CCTA-Guided Selective Invasive Coronary Catheterization: A Strategy to Reduce Contrast Volume and Improve Efficiency
Abstract
1. Introduction
2. Methods
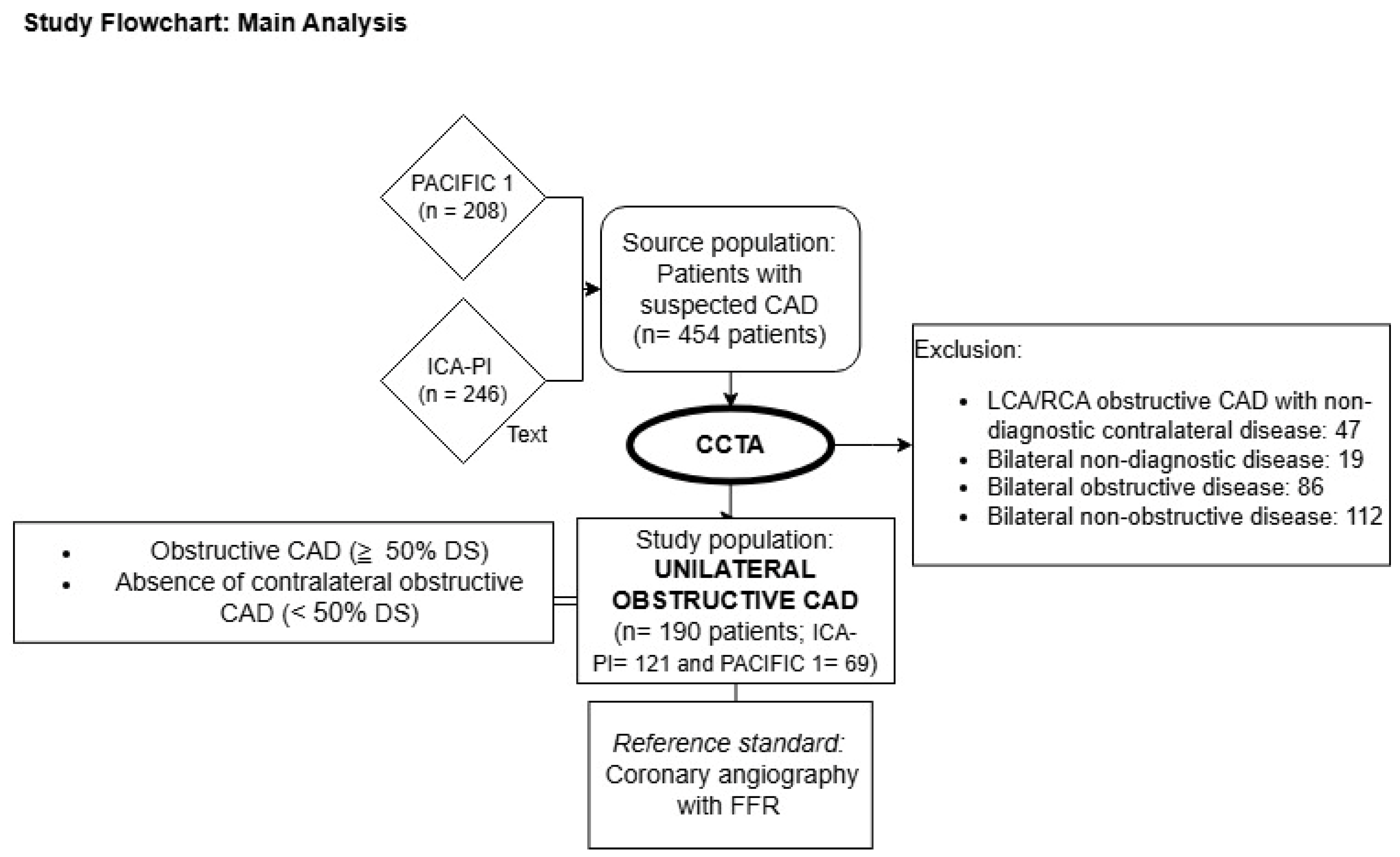
2.1. Design and Study Population
2.2. Coronary Computed Tomography Angiography
2.3. Invasive Coronary Angiography
2.4. Procedural Characteristics of the Unilateral ICA Visualization Strategy
2.5. Statistical Analysis
3. Results
3.1. CCTA’s Performance in Excluding Significant CAD
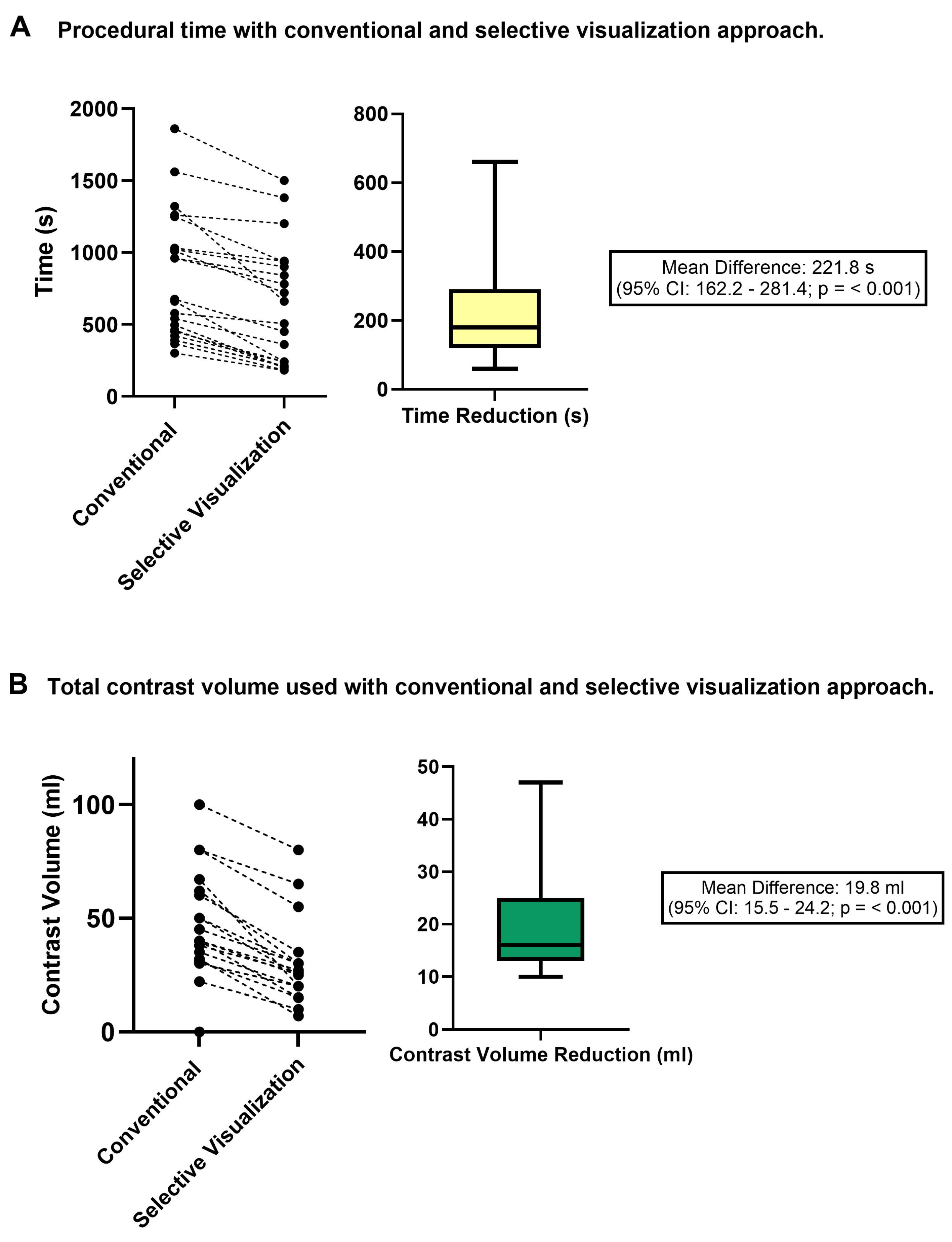
3.2. Procedural Time and Contrast Use Following a CCTA-Guided Unilateral Visualization Strategy
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary Artery Disease |
| ICA | Invasive Coronary Angiography |
| LCA | Left Coronary Artery |
| RCA | Right Coronary Artery |
| CCTA | Coronary Computed Tomography Angiography |
| DS | Diameter Stenosis |
| FFR | Fractional Flow Reserve |
| TN | True Negative |
| FN | False Negative |
| PCI | Coronary Percutaneous Intervention |
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Williams, M.C.; Newby, D.E.; Nicol, E.D. The Updated NICE Guidelines: Cardiac CT as the First-Line Test for Coronary Artery Disease. Curr. Cardiovasc. Imaging Rep. 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Federspiel, J.J.; Cowper, P.A.; Anstrom, K.J.; Hoffmann, U.; Patel, M.R.; Davidson-Ray, L.; Daniels, M.R.; Cooper, L.S.; Knight, J.D.; et al. Economic outcomes with anatomical versus functional diagnostic testing for coronary artery disease. Ann. Intern. Med. 2016, 165, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Jang, E.J.; Kim, Y.J.; Cha, M.J.; Park, S.Y.; Song, H.J.; Choi, J.E.; Shim, J.I.; Ahn, J.; Lee, H.J. Cost-effectiveness of coronary CT angiography in patients with chest pain: Comparison with myocardial single photon emission tomography. J. Cardiovasc. Comput. Tomogr. 2015, 9, 428–437. [Google Scholar] [CrossRef]
- van Beek, K.A.; de Lepper, A.G.; Van’t Veer, M.; Bouwmeester, S.; Otterspoor, L.C.; Tonino, P.A.; Lammers, J.; Winkens, M.; van Nunen, L.X. Accuracy and Potential Benefit of Ultraselective Invasive Coronary Angiography Guided by Computed Tomographic Coronary Angiography. J. Invasive Cardiol. 2022, 34, E390–E396. [Google Scholar] [CrossRef]
- Arendt, C.T.; Tischendorf, P.; Wichmann, J.L.; Messerli, M.; Jörg, L.; Ehl, N.; Gohmann, R.F.; Wildermuth, S.; Vogl, T.J.; Bauer, R.W. Using coronary CT angiography for guiding invasive coronary angiography: Potential role to reduce intraprocedural radiation exposure. Eur. Radiol. 2018, 28, 2756–2762. [Google Scholar] [CrossRef]
- World Health Organization. Environmentally Sustainable Health Systems: A Strategic Document; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Szirt, R.; Monjur, M.R.; McGovern, L.; Charlesworth, K.; O’Connor, S.; Weaver, J.C.; Coughlan, J.J. Environmental Sustainability in the Cardiac Catheter Laboratory. Heart Lung Circ. 2023, 32, 11–15. [Google Scholar] [CrossRef]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef]
- Foldyna, B.; Uhlig, J.; Mayrhofer, T.; Natale, L.; Vliegenthart, R.; Lotz, J.; Salgado, R.; Francone, M.; Nikolaou, K.; Bamberg, F.; et al. Rising utilization of coronary CT angiography across Europe over the last decade: Insights from a large prospective European registry. Eur. Heart J. 2021, 42, ehab724.0203. [Google Scholar] [CrossRef]
- Levin, D.C.; Parker, L.; Halpern, E.J.; Rao, V.M. Coronary CT Angiography: Reversal of Earlier Utilization Trends. J. Am. Coll. Radiol. 2019, 16, 147–155. [Google Scholar] [CrossRef]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijić, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.A.; Halpern, E.J.; Rao, V.M. Cardiac Imaging Trends from 2010 to 2019 in the Medicare Population. Radiol. Cardiothorac. Imaging 2021, 3, e210156. [Google Scholar] [CrossRef] [PubMed]
- Weir-McCall, J.R.; Williams, M.C.; Shah, A.S.; Roditi, G.; Rudd, J.H.; Newby, D.E.; Nicol, E.D. National Trends in Coronary Artery Disease Imaging. JACC Cardiovasc. Imaging 2023, 16, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.A.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; MacCarthy, P.A.; Van’t Veer, M.; Pijls, N.H. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron, D.J.; Stone, G.W.; Hochman, J.S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation 2020, 142, 841–857. [Google Scholar] [CrossRef]
- Al-Hijji, M.A.; Lennon, R.J.; Gulati, R.; El Sabbagh, A.; Park, J.Y.; Crusan, D.; Kanwar, A.; Behfar, A.; Lerman, A.; Holmes, D.R.; et al. Safety and Risk of Major Complications With Diagnostic Cardiac Catheterization. Circ. Cardiovasc. Interv. 2019, 12, e007791. [Google Scholar] [CrossRef]
- Tavakol, M.; Ashraf, S.; Brener, S.J. Risks and complications of coronary angiography: A comprehensive review. Glob. J. Health Sci. 2012, 4, 65–93. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef]
- Maioli, M.; Toso, A.; Gallopin, M.; Leoncini, M.; Tedeschi, D.; Micheletti, C.; Bellandi, F. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J. Cardiovasc. Med. 2010, 11, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Doshi, H.; Savage, M.P.; Ruggiero, N.J.; Walinsky, P.; Davis, M.; Troia, J.; Ahmed, B.; Fischman, D.L. Potentially recyclable material during cardiac catheterizations and coronary interventions: Curbing the carbon footprint in the cath lab. J. Am. Coll. Cardiol. 2023, 81, 2333. [Google Scholar] [CrossRef]
- Hill, K.D.; Einstein, A.J. New approaches to reduce radiation exposure. Trends Cardiovasc. Med. 2016, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Greco, M.D.; Gaita, F.; Picano, E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ. Cardiovasc. Interv. 2016, 9, e003273. [Google Scholar] [CrossRef]
- Ho, T.L.; Shieh, S.H.; Lin, C.L.; Shen, W.C.; Kao, C.H. Risk of cancer among cardiologists who frequently perform percutaneous coronary interventions: A population-based study. Eur. J. Clin. Investig. 2016, 46, 527–534. [Google Scholar] [CrossRef]
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Gutierrez-Barrios, A.; Cañadas-Pruaño, D.; Noval-Morillas, I.; Gheorghe, L.; Zayas-Rueda, R.; Calle-Perez, G. Radiation protection for the interventional cardiologist: Practical approach and innovations. World J. Cardiol. 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Klein, L.W.; Goldstein, J.A.; Haines, D.; Chambers, C.; Mehran, R.; Kort, S.; Valentine, C.M.; Cox, D. SCAI Multi-Society Position Statement on Occupational Health Hazards of the Catheterization Laboratory: Shifting the Paradigm for Healthcare Workers’ Protection. J. Am. Coll. Cardiol. 2020, 75, 1718–1724. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Age in years (mean ± SD) | 61 ± 9 |
| Male sex (%) | 129 (68%) |
| Diabetes mellitus (%) | 28 (15%) |
| Hypertension (%) | 91 (48%) |
| Hypercholesterolemia (%) | 69 (35%) |
| Current smoker (%) | 21 (11%) |
| Previous CAD (%) | 3 (1.6%) |
| BMI (mean ± SD) | 26.4 ± 4 |
| CCTA features | |
| Obstructive CAD side DS% | |
| 50–69% | 50 (26%) |
| 70–99% | 87 (46%) |
| 100% | 10 (5%) |
| Non-diagnostic | 43 (23%) |
| Non-obstructive CAD side DS% | |
| 0% | 64 (34%) |
| 1–24% | 84 (44%) |
| 25–49% | 42 (22%) |
| Absence of Hemodynamically Significant CAD | Hemodynamically Significant CAD | ||
|---|---|---|---|
| CCTA | Absence of Obstructive CAD (<50% DS) | 185 (97.4%, 95% CI: 94–99) | 5 (2.6%, 95% CI: 1–6) |
| True Negatives | False Negatives |
| True Negative (n = 185 Patients) | False Negative (n = 5 Patients) | p-Value | |
|---|---|---|---|
| Age in years (mean ± SD) | 60.5 ± 9 | 62.2 ± 12 | 0.69 |
| Male sex (%) | 122 (66%) | 5 (100%) | 0.11 |
| Diabetes mellitus (%) | 27 (32%) | 1 (20%) | 0.93 |
| Hypertension (%) | 87 (46%) | 4 (80%) | 0.35 |
| Hypercholesterolemia (%) | 61 (37%) | 4 (80%) | 0.72 |
| Smoking history (%) | 21 (11%) | 0 (0%) | 0.51 |
| Previous CAD (%) | 3 (2%) | 0 (0%) | 0.77 |
| BMI (mean ± SD) | 26 ± 4 | 29 ± 9 | 0.08 |
| CCTA characteristics | |||
| Artery with non-obstructive CAD | |||
| RCA (%) | 154 (83%) | 5 (100%) | 0.32 |
| LCA (%) | 31 (17%) | 0 (0%) | |
| Artery with non-obstructive CAD | |||
| 25–49 DS% | 41 (22%) | 1 (20%) | 0.19 |
| 1–24 DS% | 80 (43%) | 4 (80%) | |
| 0 DS% | 64 (35%) | 0 (0%) | |
| Invasive measurements | |||
| FFR (mean ± SD) | 0.94 ± 0.06 | 0.61 | <0.01 |
| Absence of Hemodynamically Significant CAD | Hemodynamically Significant CAD | ||
|---|---|---|---|
| CCTA | Absence of Obstructive CAD (0% DS) | 64 (100%, 95% CI: 94–100) | 0 (0%, 95% CI: 0–6) |
| True Negatives | False Negatives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahdal, J.; Jukema, R.; Somsen, A.G.; Kooijman, E.; Wahedi, E.; Lemkes, J.S.; Raijmakers, P.G.; Heestermans, T.; van Royen, N.; Knaapen, P.; et al. CCTA-Guided Selective Invasive Coronary Catheterization: A Strategy to Reduce Contrast Volume and Improve Efficiency. Diagnostics 2025, 15, 890. https://doi.org/10.3390/diagnostics15070890
Dahdal J, Jukema R, Somsen AG, Kooijman E, Wahedi E, Lemkes JS, Raijmakers PG, Heestermans T, van Royen N, Knaapen P, et al. CCTA-Guided Selective Invasive Coronary Catheterization: A Strategy to Reduce Contrast Volume and Improve Efficiency. Diagnostics. 2025; 15(7):890. https://doi.org/10.3390/diagnostics15070890
Chicago/Turabian StyleDahdal, Jorge, Ruurt Jukema, Aernout G. Somsen, Eline Kooijman, Ellaha Wahedi, Jorrit S. Lemkes, Pieter G. Raijmakers, Ton Heestermans, Niels van Royen, Paul Knaapen, and et al. 2025. "CCTA-Guided Selective Invasive Coronary Catheterization: A Strategy to Reduce Contrast Volume and Improve Efficiency" Diagnostics 15, no. 7: 890. https://doi.org/10.3390/diagnostics15070890
APA StyleDahdal, J., Jukema, R., Somsen, A. G., Kooijman, E., Wahedi, E., Lemkes, J. S., Raijmakers, P. G., Heestermans, T., van Royen, N., Knaapen, P., & Danad, I. (2025). CCTA-Guided Selective Invasive Coronary Catheterization: A Strategy to Reduce Contrast Volume and Improve Efficiency. Diagnostics, 15(7), 890. https://doi.org/10.3390/diagnostics15070890





