Lung Adenocarcinoma Exhibiting Thanatosomes (Hyaline Globules), Cytoplasmic Clearing, and Nuclear Pleomorphism, with a KRAS Mutation
Abstract
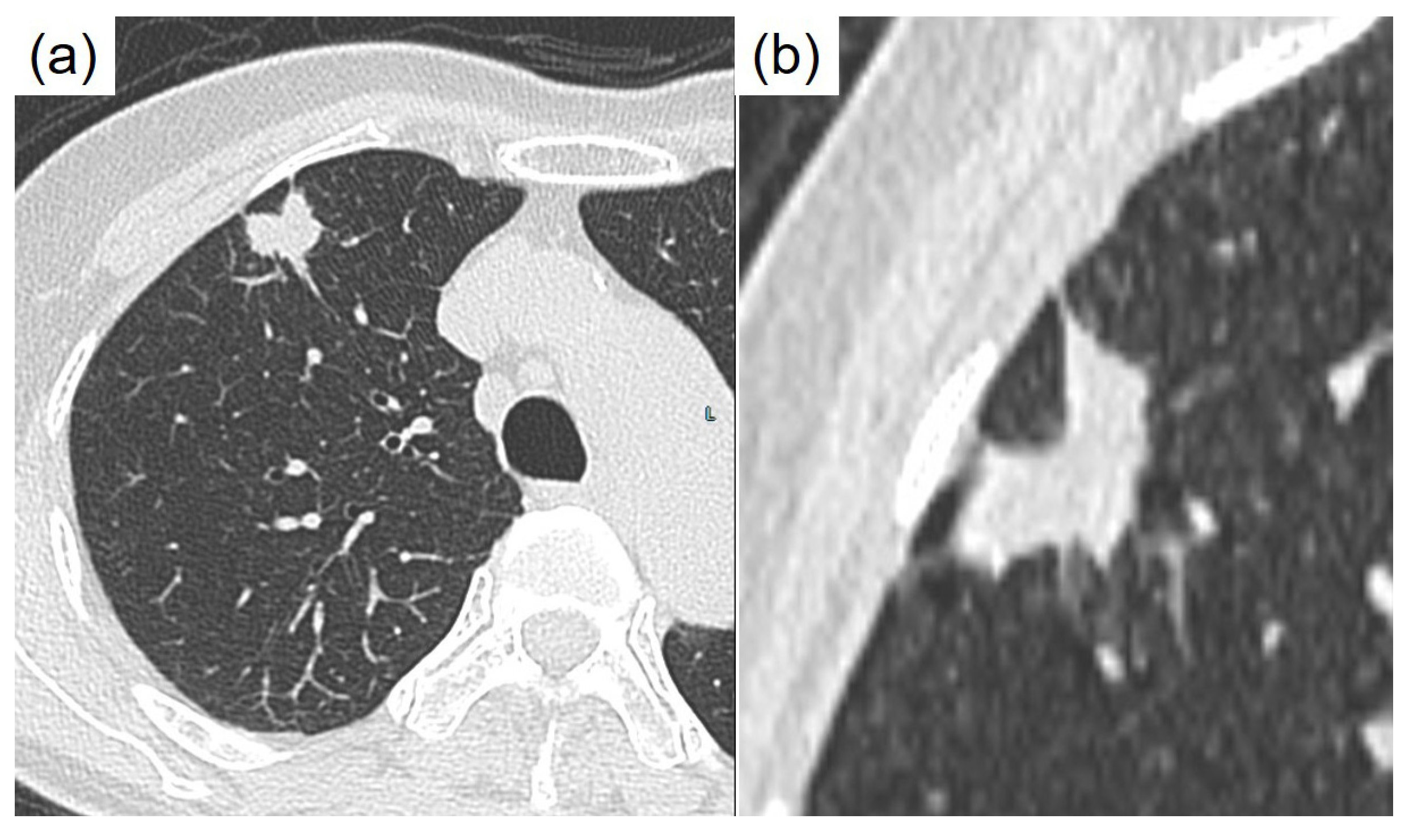
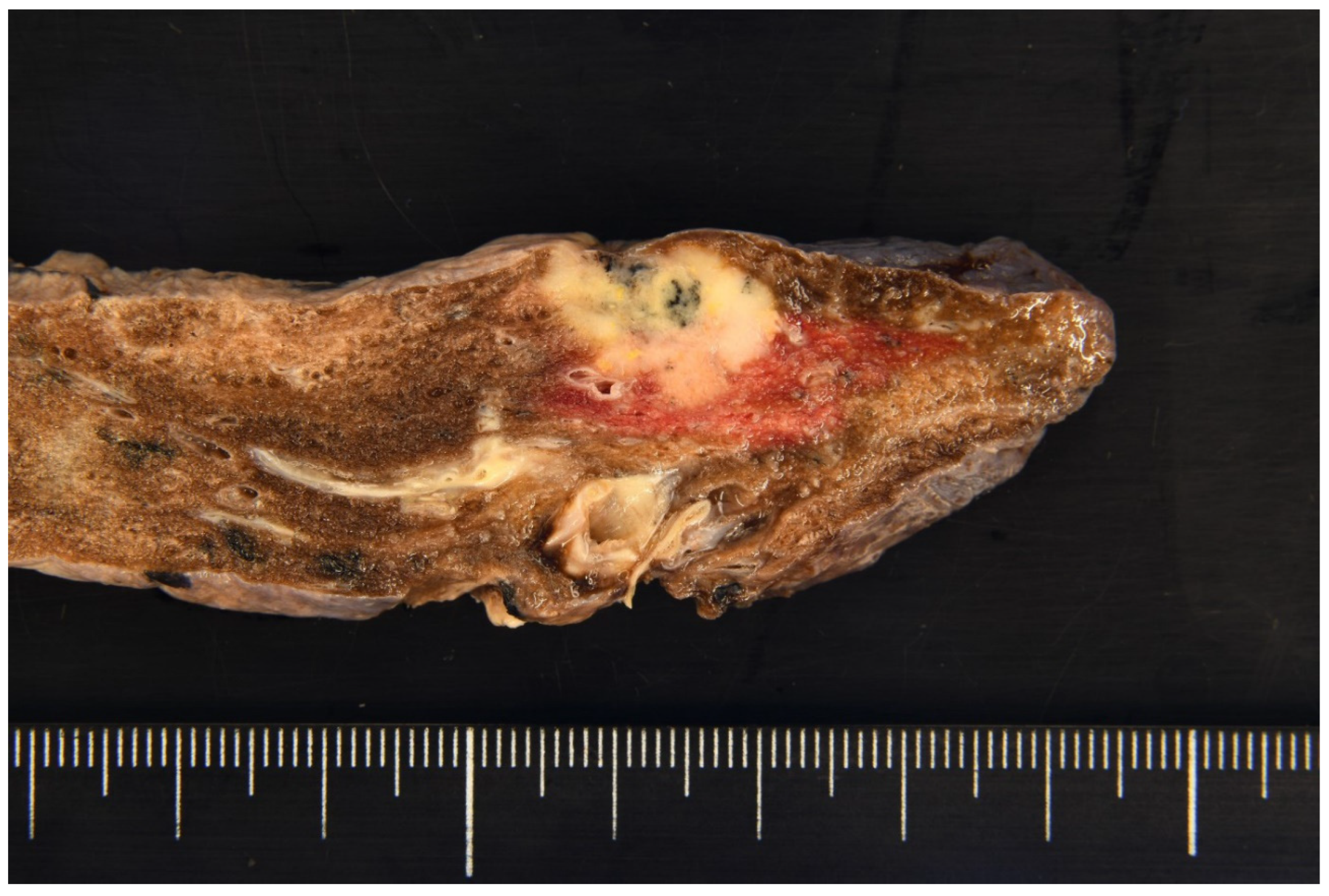

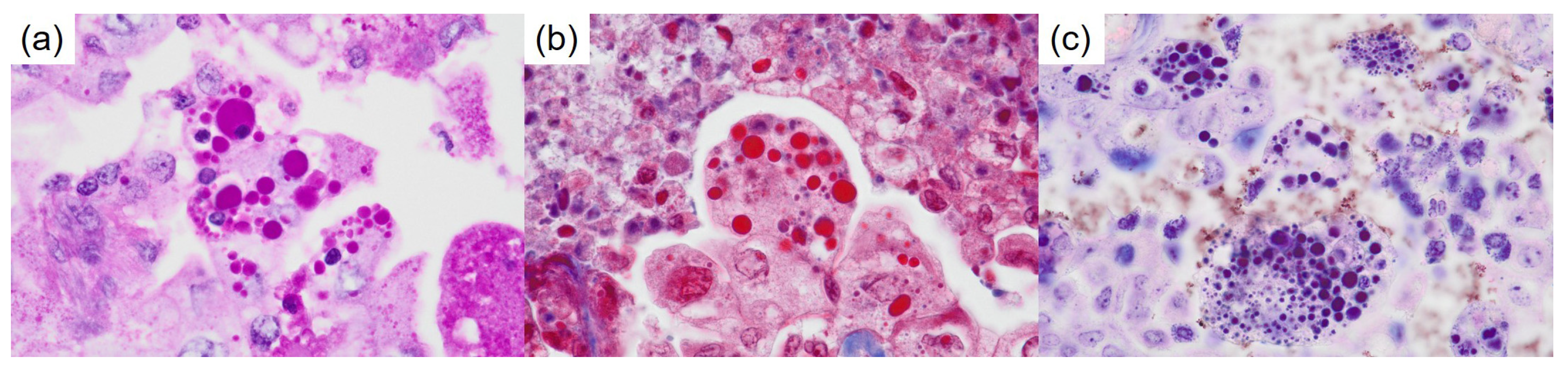
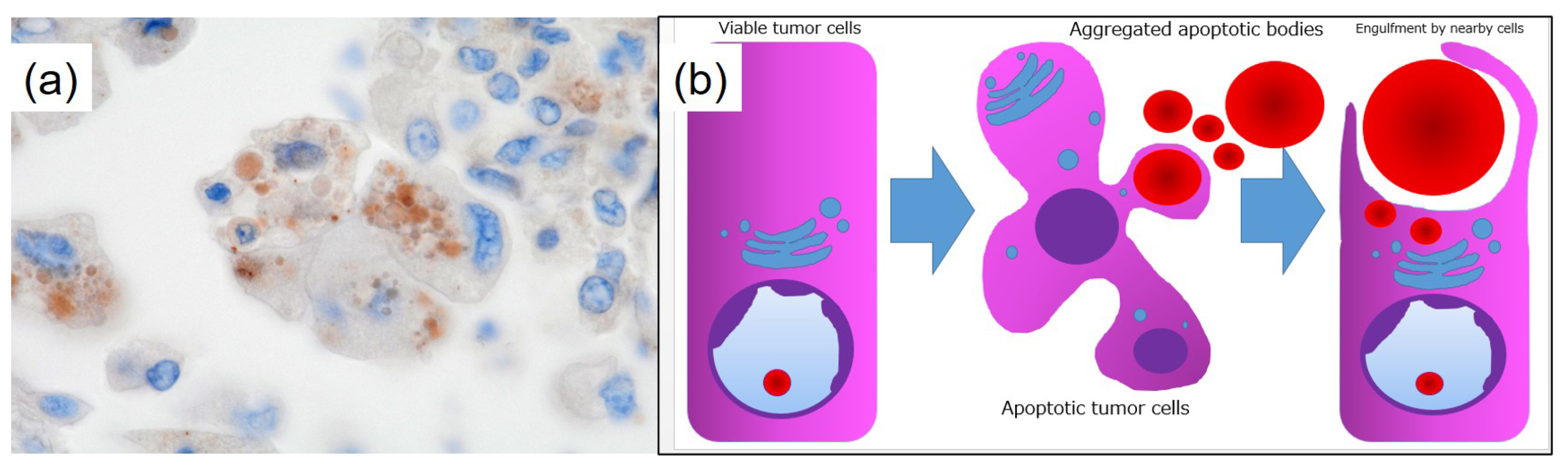
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| EGFR | epidermal growth factor receptor |
| KRAS | Kirsten rat sarcoma viral oncogene |
| HG | hyaline globule |
| TTF | thyroid transcription factor |
| AI | artificial intelligence |
| NCC | National Cancer Center |
| HE | Hematoxylin and eosin |
References
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagaratula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Takeuchi, K.; Togashi, Y.; Hatano, S.; Ninomiya, H.; Motoi, N.; Mun, M.Y.; Sakao, Y.; Okumura, S.; Nakagawa, K.; et al. EML-ALK lung cancers are characterized by rare other mutations, a TTF-1 lineage, an acinar histology, and young onset. Mod. Pathol. 2009, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Kohno, T.; Tsuta, K.; Wakai, S.; Arai, Y.; Shimada, Y.; Asamura, H.; Furuta, K.; Shibata, T.; Tsuda, H. ROS1-rearranged lung cancer: A clinicopathologic and molecular study of 15 surgical cases. Am. J. Surg. Pathol. 2013, 37, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Sumiyoshi, S.; Sonobe, M.; Kobayashi, M.; Fujimoto, M.; Kawakami, F.; Tsuruyama, T.; Travis, W.D.; Date, H.; Haga, H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J. Thorac. Oncol. 2013, 8, 52–61. [Google Scholar] [PubMed]
- Saito, M.; Shiraishi, K.; Kunitoh, H.; Takenoshita, S.; Yokota, J.; Kohno, T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016, 107, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Miyata-Morita, K.; Morita, S.; Matsutani, N.; Kondo, F.; Soejima, Y.; Sawabe, M. Frequent appearance of club cell (Clara cell)-like cells as a histological marker for ALK-positive lung adenocarcinoma. Pathol. Int. 2019, 69, 688–696. [Google Scholar] [PubMed]
- Hayashi, T.; Kohsaka, S.; Takamochi, K.; Kishikawa, S.; Ikarashi, D.; Sano, K.; Hara, K.; Onagi, H.; Suehara, Y.; Takahashi, F.; et al. Histological characteristics of lung adenocarcinoma with uncommon actionable alterations: Special emphasis on MET exon 14 skipping alterations. Histopathology 2021, 78, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Haigis, K.M. KRAS alleles: The devil is in the detail. Trends Cancer 2017, 3, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Tomizawa, K.; Mitsudomi, T. Biological and clinical significance of KRAS mutations in lung cancer: An oncogenic driver contrasting with EGFR mutation. Cancer Metastasis Rev. 2010, 29, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, D.; Ishikawa, S.; Oguni, S.; Aburatani, H.; Fukayama, M.; Niki, T. Co-activation of epidermal growth factor receptor and c-MET defines a distinct subset of lung adenocarcinomas. Am. J. Pathol. 2010, 177, 2191–2204. [Google Scholar] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS (G12C) inhibitor AMG510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar]
- Loong, H.H.F.; Du, N.; Cheng, C.; Lin, H.; Guo, J.; Lin, G.; Li, M.; Jiang, T.; Shi, Z.; Cui, Y.; et al. KRAS G12C mutations in Asia: A landscape analysis of 11,951 Chinese tumor samples. Transl. Lung Cancer Res. 2020, 9, 1759–1769. [Google Scholar] [PubMed]
- Lim, T.K.H.; Skoulids, F.; Kerr, K.M.; Ahn, M.J.; Kapp, J.R.; Soares, F.A.; Yatabe, Y. KRAS G12C in advanced NSCLC: Prevalence, co-mutations, and testing. Lung Cancer 2023, 184, 107293. [Google Scholar] [PubMed]
- Papadimitriou, J.C.; Drachenberg, C.B.; Brenner, D.S.; Newkirk, C.; Trump, B.F.; Silverberg, S.G. “Thanatosomes”: A unifying morphogenetic concept for tumor hyaline globules related to apoptosis. Hum. Pathol. 2000, 31, 1455–1465. [Google Scholar] [PubMed]
- Tachibana, M.; Koreyasu, R.; Kamimura, K.; Tsutsumi, Y. Pancreatic intraductal papillary mucinous neoplasm with hyaline globules (thanatosomes): Report of two cases. Int. Med. Case Rep. J. 2021, 14, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jung, A.W.; Torne, R.V.; Gonzalez, S.; Vöhringer, H.; Shmatko, A.; Yates, L.R.; Jimenez-Linan, M.; Moore, L.; Gerstung, M. Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis. Nat. Cancer 2020, 1, 800–810. [Google Scholar]
- Kather, J.N.; Heij, L.R.; Grabsch, H.I.; Loeffler, C.; Echle, A.; Muti, H.S.; Krause, J.; Niehues, J.M.; Sommer, K.A.J.; Bankhead, P.; et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat. Cancer 2020, 1, 789–799. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, M.; Ito, Y.; Fujikawa, R.; Tsukamoto, K.; Uehara, M.; Kobayashi, J.; Hayashi, T. Lung Adenocarcinoma Exhibiting Thanatosomes (Hyaline Globules), Cytoplasmic Clearing, and Nuclear Pleomorphism, with a KRAS Mutation. Diagnostics 2025, 15, 894. https://doi.org/10.3390/diagnostics15070894
Tachibana M, Ito Y, Fujikawa R, Tsukamoto K, Uehara M, Kobayashi J, Hayashi T. Lung Adenocarcinoma Exhibiting Thanatosomes (Hyaline Globules), Cytoplasmic Clearing, and Nuclear Pleomorphism, with a KRAS Mutation. Diagnostics. 2025; 15(7):894. https://doi.org/10.3390/diagnostics15070894
Chicago/Turabian StyleTachibana, Mitsuhiro, Yutaro Ito, Ryo Fujikawa, Kei Tsukamoto, Masahiro Uehara, Jun Kobayashi, and Takuo Hayashi. 2025. "Lung Adenocarcinoma Exhibiting Thanatosomes (Hyaline Globules), Cytoplasmic Clearing, and Nuclear Pleomorphism, with a KRAS Mutation" Diagnostics 15, no. 7: 894. https://doi.org/10.3390/diagnostics15070894
APA StyleTachibana, M., Ito, Y., Fujikawa, R., Tsukamoto, K., Uehara, M., Kobayashi, J., & Hayashi, T. (2025). Lung Adenocarcinoma Exhibiting Thanatosomes (Hyaline Globules), Cytoplasmic Clearing, and Nuclear Pleomorphism, with a KRAS Mutation. Diagnostics, 15(7), 894. https://doi.org/10.3390/diagnostics15070894




