Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Tumor Characteristics
2.3. Rectal MRI Methodology
- Craniocaudal length of the tumor and short-dimension thickness of the mass;
- Distance of the tumor from the anal verge and from the top of the anal sphincter complex;
- Mucinous nature of the tumor;
- Extramural depth of invasion;
- Relationship to peritoneal reflections;
- Invasion of peritoneal reflections;
- Invasion of adjacent organs;
- Invasion of anal canal;
- Number of visible mesorectal lymph nodes and size of largest lymph node;
- Minimum distance of primary tumor/metastatic lymph node/mesorectal deposit to mesorectal fascia;
- Presence of extramural vascular invasion (EMVI);
- Presence of mesorectal fascia invasion (status of the circumferential resection margin (CRM)).
2.4. Immunohistochemical Methodology
2.5. Statistical Analysis
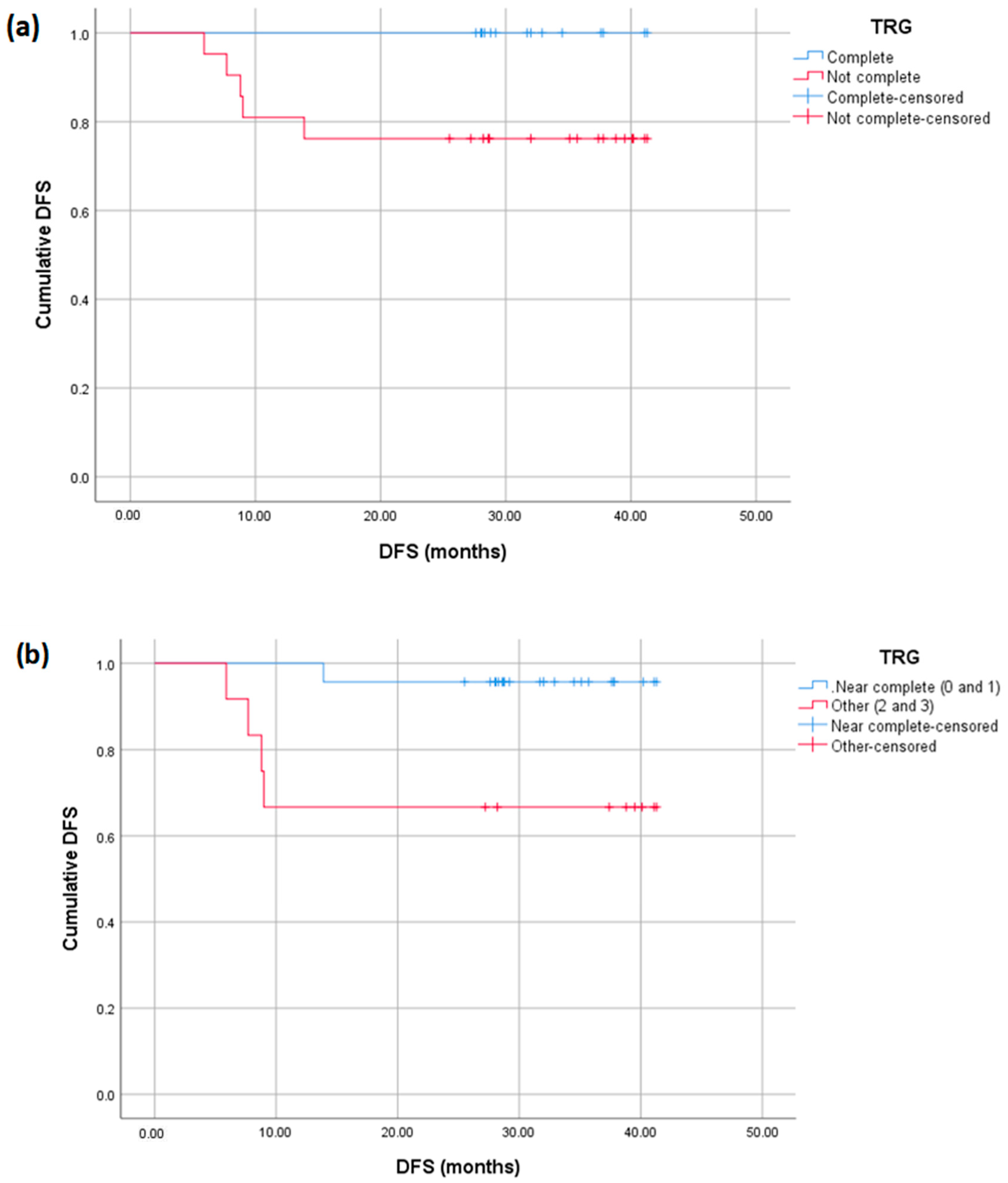
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar]
- Horvat, N.; Carlos Tavares Rocha, C.; Clemente Oliveira, B.; Petkovska, I.; Gollub, M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. RadioGraphics 2019, 39, 367–387. [Google Scholar] [PubMed]
- São Julião, G.P.; Habr-Gama, A.; Vailati, B.B.; Araujo, S.E.A.; Fernandez, L.M.; Perez, R.O. New Strategies in Rectal Cancer. Surg. Clin. N. Am. 2017, 97, 587–604. [Google Scholar]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar]
- Morais, M.; Pinto, D.M.; Machado, J.C.; Carneiro, S. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: A systematic review. Eur. J. Surg. Oncol. 2022, 48, 218–227. [Google Scholar]
- Benson, A.B.; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 370–398. [Google Scholar] [PubMed]
- Chen, M.; Chen, L.Z.; Xu, L.; Zhang, J.S.; Song, X. Neoadjuvant chemoradiation for locally advanced rectal cancer: A systematic review of the literature with network meta-analysis. Cancer Manag. Res. 2019, 11, 741–758. [Google Scholar]
- Marr, R.; Birbeck, K.; Garvican, J.; Macklin, C.P.; Tiffin, N.J.; Parsons, W.J.; Dixon, M.F.; Mapstone, N.P.; Sebag-Montefiore, D.; Scott, N.; et al. The modern abdominoperineal excision: The next challenge after total mesorectal excision. Ann. Surg. 2005, 242, 74–82. [Google Scholar]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; e Sousa, A.H.S., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717, discussion 7–8. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar]
- Rahma, O.E.; Yothers, G.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Colangelo, L.H.; Lucas, P.C.; Gollub, M.J.; et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results From the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Grainger, J.; Harrison, M.; Ostler, P.; Makris, A. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious? Br. J. cancer 2006, 94, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Azin, A.; Khorasani, M.; Quereshy, F.A. Neoadjuvant chemoradiation in locally advanced rectal cancer: The surgeon’s perspective. J. Clin. Pathol. 2019, 72, 133–134. [Google Scholar] [CrossRef]
- Goodman, K.A. Total neoadjuvant therapy for rectal cancer. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2018, 22, 459–465. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, N.K. Challenges and shifting treatment strategies in the surgical treatment of locally advanced rectal cancer. Ann. Gastroenterol. Surg. 2020, 4, 379–385. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Palta, M.; Willett, C.G.; Czito, B.G. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer 2017, 123, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Kerr, J.; Schlesinger-Raab, A.; Eckel, R.; Sauer, H.; Hölzel, D. Quality of life in rectal cancer patients: A four-year prospective study. Ann. Surg. 2003, 238, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Dinaux, A.M.; Amri, R.; Bordeianou, L.G.; Hong, T.S.; Wo, J.Y.; Blaszkowsky, L.S.; Allen, J.; Murphy, J.; Kunitake, H.; Berger, D. The Impact of Pathologic Complete Response in Patients with Neoadjuvantly Treated Locally Advanced Rectal Cancer-a Large Single-Center Experience. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2017, 21, 1153–1158. [Google Scholar] [CrossRef]
- Tan, Y.; Fu, D.; Li, D.; Kong, X.; Jiang, K.; Chen, L.; Yuan, Y.; Ding, K. Predictors and Risk Factors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Population-Based Analysis. Front. Oncol. 2019, 9, 497. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, J.; Chen, J.; Mei, S.; Wang, Z. Predictive Factors for Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1607–1611. [Google Scholar] [PubMed]
- Bitterman, D.S.; Resende Salgado, L.; Moore, H.G.; Sanfilippo, N.J.; Gu, P.; Hatzaras, I.; Du, K.L. Predictors of Complete Response and Disease Recurrence Following Chemoradiation for Rectal Cancer. Front. Oncol. 2015, 5, 286. [Google Scholar]
- Shin, J.K.; Huh, J.W.; Lee, W.Y.; Yun, S.H.; Kim, H.C.; Cho, Y.B.; Park, Y.A. Clinical prediction model of pathological response following neoadjuvant chemoradiotherapy for rectal cancer. Sci. Rep. 2022, 12, 7145. [Google Scholar]
- Wang, Y.; Yang, L.; Bao, H.; Fan, X. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021, 18, e1003741. [Google Scholar]
- George, T.J., Jr.; Allegra, C.J.; Yothers, G. Neoadjuvant Rectal (NAR) Score: A New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr. Color. Cancer Rep. 2015, 11, 275–280. [Google Scholar]
- George, T.J.; Yothers, G.; Rahma, O.E.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Lucas, P.C.; Colangelo, L.H.; et al. Long-term results from NRG-GI002: A phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC). J. Clin. Oncol. 2023, 41 (Suppl. S4), 7. [Google Scholar]
- Hall, W.A.; Li, J.; You, Y.N.; Gollub, M.J.; Grajo, J.R.; Rosen, M.; Deprisco, G.; Yothers, G.; Dorth, J.A.; Rahma, O.E.; et al. Prospective Correlation of Magnetic Resonance Tumor Regression Grade with Pathologic Outcomes in Total Neoadjuvant Therapy for Rectal Adenocarcinoma. J. Clin. Oncol. 2023, 41, 4643–4651. [Google Scholar]
- Shamseddine, A.; Khalifeh, I.M.; Elias, C.; Turfa, R.; Kattan, J.G.; Mukherji, D.; Temraz, S.N.; Alqasem, K.; Amarin, R.; Alawabdeh, T.; et al. High immunoscore as a predictor of outcome in patients who underwent chemoimmuno-therapy in locally advanced rectal cancer: A post-hoc analysis of the correlation between immunoscore and pCR in the Averectal study. J. Clin. Oncol. 2022, 40 (Suppl. S4), 184. [Google Scholar]
- Kalisz, K.R.; Enzerra, M.D.; Paspulati, R.M. MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy. Radiographics 2019, 39, 538–556. [Google Scholar]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar]
- Jang, J.K.; Choi, S.H.; Park, S.H.; Kim, K.W.; Kim, H.J.; Lee, J.S.; Kim, A.Y. MR tumor regression grade for pathological complete response in rectal cancer post neoadjuvant chemoradiotherapy: A systematic review and meta-analysis for accuracy. Eur. Radiol. 2020, 30, 2312–2323. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, Z.; Chen, J.; Ye, J.; Tang, Z.; Fang, Y.; Yao, K.; Zeng, Q.; Yang, Y.; Tang, H.; et al. A nomogram for predicting good response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer: A retrospective, double-center, cohort study. Int. J. Color. Dis. 2022, 37, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Dizdarevic, E.; Hansen, T.F.; Jakobsen, A. The Prognostic Importance of ctDNA in Rectal Cancer: A Critical Reappraisal. Cancers 2022, 14, 2252. [Google Scholar] [CrossRef] [PubMed]
- Orhan, A.; Khesrawi, F.; Tvilling Madsen, M.; Peuliche Vogelsang, R.; Dohrn, N.; Kanstrup Fiehn, A.-M.; Gögenur, I. Tumor-Infiltrating Lymphocytes as Biomarkers of Treatment Response and Long-Term Survival in Patients with Rectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 636. [Google Scholar] [CrossRef]
- Alkan, A.; Hofving, T.; Angenete, E.; Yrlid, U. Biomarkers and cell-based models to predict the outcome of neoadjuvant therapy for rectal cancer patients. Biomark. Res. 2021, 9, 60. [Google Scholar]
- Galon, J.; Hermitte, F.; Mlecnik, B.; Marliot, F.; Bifulco, C.; Lugli, A.; Nagtegaal, I.; Hartmann, A.; Eynde, M.v.D.; Roehrl, M.; et al. Immunoscore predicts significant differences in time to recurrence in stage I colon cancer patients. Ann. Oncol. 2019, 30, ix30. [Google Scholar] [CrossRef]
- Sahu, A.; Kose, K.; Kraehenbuehl, L.; Byers, C.; Holland, A.; Tembo, T.; Santella, A.; Alfonso, A.; Li, M.; Cordova, M.; et al. In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response. Nat. Commun. 2022, 13, 5312. [Google Scholar]
- Anitei, M.G.; Zeitoun, G.; Mlecnik, B.; Marliot, F.; Haicheur, N.; Todosi, A.M.; Kirilovsky, A.; Lagorce, C.; Bindea, G.; Ferariu, D.; et al. Prognostic and Predictive Values of the Immunoscore in Patients with Rectal Cancer. Clin. Cancer Res. 2014, 20, 1891–1899. [Google Scholar] [CrossRef]
- Kirilovsky, A.; El Sissy, C.; Zeitoun, G.; Marliot, F.; Haicheur, N.; Lagorce-Pagès, C.; Taieb, J.; Karoui, M.; Custers, P.; Dizdarevic, E.; et al. The “Immunoscore” in rectal cancer: Could we search quality beyond quantity of life? Oncotarget 2022, 13, 18–31. [Google Scholar] [CrossRef]
- A Sinicrope, F.; Shi, Q.; Hermitte, F.; Zemla, T.J.; Mlecnik, B.; Benson, A.B.; Gill, S.; Goldberg, R.M.; Kahlenberg, M.S.; Nair, S.G.; et al. Contribution of Immunoscore and Molecular Features to Survival Prediction in Stage III Colon Cancer. JNCI Cancer Spectr. 2020, 4, pkaa023. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Van den Eynde, M.; Muşină, A.M.; Anitei, M.G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef] [PubMed]
- Leow, Y.C.; Roslani, A.C. Pathological Complete Response After Neoadjuvant Therapy in Rectal Adenocarcinoma: A 5-Year Follow-up. Indian J. Surg. 2021, 83 (Suppl. S3), 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, S.; Guo, Y.; Luo, Y.; Li, L. Total neoadjuvant therapy versus standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis of 15 trials. PLoS ONE 2022, 17, e0276599. [Google Scholar] [CrossRef]
- Yothers, G.; George, T.J.; Petrelli, N.J.; O’Connell, M.J.; Beart, R.W.; Allegra, C.J.; Roh, M.S.; Lopa, S.H.; Colangelo, L.H.; Sharif, S.; et al. Neoadjuvant rectal cancer (RC) score to predict survival: Potential surrogate endpoint for early phase trials. J. Clin. Oncol. 2014, 32 (Suppl. S15), 3533. [Google Scholar]
- Hong, T.S.; Szymonifka, J.; Yothers, G.; Blaszkowsky, L.S.; Russo, A.L.; Wo, J.Y.; Mamon, H.J.; Kwak, E.L.; Zhu, A.X.; Allen, J.N.; et al. Validation of the NSABP neoadjuvant rectal score (NAR) in a prospective phase II study evaluating an experimental regimen and a standard chemoradiation cohort with molecular genotyping. J. Clin. Oncol. 2014, 32 (Suppl. S15), 3599. [Google Scholar] [CrossRef]
- Roy, A.; Olsen, J.R.; Myerson, R.J.; Markovina, S.; DeWees, T.A.; Parikh, P.J. Short-Term Endpoints for Neoadjuvant Rectal Cancer Therapy: Pathologic Complete Response or Neoadjuvant Rectal Cancer Score? Int. J. Radiat. Oncol. Biol. Phys. 2016, 96 (Suppl. S2), E201. [Google Scholar] [CrossRef]
- Fokas, E.; Fietkau, R.; Hartmann, A.; Hohenberger, W.; Grützmann, R.; Ghadimi, M.; Liersch, T.; Ströbel, P.; Grabenbauer, G.; Graeven, U.; et al. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Chehade, L.; Turfa, R.; Kattan, J.; Temraz, S.; Zeidan, Y.; Amarin, R.; Alawabdeh, T.; Deeba, S.; Doughan, S.; Mohamad, I.; et al. 250P Short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma: Final analysis of the phase II Averectal trial. Ann. Oncol. 2024, 35, S107. [Google Scholar] [CrossRef]
- Shamseddine, A.; Machmouchi, A.; Natout, M.; Turfa, R.; Kattan, J.G.; Temraz, S.N.; Tawil, A.; Elkhaldi, M.; Jaber, O.; Amarin, R.; et al. Assessment of immunoscore and MRI tumor regression grade to predict complete pathologic response in patients with locally advanced rectal cancer: Data from phase II Averectal study. J. Clin. Oncol. 2023, 41 (Suppl. S4), 212. [Google Scholar] [CrossRef]


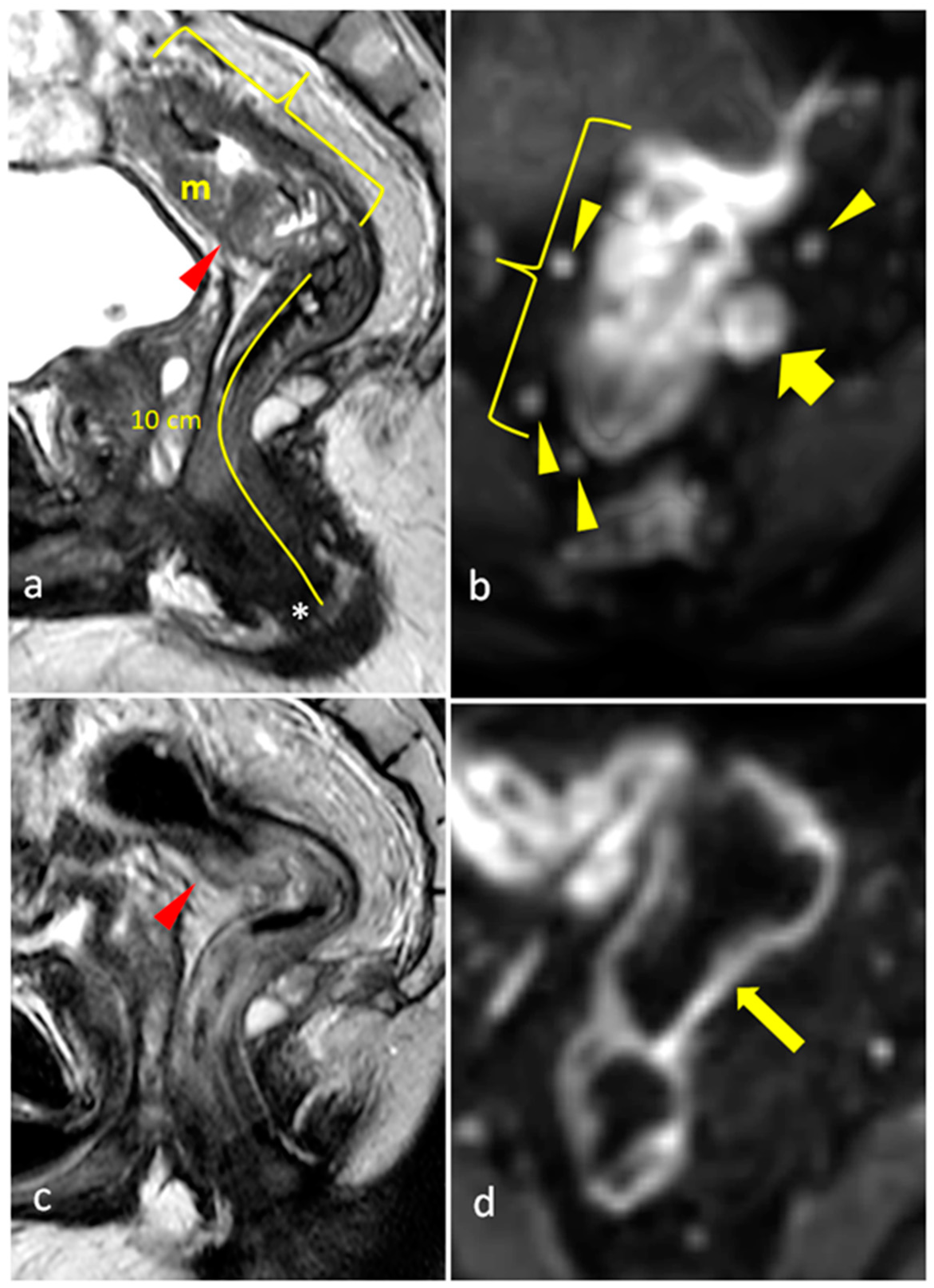

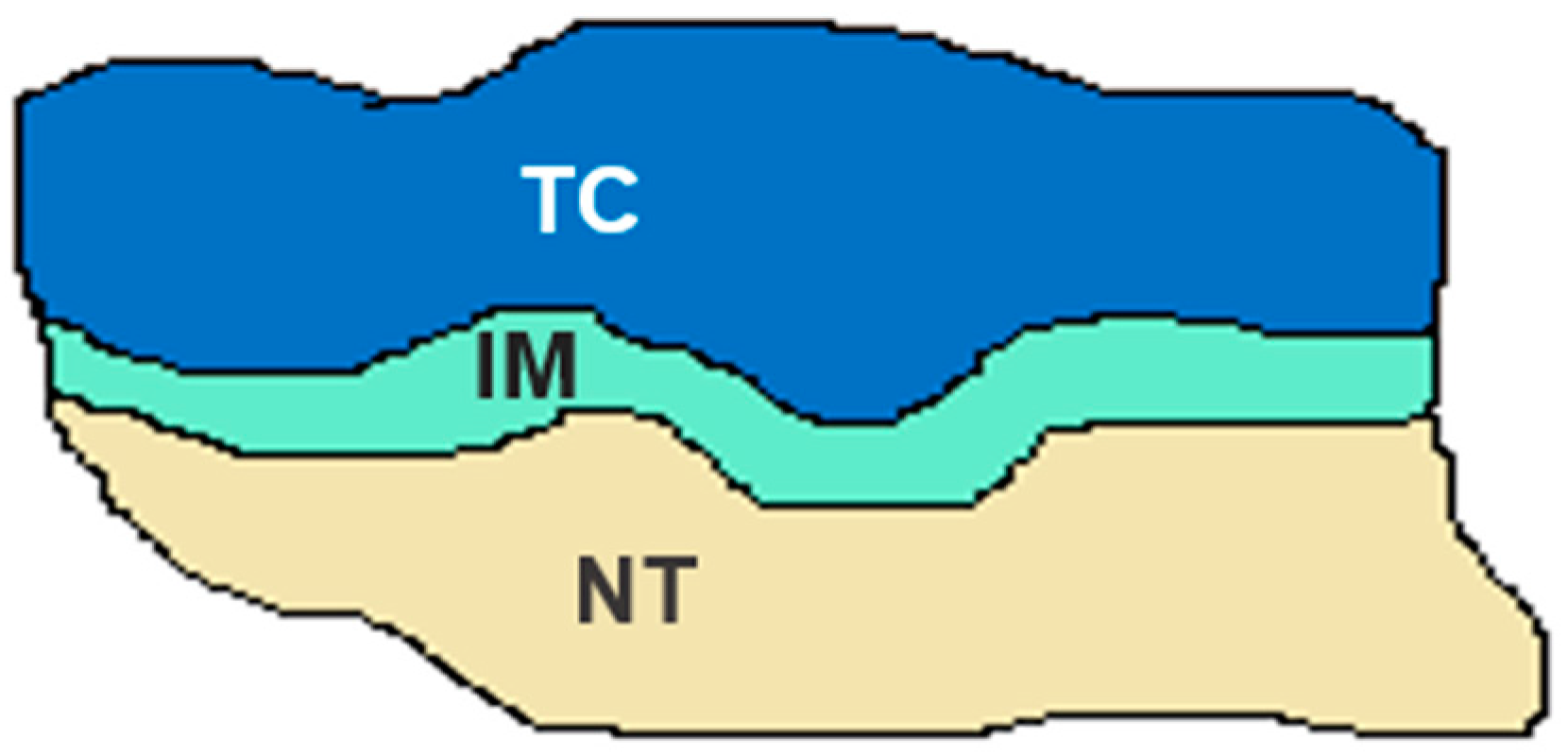




| Grade | Definition | |
|---|---|---|
| mrTRG | 1 | Complete Radiologic Response (i.e., no evidence of tumor) |
| 2 | Good Response (i.e., dense >75% fibrosis with no obvious residual tumor) | |
| 3 | Moderate Response (i.e., >50% fibrosis or mucin with a minority of visible tumor) | |
| 4 | Slight Regression (i.e., <50% fibrosis or mucin with a majority of visible tumor) | |
| 5 | No post-treatment changes | |
| pTRG | 0 | No residual tumor cells |
| 1 | <10% residual tumor cells | |
| 2 | 10% to 50% residual tumor cells | |
| 3 | >50% residual tumor cells | |
| IS | 0 | >62% mean density percentiles of CD3- and CD8-positive T-cells |
| 1 | <62% mean density percentiles of CD3- and CD8-positive T-cells | |
| NAR | Low | <8 |
| Intermediate | 8 to 16 | |
| High | >16 |
| Pathologic NAR | Low, n (%) | Intermediate, n (%) | High, n (%) | p-Value |
|---|---|---|---|---|
| Non-pCR | 4 (18.2%) | 9 (40.9%) | 9 (40.9%) | <0.0001 |
| pCR | 12 (92.3%) | 1 (7.7%) | 0 (0%) | |
| Radiologic NAR | Low | Intermediate | High | p-value |
| Non-pCR | 10 (45.5%) | 6 (27.3%) | 6 (27.3%) | 0.128 |
| pCR | 8 (61.5%) | 5 (38.5%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natout, M.; Machmouchi, A.; Hussain, H.; Chehade, L.; Abbas, N.; Turfa, R.; Kattan, J.; Temraz, S.; Tawil, A.; Elkhaldi, M.; et al. Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study. Diagnostics 2025, 15, 913. https://doi.org/10.3390/diagnostics15070913
Natout M, Machmouchi A, Hussain H, Chehade L, Abbas N, Turfa R, Kattan J, Temraz S, Tawil A, Elkhaldi M, et al. Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study. Diagnostics. 2025; 15(7):913. https://doi.org/10.3390/diagnostics15070913
Chicago/Turabian StyleNatout, Mustafa, Ahmad Machmouchi, Hero Hussain, Laudy Chehade, Noura Abbas, Rim Turfa, Joseph Kattan, Sally Temraz, Ayman Tawil, Mousa Elkhaldi, and et al. 2025. "Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study" Diagnostics 15, no. 7: 913. https://doi.org/10.3390/diagnostics15070913
APA StyleNatout, M., Machmouchi, A., Hussain, H., Chehade, L., Abbas, N., Turfa, R., Kattan, J., Temraz, S., Tawil, A., Elkhaldi, M., Jaber, O., Amarin, R., Alawabdeh, T., Charafeddine, M., Al Darazi, M., & Shamseddine, A. (2025). Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study. Diagnostics, 15(7), 913. https://doi.org/10.3390/diagnostics15070913








