Contribution of the EEG in the Diagnostic Workup of Patients with Transient Neurological Deficit and Acute Confusional State at the Emergency Department: The EMINENCE Study
Abstract
Highlights
- An emergent EEG is often required in the differential diagnosis of transient neurological deficit (TND) and acute confusional state (ACS).
- An EEG is useful not only to confirm a diagnostic suspicion but also to help in ruling out initial working diagnoses.
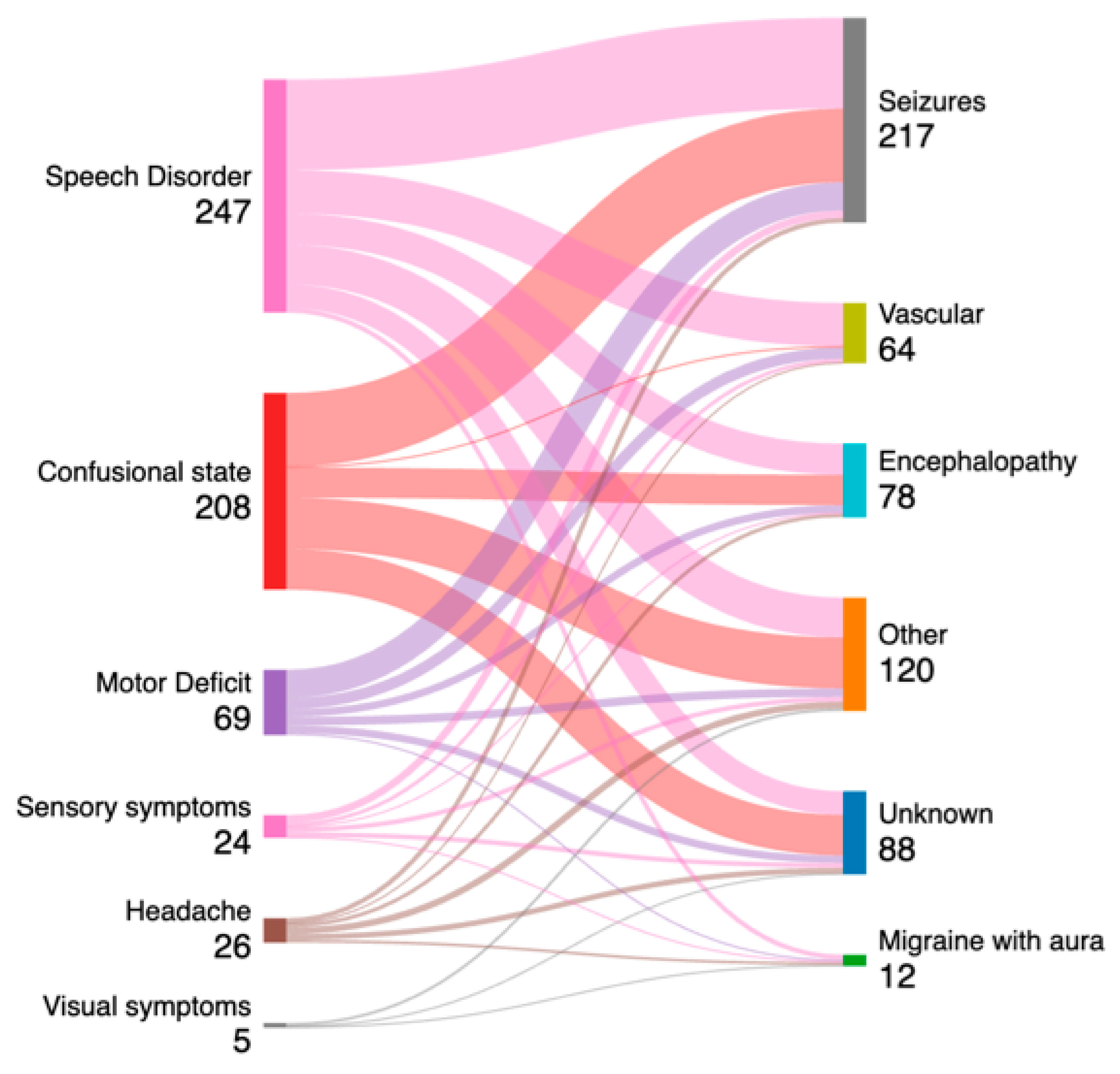
- The emergent EEG mainly contributes to a diagnosis when speech disorder, hyposthenia, and ACS are the admission signs/symptoms.
- The emergent EEG shows its greatest usefulness in the final diagnosis of seizures and encephalopathy.
Abstract
1. Introduction
2. Materials and Methods
2.1. The EMINENCE Study
2.2. EmEEG Recording and Classification
2.3. Final TND/ACS Classification
2.4. Decision-Making Criteria
2.5. Outcome Assessment
2.6. Statistical Analysis
2.7. Standard Protocol Approvals, Registrations, and Patient Consent
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bots, M.L.; van der Wilk, E.C.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Transient neurological attacks in general population. Prevalence, risk factors, and clinical relevance. Stroke 1997, 28, 768–773. [Google Scholar] [PubMed]
- Lozeron, P.; Tcheumeni, N.C.; Turki, S.; Amiel, H.; Meppiel, E.; Masmoudi, S.; Roos, C.; Crassard, I.; Plaisance, P.; Benbetka, H.; et al. Contribution of EEG in transient neurological deficits. J. Neurol. 2018, 265, 89–97. [Google Scholar] [PubMed]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S.; Kimchi, E.Y.; Cleinman, A.A. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [PubMed]
- Correia, M.; Fonseca, A.C.; Canhao, P. Short-term outcome of patients with possible transient ischemic attacks: A prospective study. BMC Neurol. 2015, 15, 78. [Google Scholar]
- Fonseca, A.C.; Canhão, P. Diagnostic difficulties in the classification of transient neurological attacks. Eur. J. Neurol. 2011, 18, 644–648. [Google Scholar]
- Kaplan, P.W. Focal seizures resembling transient ischemic attacks due to subclinical ischemia. Cerebrovasc. Dis. 1993, 3, 241–243. [Google Scholar]
- Fisher, C.M. Concerning recurrent transient cerebral ischemic attacks. Can. Med. Assoc. J. 1962, 86, 1091–1099. [Google Scholar]
- Fisher, C.M. Transient paralytic attacks of obscure nature: The question of non-convulsive seizures paralysis. Can. J. Neurol. Sci. 1978, 5, 267–273. [Google Scholar] [CrossRef]
- Primavera, A.; Giberti, L.; Cocito, L. Focal inhibitory seizures as the persisting sign of ischemic cerebrovascular disease. Ital. J. Neurol. Sci. 1993, 14, 381–384. [Google Scholar] [CrossRef]
- Lee, H.; Lerner, A. Transient inhibitory seizures mimicking crescendo TIAs. Neurology 1990, 40, 165–166. [Google Scholar] [CrossRef]
- Muraga, K. Limb-shaking TIA: Cortical myoclonus associated with ICA stenosis. Neurology 2016, 86, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Prud’hon, S.; Amiel, H.; Zanin, A.; Revue, E.; Kubis, N.; Lozeron, P. EEG and acute confusional state at the emergency department. Neurophysiol. Clin. 2024, 54, 102966. [Google Scholar] [PubMed]
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293. [Google Scholar] [PubMed]
- Amort, M.; Fluri, F.; Schäfer, J.; Weisskopf, F.; Katan, M.; Burow, A.; Bucher, H.C.; Bonati, L.H.; Lyrer, P.A.; Engelter, S.T. Transient ischemic attack versus transient ischemic attack mimics: Frequency, clinical characteristics and outcome. Cerebrovasc. Dis. 2011, 32, 57–64. [Google Scholar]
- Solenski, N.J. Transient ischemic attacks: Part II. Treatment. Am. Fam. Phys. 2004, 69, 1681–1688. [Google Scholar]
- Vellieux, G.; Amiel, H.; Roos, C.; Crassard, I.; Houdart, E.; Kubis, N.; Lozeron, P. Spectral analysis of EEG in etiological assessment of patients with transient neurological deficits. Neurophysiol. Clin. 2021, 51, 225–232. [Google Scholar] [CrossRef]
- Edlow, J.A.; Selim, M.H. Atypical presentations of acute cerebrovascular syndromes. Lancet Neurol. 2011, 10, 550–560. [Google Scholar] [CrossRef]
- Hemmen, T.M.; Meyer, B.C.; McClean, T.L.; Lyden, P.D. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J. Stroke Cerebrovasc. Dis. 2008, 17, 23–25. [Google Scholar]
- Floery, D.; Vosko, M.R.; Fellner, F.A.; Fellner, C.; Ginthoer, C.; Gruber, F.; Ransmayr, G.; Doerfler, A.; Uder, M.; Bradley, W.G. Acute-onset migrainous aura mimicking acute stroke: MR perfusion imaging features. AJNR Am. J. Neuroradiol. 2012, 33, 1546–1552. [Google Scholar]
- Wallis, W.E.; Donaldson, I.; Scott, R.S.; Wilson, J. Hypoglycemia masquerading as cerebrovascular disease (hypoglycemic hemiplegia). Ann. Neurol. 1985, 18, 510–512. [Google Scholar]
- Vilella, L.; Cuevas, M.G.; Luque, M.Q.; Toledo, M.; Gil, M.S.; Guzmán, L.; Puig, J.S.; Pérez, E.S. Prognosis of status epilepticus in elderly patients. Act. Neurol. Scand. 2018, 137, 321–328. [Google Scholar]
- Carreño, M. Recognition of nonepileptic events. Semin. Neurol. 2008, 28, 297–304. [Google Scholar] [PubMed]
- Tatemichi, T.K.; Young, W.L.; Prohovnik, I.; Gitelman, D.R.; Correll, J.W.; Mohr, J.P. Perfusion insufficiency in limb-shaking transient ischemic attacks. Stroke 1990, 21, 341–347. [Google Scholar]
- Kanich, W.; Brady, W.J.; Huff, J.S.; Perron, A.D.; Holstege, C.; Lindbeck, G.; Carter, C.T. Altered mental status: Evaluation and etiology in the ED. Am. J. Emerg. Med. 2002, 20, 613–617. [Google Scholar]
- Maldonado, J.R. Acute Brain Failure: Pathophysiology, Diagnosis, Management, and Sequelae of Delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar]
- Rosen, T.; Connors, S.; Clark, S.; Halpern, A.; Stern, M.E.; DeWald, J.; Lachs, M.S.; Flomenbaum, N. Assessment and Management of Delirium in Older Adults in the Emergency Department: Literature Review to Inform Development of a Novel Clinical Protocol. Adv. Emerg. Nurs. J. 2015, 37, 183–196. [Google Scholar]
- Nor, A.M.; Davis, J.; Sen, B.; Shipsey, D.; Louw, S.J.; Dyker, A.G.; Davis, M.; Ford, G.A. The Recognition of Stroke in the Emergency Room (ROSIER) scale: Development and validation of a stroke recognition instrument. Lancet Neurol. 2005, 4, 727–734. [Google Scholar]
- Krishnaswamy, A.; Klein, J.P.; Kapadia, S.R. Clinical cerebrovascular anatomy. Catheter. Cardiovasc. Interv. 2010, 75, 530–539. [Google Scholar]
- Moreau, F.; Asdaghi, N.; Modi, J.; Goyal, M.; Coutts, S.B. Magnetic resonance imaging versus computed tomography in transient ischemic attack and minor stroke: The more υou see the more you know. Cerebrovasc. Dis. Extra 2013, 3, 130–136. [Google Scholar]
- Souillard-Scemama, R.; Tisserand, M.; Calvet, D.; Jumadilova, D.; Lion, S.; Turc, G.; Edjlali, M.; Mellerio, C.; Lamy, C.; Naggara, O.; et al. An update on brain imaging in transient ischemic attack. J. Neuroradiol. 2015, 42, 3–11. [Google Scholar]
- Rodriguez Quintana, J.H.; Silvia Juliana Bueno, F.; Zuleta-Motta, J.L.; Ramos, M.F.; V’elez-van-Meerbeke, A.; the Neuroscience Research Group (NeuRos). Utility of Routine EEG in Emergency Department and Inpatient Service. Neurol. Clin. Pract. 2021, 11, e677–e681. [Google Scholar] [CrossRef] [PubMed]
- Praline, J.; Grujic, J.; Corcia, P.; Lucas, B.; Hommet, C.; Autret, A.; de Toffol, B. Emergent EEG in clinical practice. Clin. Neurophysiol. 2007, 118, 2149–2155. [Google Scholar] [PubMed]
- Scarpino, M.; Verna, M.T.; Grippo, A.; Lolli, F.; Piccardi, B.; Nazerian, P.; Nencini, P.; Boccardi, C.; Nencioni, A. The role of EEG in the Emergency Department: Its contribution to the patient’s diagnostic-therapeutic pathway. Eminence Study Clin. Neurophysiol. Pract. 2025, 10, 70–77. [Google Scholar]
- Scarpino, M.; Grippo, A.; Lanzo, G.; Lolli, F. The burden of clinical neurophysiology for the neurological prognosis of coma. Future Med. 2018, 13, 127–129. [Google Scholar]
- Scarpino, M.; Lolli, F.; Hakiki, B.; Atzori, T.; Lanzo, G.; Sterpu, R.; Portaccio, E.; Romoli, A.; Morocchesi, A.; Amantini, A.; et al. The prognostic value of the EEG, according to the American Clinical Neurophysiology Society terminology, recorded at the post-acute stage in patients with unresponsive wakefulness syndrome. Neurophysiol. Clin. 2019, 49, 317–327. [Google Scholar]
- Scarpino, M.; Lolli, F.; Hakiki, B.; Lanzo, G.; Sterpu, R.; Atzori, T.; Portaccio, E.; Draghi, F.; Amantini, A.; Grippo, A.; et al. for the Intensive Rehabilitation Unit Study Group of the IRCCS Don Gnocchi Foundation, Italy. EEG and Coma Recovery Scale-Revised prediction of neurological outcome in Disorder of Consciousness patients. Acta Neurol. Scand. 2020, 142, 221–228. [Google Scholar]
- Hirsch, L.J.; Fong, M.W.; Leitinger, M.; LaRoche, S.M.; Beniczky, S.; Abend, N.S.; Lee, J.W.; Wusthoff, C.J.; Hahn, C.D.; Westover, M.B.; et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J. Clin. Neurophysiol. 2013, 30, 1–27. [Google Scholar]
- Ad hoc Committee on Cerebrovascular Disease of National Institute of Neurological Diseases and Blindness (NIBDN). A classification and outline of cerebrovascular diseases II. Stroke 1975, 6, 564–616. [Google Scholar]
- Dudek, F.E.; Bertram, E.H. Counterpoint to “what is an epileptic seizure?” by D’Ambrosio and Miller. Epilepsy Curr. 2010, 10, 91–94. [Google Scholar]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar]
- Headache Classification Committee of the International Headache Society (HIS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar]
- Bentes, C.; Canhão, P.; Peralta, A.R.; Viana, P.; Fonseca, A.C.; Geraldes, R.; Pinho EMelo, T.; Paiva, T.; Ferro, J.M. Usefulness of EEG for the differential diagnosis of possible transient ischemic attack. Clin. Neurophysiol. Pract. 2017, 3, 11–19. [Google Scholar] [PubMed]
- Caplan, L. Transient global amnesia. In Handbook of Clinical Neurology; Vinken, P.J., Bruyn, G.W., Klawans, H.I., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 205–218. [Google Scholar]
- Hodges, J.R.; Warlow, C.P. Syndromes of transient amnesia: Towards a classification. A study of 153 cases. J. Neurol. Neurosurg. Psychiatry 1990, 53, 834–843. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR; American Psychiatric Publishing Inc.: Washington, DC, USA, 2000. [Google Scholar]
- Lee, S.Y.; Lee, S.J.; Kim, S.S.; Jun, H.S.; Oh, C.; Lin, C.; Phi, J.H. Post-traumatic Transient Neurological Dysfunction: A Proposal for Pathophysiology. J. Neurotrauma 2024, 41, e1695–e1707. [Google Scholar]
- Phi, J.H.; Lee, S.J.; Kang, H.S.; Kim, J.E.; Kim, S.K.; Cho, W.S.; Lee, S.Y. Postoperative Transient Neurologic Dysfunction: A Proposal for Pathophysiology. J. Clin. Neurol. 2018, 14, 393–400. [Google Scholar]
- Madkour, O.; Elwan, O.; Hamdy, H.; Elwan, H.; Abbas, A.; Taher, M.; Abdel-Kader, A. Transient ischemic attacks: Electrophysiological (conventional and topographic EEG) and radiological (CCT) evaluation. J. Neurol. Sci. 1993, 119, 8–17. [Google Scholar]
- Bellini, A.; Curti, D.G.; Cursi, M.; Cecchetti, G.; Agosta, F.; Fanelli, G.F.; Filippi, M. Predictors of seizure detection and EEG clinical impact in an Italian tertiary emergency department. J. Neurol. 2024, 271, 5137–5145. [Google Scholar] [CrossRef]
- Zawar, I.; Briskin, I.; Hantus, S. Risk factors that predict delayed seizure detection on continuous electroencephalogram (cEEG) in a large sample size of critically ill patients. Epilepsia Open 2022, 7, 131–143. [Google Scholar]
- Leitinger, M.; Beniczky, S.; Rohracher, A.; Gardella, E.; Kalss, G.; Qerama, E.; Höfler, J.; Lindberg-Larsen, A.H.; Kuchukhidze, G.; Dobesberger, J.; et al. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus—Approach to clinical application. Epilepsy Behav. 2015, 49, 158–163. [Google Scholar]
| Patients, n | 579 |
|---|---|
| Age year, median (IQR) | 73 (IQR 24) |
| Female gender, n (%) | 290 (49.2%) |
| Previous epileptic seizures | 124 (21.0%) |
| Unknown aetiology | 47 (8.1%) |
| Structural aetiology | 77 (13.3%) |
| Antiseizure medication | 106 (18.3%) |
| Under-dosed antiseizure medication | 22 (3.8%) |
| Fever | 65 (11.2%) |
| Sepsis | 76 (12.9%) |
| Metabolic disturbance | 39 (6.7%) |
| Electrolyte disturbance | 80 (13.8%) |
| Drug abuse | 13 (2.2%) |
| Previous neurological history | 388 (67.0%) |
| Stroke | 54 (9.1%) |
| Neurosurgery | 69 (13.9%) |
| Cardiac disorders | 114 (19.7%) |
| Diabetes | 105 (18.1%) |
| Dyslipidemia | 154 (26.6%) |
| Thyroid disease | 69 (11.9%) |
| Brain computer tomography | 558 (96.4%) |
| Recent brain computed tomography lesions | 90 (15.2%) |
| Lumbar puncture | 24 (3.8%) |
| Positive | 2 (8.3%) |
| Home discharge | 391 (67.5%) |
| Hospitalization | 185 (32.0%) |
| Hospitalization refused | 3 (0.5%) |
| Epileptic Discharges/ Seizures | Major Focal Waves | Major Bilateral Waves | Generalized Periodic Discharges with Triphasic Morphology (GPDTM) | |
|---|---|---|---|---|
| Cramer’s V | 0.12 | 0.14 | 0.13 | 0.07 |
| Initial Symptoms on Admission | ||||
| Speech Disorder (n = 247) | 42 (17.0%) | 117 (47.3%) | 117 (47.3%) | 3 (1.2%) |
| Acute Confusional State (n = 208) | 31 (14.9%) | 57 (27.4%) | 68 (32.6%) | 5 (2.4%) |
| Motor deficit (n = 69) | 16 (23.1%) | 30 (43.4%) | 30 (43.4%) | 1 (1.4%) |
| Headache (n = 26) | 1 (3.8%) | 8 (30.7%) | 3 (11.5%) | 1 (3.8%) |
| Sensory deficit (n = 24) | 1 (4.1%) | 6 (25.0%) | 3 (11.5%) | 0 (0.0%) |
| Visual disorder (n = 5) | 0 (0.0%) | 0 (0%) | 1 (20%) | 0 (0.0%) |
| Epileptic Discharges/ Seizures | Major Focal Waves | Major Bilateral Waves | Generalized Periodic Discharges with Triphasic Morphology (GPDTM) | |
|---|---|---|---|---|
| Cramer’s V | 0.46 | 0.25 | 0.15 | 0.14 |
| Final Diagnosis | ||||
| Seizures (n = 217) | 82 (37.7%) | 129 (59.4%) | 115 (52.9%) | 4 (1.8%) |
| Vascular Disease (n = 64) | 1 (1.5%) | 24 (37.5%) | 20 (31.2%) | 0 (0%) |
| Migraine with aura (n = 12) | 0 (0%) | 3 (25.0%) | 1 (8.3%) | 0 (0%) |
| Encephalopathies (n = 78) | 5 (6.4%) | 15 (19.2%) | 49 (62.8%) | 6 (7.6%) |
| Other (n = 120) | 1 (0.8%) | 31 (25.8%) | 37 (30.8%) | 0 (0.0%) |
| Unknown (n = 88) | 3 (3.4%) | 17 (19.3%) | 27 (30.6%) | 0 (0.0%) |
| Final Diagnosis | Seizures | Encephalopathies | Vascular Disease | Migraine with Aura | Other | Unknown |
|---|---|---|---|---|---|---|
| Initial Symptoms on Admission | ||||||
| Epileptic discharges/ Seizures | ||||||
| Speech Disorder (n = 247) | 38/96 | 2/33 | 1/46 | 0/5 | 1/42 | 0/25 |
| Acute Confusional State (n = 208) | 26/78 | 3/32 | 0/1 | 0/0 | 0/54 | 2/43 |
| Motor deficit (n = 69) | 15/30 | 0/8 | 0/12 | 0/2 | 0/9 | 1/8 |
| Major Focal Waves | ||||||
| Speech Disorder (n = 247) | 71/96 | 9/33 | 18/46 | 1/5 | 14/42 | 4/25 |
| Acute Confusional State (n = 208) | 35/78 | 4/32 | 0/1 | 0/0 | 8/55 | 10/43 |
| Motor Deficit (n = 69) | 17/30 | 2/8 | 4/12 | 1/2 | 3/9 | 3/8 |
| Major Bilateral Waves | ||||||
| Speech Disorder (n = 247) | 55/96 | 24/33 | 17/46 | 1/5 | 23/42 | 4/25 |
| Acute Confusional State (n = 208) | 40/78 | 22/32 | 2/2 | 0/0 | 10/55 | 19/43 |
| Motor deficit (n = 69) | 2/30 | 5/8 | 3/12 | 0/2 | 3/9 | 1/8 |
| Generalized Periodic Discharges with Triphasic Morphology (GPDTM) | ||||||
| Speech Disorder (n = 247) | 1/96 | 2/33 | 0/46 | 0/5 | 0/42 | 0/25 |
| Acute Confusional State (n = 208) | 3/78 | 2/32 | 0/1 | 0/0 | 0/54 | 4/43 |
| Motor Deficit (n = 69) | 0/30 | 1/8 | 0/12 | 0/2 | 0/9 | 0/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpino, M.; Grippo, A.; Verna, M.T.; Lolli, F.; Piccardi, B.; Nazerian, P.; Nencini, P.; Ielapi, C.; Nencioni, A. Contribution of the EEG in the Diagnostic Workup of Patients with Transient Neurological Deficit and Acute Confusional State at the Emergency Department: The EMINENCE Study. Diagnostics 2025, 15, 863. https://doi.org/10.3390/diagnostics15070863
Scarpino M, Grippo A, Verna MT, Lolli F, Piccardi B, Nazerian P, Nencini P, Ielapi C, Nencioni A. Contribution of the EEG in the Diagnostic Workup of Patients with Transient Neurological Deficit and Acute Confusional State at the Emergency Department: The EMINENCE Study. Diagnostics. 2025; 15(7):863. https://doi.org/10.3390/diagnostics15070863
Chicago/Turabian StyleScarpino, Maenia, Antonello Grippo, Maria Teresa Verna, Francesco Lolli, Benedetta Piccardi, Peiman Nazerian, Patrizia Nencini, Carmela Ielapi, and Andrea Nencioni. 2025. "Contribution of the EEG in the Diagnostic Workup of Patients with Transient Neurological Deficit and Acute Confusional State at the Emergency Department: The EMINENCE Study" Diagnostics 15, no. 7: 863. https://doi.org/10.3390/diagnostics15070863
APA StyleScarpino, M., Grippo, A., Verna, M. T., Lolli, F., Piccardi, B., Nazerian, P., Nencini, P., Ielapi, C., & Nencioni, A. (2025). Contribution of the EEG in the Diagnostic Workup of Patients with Transient Neurological Deficit and Acute Confusional State at the Emergency Department: The EMINENCE Study. Diagnostics, 15(7), 863. https://doi.org/10.3390/diagnostics15070863






