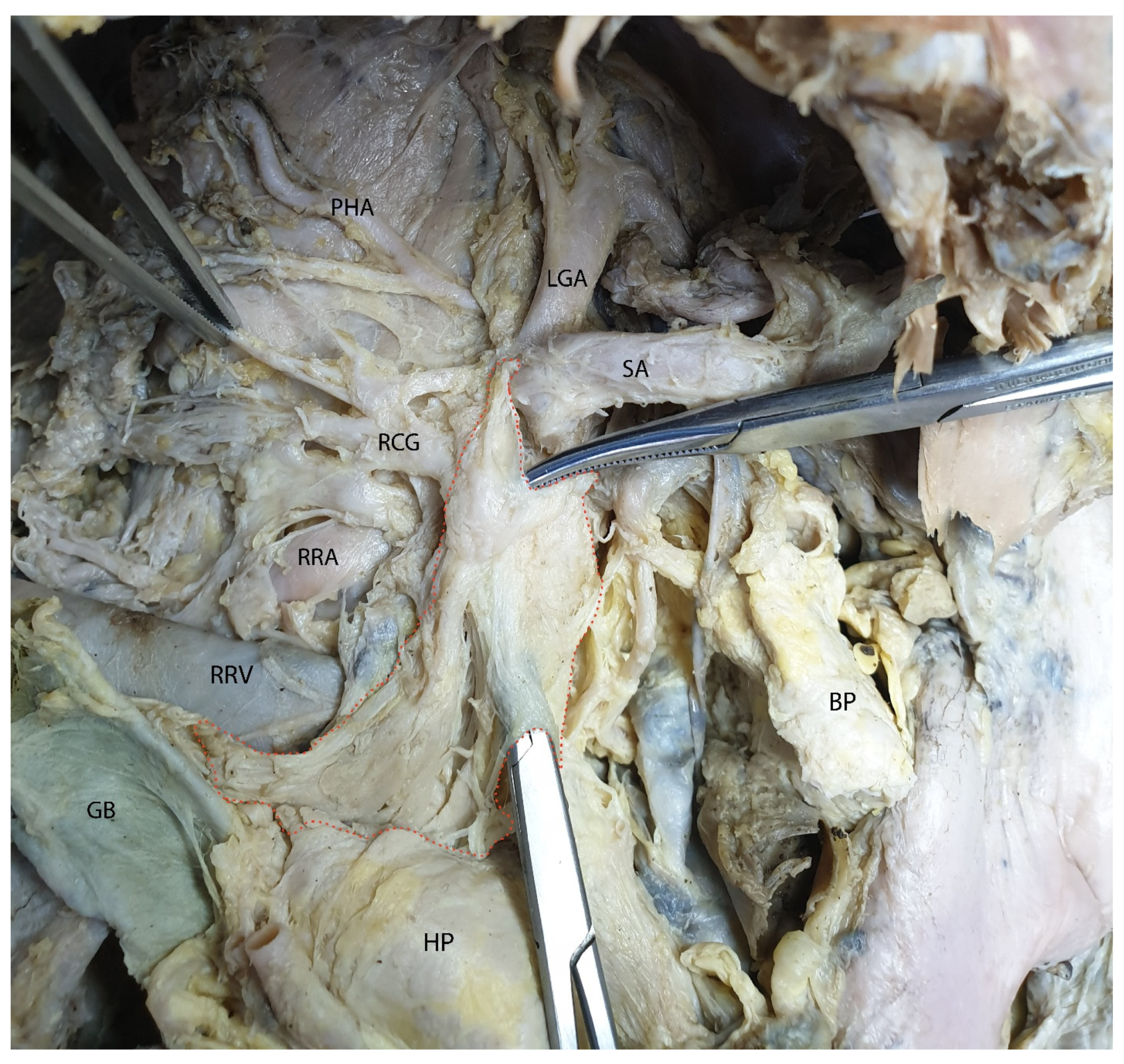
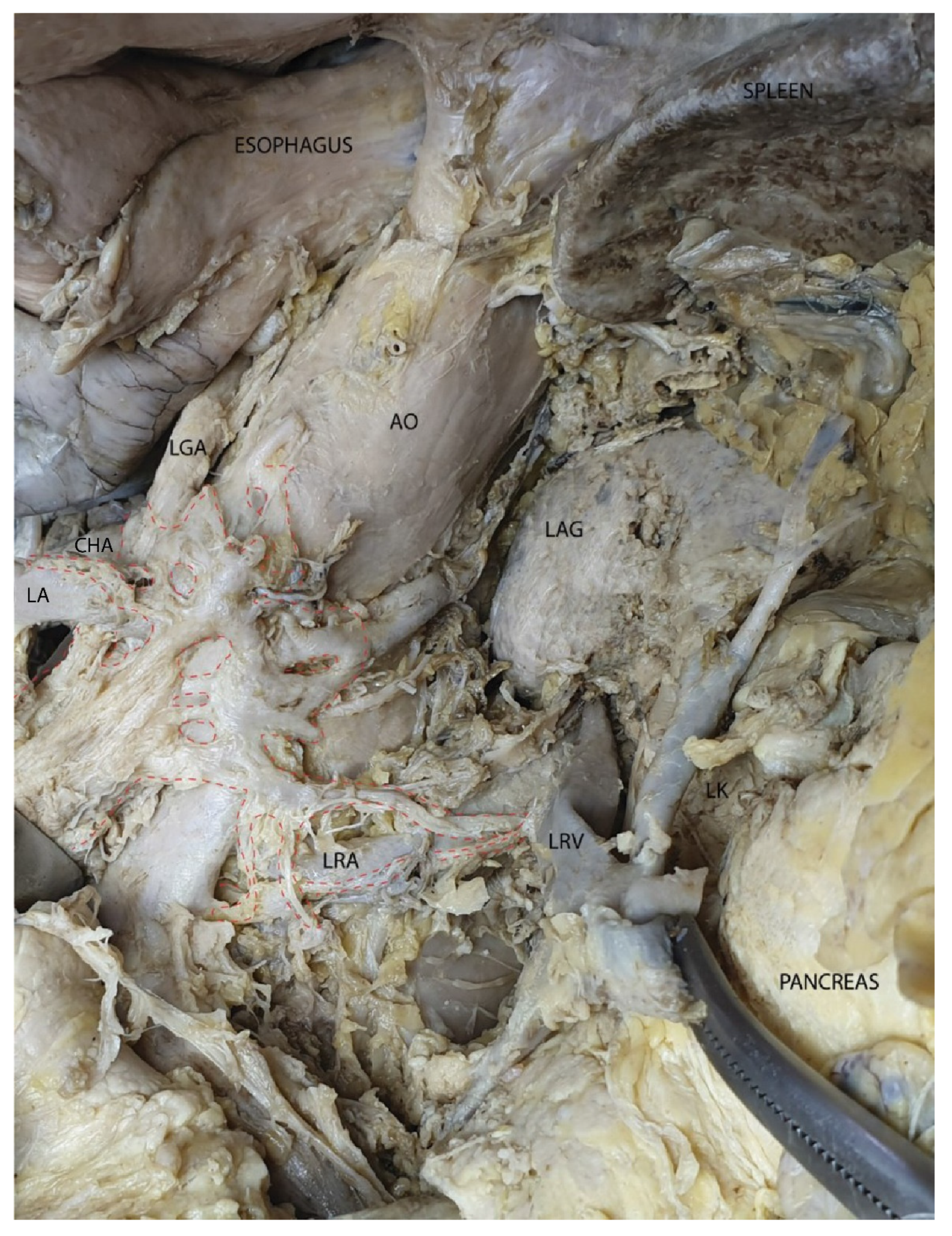
3.2. Mesopancreas—Definition and Demonstration Through Dissection: Dissection of the Celiac Ganglia
4. Discussion
4.1. Surgical Complications
4.2. Lymphatic Spread
4.3. Oncological Outcomes
4.4. Comparison with the Mesorectum
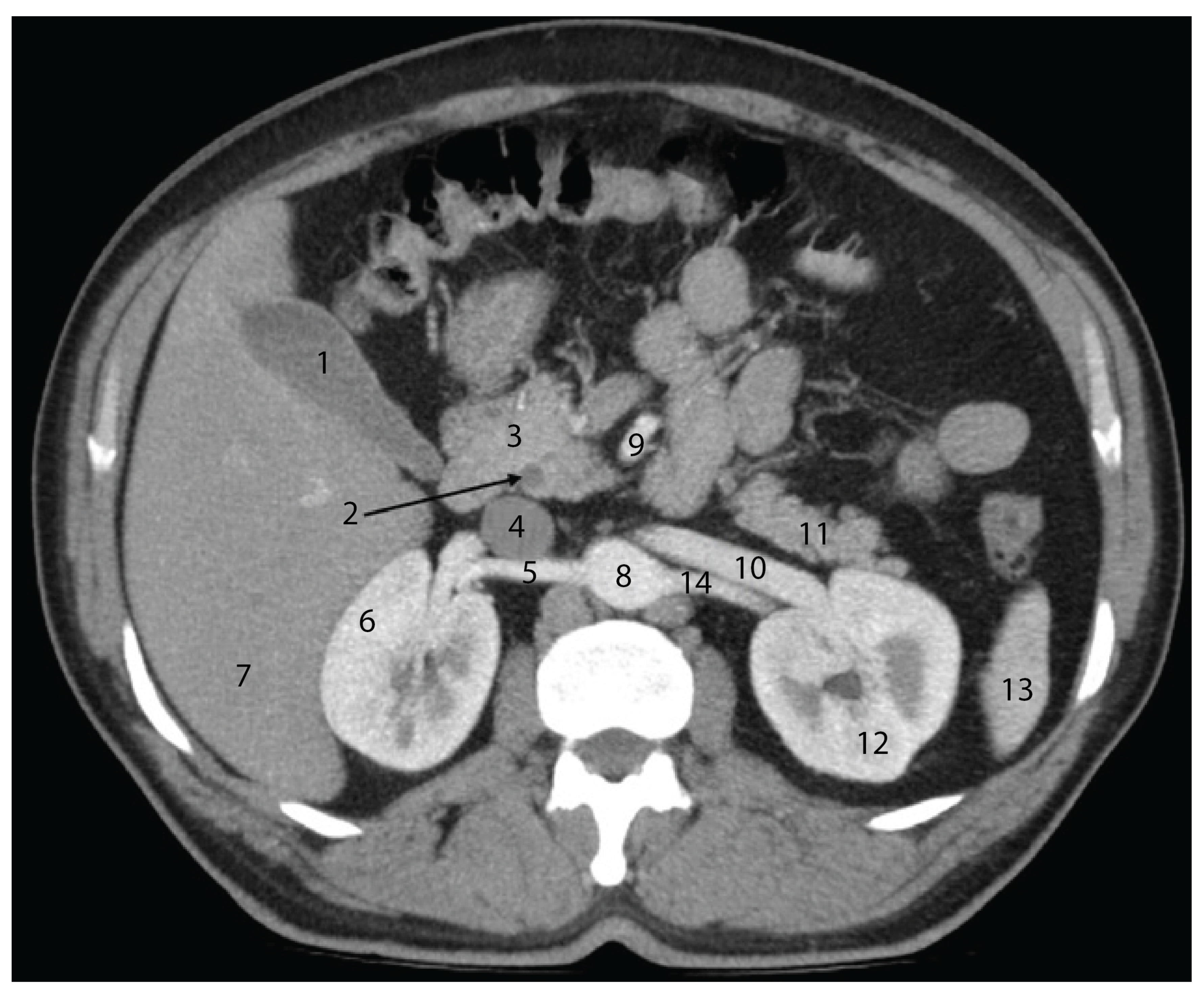
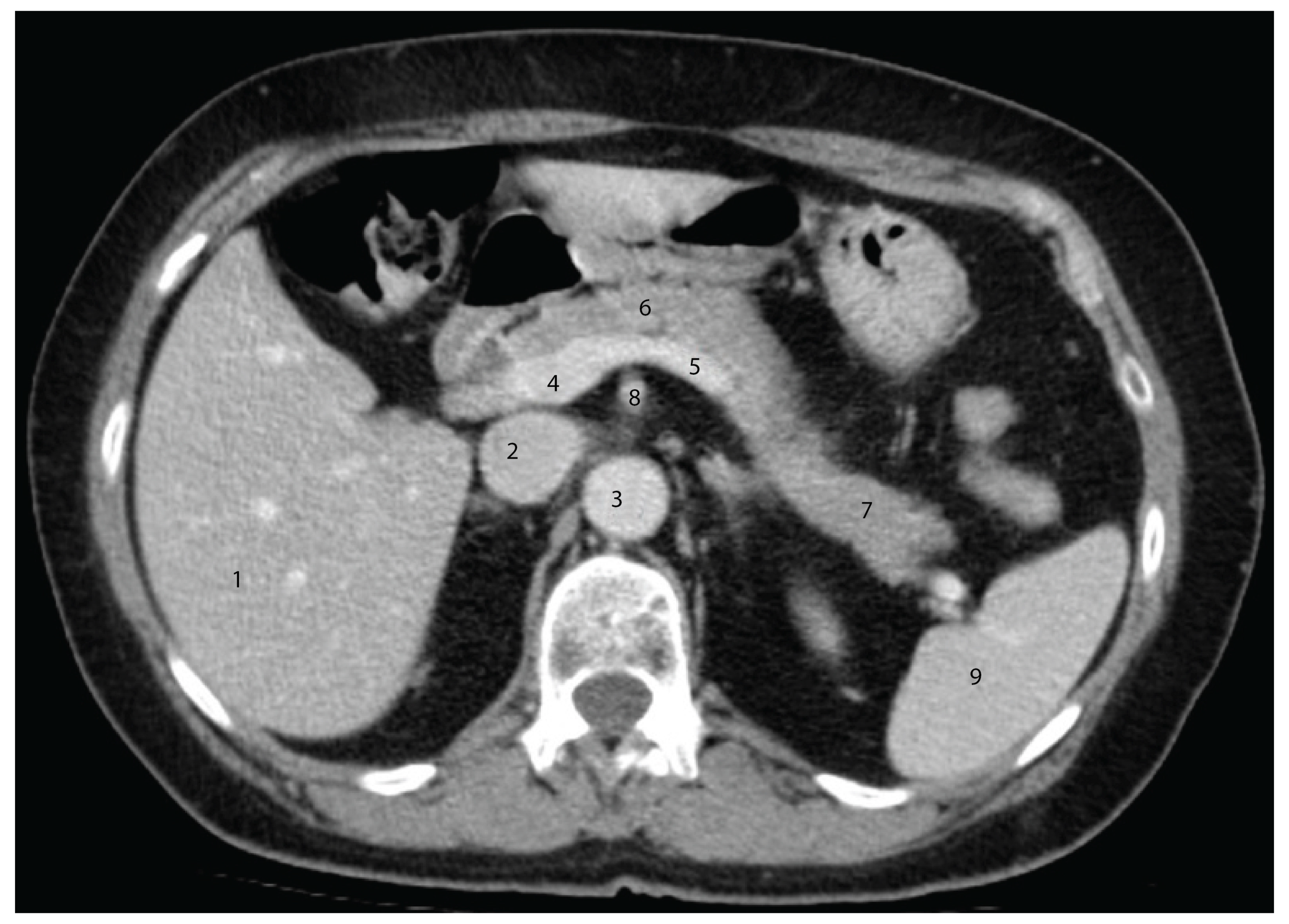
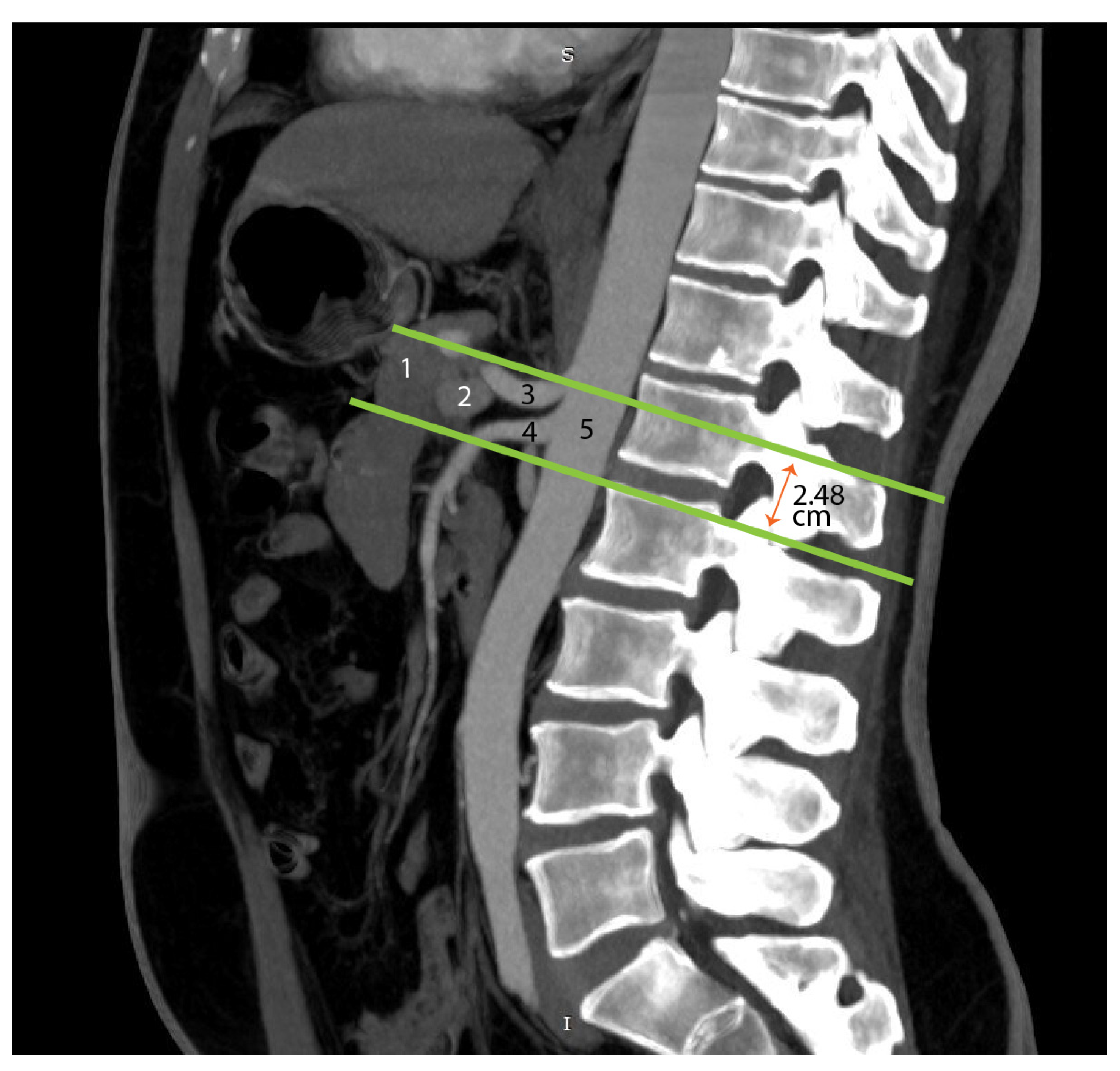
4.5. Advances in Imaging
4.6. Technological Innovations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva, L.F.L.; Belotto, M.; de Almeida, L.F.C.; Samuel, J.; Pereira, L.H.; Albagli, R.O.; Ramia, J.M. Radicality and safety of total mesopancreatic excision in pancreatoduodenectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2024, 22, 217. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, S.W. En bloc proximal peri-mesenteric clearance for pancreatic head cancer surgery. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Domeyer, M.; Wolloscheck, T.; Konerding, M.A.; Junginger, T. Resection of the mesopancreas (RMP): A new surgical classification of a known anatomical space. World J. Surg. Oncol. 2007, 5, 44. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Members of the European Study Group for Pancreatic Cancer. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef]
- Lüttges, J.; Vogel, I.; Menke, M.; Henne-Bruns, D.; Kremer, B.; Klöppel, G. The retroperitoneal resection margin and vessel involvement are important factors determining survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Virchows Arch. 1988, 433, 237–242. [Google Scholar] [CrossRef]
- Olakowski, M. Mesopancreas—New unknown land or a mirage? In Langenbeck’s Archives of Surgery; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2021; Volume 406, pp. 2899–2901. [Google Scholar]
- Luberice, K.; Downs, D.; Sadowitz, B.; Ross, S.; Rosemurgy, A. Has survival improved following resection for pancreatic adenocarcinoma? Am. J. Surg. 2017, 214, 341–346. [Google Scholar] [CrossRef]
- Popescu, I.; Dumitrascu, T. What Is the Value of Total Mesopancreas Excision in Pancreatic Ductal Adenocarcinoma? Current Evidence of the Literature; Editura Celsius: Chirurgia, Romania, 2018; Volume 113, pp. 335–343. [Google Scholar]
- Adham, M.; Singhirunnusorn, J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur. J. Surg. Oncol. 2012, 38, 340–345. [Google Scholar] [CrossRef] [PubMed]
- DeRoss, A.L. Surgical Diseases of the Stomach and Duodenum in Infants and Children. In Shackelford’s Surgery of the Alimentary Tract Volume 1-2, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 807–820. [Google Scholar]
- Godlewski, G.; Gaubert, J.; Cristol-Gaubert, R.; Radi, M.; Baecker, V.; Travo, P.; Prat-Pradal, D. Moving and fusion of the pancreatic buds in the rat embryos during the embryonic period (carnegie stages 13-17) bya three-dimensional computer-assisted reconstruction. Surg. Radiol. Anat. 2011, 33, 659–664. [Google Scholar] [CrossRef] [PubMed]
- PARLAMENTUL ROMANIEI, “LEGE nr. 104 din 27 Martie 2003 (*Republicată*) Privind Manipularea Cadavrelor Umane și Prelevarea Organelor și Țesuturilor de la Cadavre în Vederea Transplantului”. Available online: https://legislatie.just.ro/public/DetaliiDocument/42803 (accessed on 12 October 2022).
- Waasdorp Hurtado, C.; Christine Waasdorp, F.; Reviewers Sferra, T.; Polk, B. Naspghan Physiology Lecture Series Embryology and Anatomy of the Gastrointestinal Tract. Available online: http://www.ajronline.org (accessed on 12 October 2022).
- Cochard, L.R. Netter’s Atlas of Human Embryology; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Cho, B.H.; Kimura, W.; Song, C.H.; Fujimiya, M.; Murakami, G. An investigation of the embryologic development of the fascia used as the basis for pancreaticoduodenal mobilization. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 824–831. [Google Scholar] [CrossRef]
- Donovan, M.F.; Cascella, M. Embryology, Weeks 6-8. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK563181/ (accessed on 10 October 2022).
- Karim, S.A.M.; Abdulla, K.S.; Abdulkarim, Q.H.; Rahim, F.H. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int. J. Surg. 2018, 52, 383–387. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S.; Viola, M.G. Outcomes and Complications of Duodenopancreatectomy in Octogenarian Patients: A Review. Maedica 2023, 18, 705. [Google Scholar] [PubMed]
- Dai, M.; Chen, L.; Xu, Q.; Cui, M.; Li, P.; Liu, W.; Yuan, S. Robotic Versus Laparoscopic Pancreaticoduodenectomy for Pancreatic Cancer: Evaluation and Analysis of Surgical Efficacy. Ann. Surg. Oncol. 2024, 31, 7043–7051. [Google Scholar]
- Feng, Q.; Jiang, C.; Feng, X.; Du, Y.; Liao, W.; Jin, H.; Liao, M.; Zeng, Y.; Huang, J. Robotic Versus Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 752236. [Google Scholar]
- Cesmebasi, A.; Malefant, J.; Patel, S.D.; Plessis, M.D.; Renna, S.; Tubbs, R.S.; Loukas, M. The surgical anatomy of the lymphatic system of the pancreas. Clin. Anat. 2015, 28, 527–537. [Google Scholar]
- Fink, D.M.; Steele, M.M.; Hollingsworth, M.A. The Lymphatic System and Pancreatic Cancer. Cancer Lett. 2015, 381, 217. [Google Scholar] [PubMed]
- Kocher, H.M.; Sohail, M.; Benjamin, I.S.; Patel, A.G. Technical limitations of lymph node mapping in pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 887–891. [Google Scholar]
- Durczyński, A.; Hogendorf, P.; Szymański, D.; Grzelak, P.; Strzelczyk, J. Sentinel lymph node mapping in tumors of the pancreatic body: Preliminary report. Contemp. Oncol. 2012, 16, 206–209. [Google Scholar]
- Tummers, W.S.; Groen, J.V.; Sibinga Mulder, B.G.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; Swijnenburg, R.J. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar]
- Fernandes, E.D.S.M.; Strobel, O.; Girão, C.; Moraes-Junior, J.M.A.; Torres, O.J.M. What do surgeons need to know about the mesopancreas. In Langenbeck’s Archives of Surgery; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2021; Volume 406, pp. 2621–2632. [Google Scholar]
- MacFarlane, J.K.; Ryall, R.D.H.; Heald, R.J. Mesorectal excision for rectal cancer. Lancet 1993, 341, 457–460. [Google Scholar]
- Phang, P.T. Total mesorectal excision: Technical aspects. Can. J. Surg. 2004, 47, 130–137. [Google Scholar]
- Popescu, I.; Dumitrascu, T. Total meso-pancreas excision: Key point of resection in pancreatic head adenocarcinoma. Hepatogastroenterology 2011, 58, 202–207. [Google Scholar] [PubMed]
- Ramia, J.M.; De-la-Plaza, R.; Manuel-Vazquez, A.; Lopez-Marcano, A.; Morales, R. Systematic review of the mesopancreas: Concept and clinical implications. Clin. Transl. Oncol. 2018, 20, 1385–1391. [Google Scholar]
- Ohigashi, H.; Ishikawa, O.; Eguchi, H.; Yamada, T.; Sasaki, Y.; Noura, S.; Imaoka, S. Early ligation of the inferior pancreaticoduodenal artery to reduce blood loss during pancreaticoduodenectomy. Hepatogastroenterology 2004, 51, 4–5. [Google Scholar]
- Zimmitti, G.; Manzoni, A.; Addeo, P.; Garatti, M.; Zaniboni, A.; Bachellier, P.; Rosso, E. Laparoscopic pancreatoduodenectomy with superior mesenteric artery-first approach and pancreatogastrostomy assisted by mini-laparotomy. Surg. Endosc. 2016, 30, 1670–1671. [Google Scholar] [PubMed]
- Lee, J.M.; Yoon, J.H. Imaging Diagnosis of Pancreatic Cancer: CT and MRI. In Pancreatic Cancer: With Special Focus on Topical Issues and Surgical Techniques; Springer Nature: Berlin, Germany, 2017; pp. 95–114. [Google Scholar]
- Tamm, E.P.; Balachandran, A.; Bhosale, P.R.; Katz, M.H.; Fleming, J.B.; Lee, J.H.; Varadhachary, G.R. Imaging of Pancreatic Adenocarcinoma: Update on Staging/Resectability. Radiol. Clin. 2012, 50, 407–428. [Google Scholar]
- Raman, S.P.; Horton, K.M.; Fishman, E.K. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012, 18, 511–522. [Google Scholar]
- Sahani, D.V.; Bonaffini, P.A.; Catalano, O.A.; Guimaraes, A.R.; Blake, M.A. State-of-the-art PET/CT of the pancreas: Current role and emerging indications. Radiographics 2012, 32, 1133–1158. [Google Scholar] [PubMed]
- Puranik, A.D.; Agrawal, A.; Shah, S.; Purandare, N.; Rangarajan, V. PET/CT in Pancreatic Malignancies. In PET/CT in Hepatobiliary and Pancreatic Malignancies; Springer International Publishing: Cham, Switzerland, 2018; pp. 65–74. [Google Scholar]
- Maroldi, R.; Farina, D.; Borghesi, A.; Marconi, A.; Gatti, E. Perineural Tumor Spread. Neuroimaging Clin. N. Am. 2008, 18, 413–429. [Google Scholar] [CrossRef]
- Bilreiro, C.; Matos, C. Diffusion-Weighted Imaging of the Pancreas. In Diffusion Weighted Imaging of the Hepatobiliary System: Techniques and Clinical Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 113–130. [Google Scholar]
- Wegner, C.S.; Gaustad, J.V.; Andersen, L.M.K.; Simonsen, T.G.; Rofstad, E.K. Diffusion-weighted and dynamic contrast-enhanced MRI of pancreatic adenocarcinoma xenografts: Associations with tumor differentiation and collagen content. J. Transl. Med. 2016, 14, 1–11. [Google Scholar]
- Ielpo, B.; d’Addetta, M.V.; Anselmo, A.; Rosso, E.; de Blasi, V.; Sanchez-Velazquez, P.; Burdio, F. Levels of Robotic Mesopancreas Dissection According to Malignancy and Vascular Anatomy: What Surgeons Need to Know. Ann. Surg. Oncol. 2024, 31, 1916–1918. [Google Scholar] [CrossRef]
- Kitano, Y.; Inoue, Y.; Kobayashi, H.; Kobayashi, K.; Oba, A.; Ono, Y.; Takahashi, Y. Development of the multiple scope transition method in robotic pancreaticoduodenectomy. Surg. Endosc. 2024, 38, 6169–6176. [Google Scholar] [CrossRef] [PubMed]
- Helton, W.S.; Rose, J.B. Intraoperative and Laparoscopic Ultrasound During Pancreatic Surgery. In Abdominal Ultrasound for Surgeons; Springer International Publishing: Cham, Switzerland, 2014; pp. 161–176. [Google Scholar]
- Chang, J.H.; Wehrle, C.; Woo, K.; Naples, R.; Stackhouse, K.A.; Dahdaleh, F.; Naffouje, S.A. Comparing oncologic and surgical outcomes of robotic and laparoscopic distal pancreatectomy: A propensity-matched analysis. Surg. Endosc. 2024, 38, 5678–5685. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipoiu, F.-M.; Badea, G.-T.; Enyedi, M.; Oprea, Ș.; Filipoiu, Z.-F.; Mutu, D.-E.G. Mesopancreas—Anatomical Insights and Its Implications for Diagnosis and Clinical and Surgical Practice. Diagnostics 2025, 15, 914. https://doi.org/10.3390/diagnostics15070914
Filipoiu F-M, Badea G-T, Enyedi M, Oprea Ș, Filipoiu Z-F, Mutu D-EG. Mesopancreas—Anatomical Insights and Its Implications for Diagnosis and Clinical and Surgical Practice. Diagnostics. 2025; 15(7):914. https://doi.org/10.3390/diagnostics15070914
Chicago/Turabian StyleFilipoiu, Florin-Mihail, Georgian-Theodor Badea, Mihaly Enyedi, Ștefan Oprea, Zoran-Florin Filipoiu, and Daniela-Elena Gheoca Mutu. 2025. "Mesopancreas—Anatomical Insights and Its Implications for Diagnosis and Clinical and Surgical Practice" Diagnostics 15, no. 7: 914. https://doi.org/10.3390/diagnostics15070914
APA StyleFilipoiu, F.-M., Badea, G.-T., Enyedi, M., Oprea, Ș., Filipoiu, Z.-F., & Mutu, D.-E. G. (2025). Mesopancreas—Anatomical Insights and Its Implications for Diagnosis and Clinical and Surgical Practice. Diagnostics, 15(7), 914. https://doi.org/10.3390/diagnostics15070914





