1. Introduction
Neurodevelopmental disorders, including learning disabilities (LDs), have traditionally been attributed to genetic predispositions [
1,
2]. However, increasing evidence suggests that environmental and immune-related factors also play a crucial role in shaping cognitive development [
3,
4,
5,
6,
7]. Maternal immune activation [
8,
9], stress-induced dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis [
10], and vitamin D deficiency contribute to persistent neuroinflammation, which in turn affects synaptic plasticity and neural connectivity [
3,
11,
12,
13,
14,
15,
16,
17].
Neuroinflammation is characterized by the chronic activation of microglia and astrocytes, leading to the excessive production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [
11]. This prolonged inflammatory state disrupts key neurodevelopmental processes, including synaptogenesis, myelination, and neurotransmission, which are essential for learning and memory functions. Elevated levels of IL-6, TNF-α, and C-reactive protein (CRP) have been documented in individuals with LDs, suggesting that immune dysregulation may contribute to cognitive impairments [
11,
18,
19,
20,
21].
The impact of neuroinflammation on learning and memory extends to various cognitive domains, including executive function, working memory, and processing speed [
22]. Chronic inflammation disrupts synaptic pruning, a process that refines neural circuits during early development. Overactivation of microglia leads to excessive synaptic elimination, resulting in weakened neural connectivity and reduced cognitive efficiency. Studies in both animal models and human populations have linked prolonged neuroinflammatory states to deficits in verbal reasoning, problem-solving, and reading comprehension [
22].
Although research suggests that neuroinflammation may contribute to LDs, this remains a hypothesis requiring further validation rather than a confirmed causal relationship. Neuroinflammatory mechanisms, driven by microglial and astrocytic activation, have been implicated in synaptic dysfunction, impaired neural connectivity, and cognitive deficits, which are also key features of LDs. While associations between elevated inflammatory markers (e.g., IL-6, TNF-α) and cognitive impairments have been observed, direct evidence establishing neuroinflammation as a primary cause of LDs is still limited. More longitudinal studies and mechanistic investigations are necessary to determine whether neuroinflammation is a driving factor or a secondary consequence of cognitive deficits.
LDs are heterogeneous neurodevelopmental disorders that affect approximately 5–15% of school-aged children. The core symptoms of LDs vary depending on the specific type of disorder. Dyslexia, the most common form, is characterized by persistent difficulties in reading, phonological processing, and spelling. Attention deficit hyperactivity disorder (ADHD) frequently coexists with LDs and is associated with inattention, hyperactivity, and executive function deficits, which can interfere with learning processes. Other LD subtypes, such as dyscalculia and dysgraphia, primarily affect mathematical reasoning and written expression, respectively. LDs are typically diagnosed between the ages of 6 and 10 when children begin formal education and persistent academic difficulties become evident. Understanding the underlying mechanisms of LDs, including potential neuroinflammatory contributions, is essential for developing targeted diagnostic approaches and interventions aimed at improving cognitive and learning outcomes.
1.1. EEG Biomarkers and Neuroinflammation
Electroencephalography (EEG) provides a functional assessment of brain activity, capturing frequency band patterns that reflect underlying neurophysiological states. These patterns arise from the synchronized neural firing of pyramidal neurons in the cerebral cortex and are shaped by the dynamic interplay between excitatory and inhibitory neural circuits. EEG serves as a non-invasive and cost-effective tool for detecting alterations in neural connectivity, processing efficiency, and neuroinflammation-related disruptions.
While traditional EEG frequency bands are based on adult norms, research has shown that children’s EEG band powers differ significantly, necessitating developmentally adjusted interpretations. In particular, the peak alpha frequency (PAF) is lower in children with learning disabilities (LDs) and ADHD compared to their typically developing peers. This indicates that using fixed EEG band definitions may overlook subtle but critical neurophysiological differences, highlighting the need for individualized and developmentally appropriate EEG analysis in pediatric populations [
19,
23].
1.2. Key EEG Markers Associated with Neuroinflammation and Learning Disabilities
Elevated theta (4–8 Hz) power reflects reduced cortical arousal and impaired cognitive processing, often observed in children with attention deficits and learning disabilities [
24,
25].
Reduced alpha power (developmentally shifted range) indicates disrupted inhibitory–excitatory balance and diminished neural synchronization. Since alpha frequency is lower in children, its reduction must be interpreted in the context of age-specific norms [
5].
Increased gamma (30–100 Hz) power may represent compensatory neural hyperactivity, often linked to synaptic dysfunction and neuroinflammatory processes. However, gamma power is also highly susceptible to EMG contamination, which must be carefully considered in interpretation [
26].
These patterns are thought to correlate with neuroinflammatory states, providing an accessible and objective tool for early detection of cognitive dysfunction in LDs. Further research highlights that EEG band power abnormalities reflect disruptions in neurotransmitter systems, particularly GABAergic and glutamatergic signaling, which are closely linked to neuroinflammatory processes. For example, excessive theta activity may indicate increased cortical excitability due to an imbalance between inhibitory and excitatory networks; reduced alpha power suggests deficiencies in cortical inhibition, which may be further exacerbated by neuroinflammation and neurodevelopmental disorders [
5,
27,
28].
1.3. Implications for EEG-Based LD Diagnosis
Given the developmental shifts in EEG frequency bands, it is critical to consider individualized EEG band definitions when analyzing children’s neural activity. Future studies should explore adaptive EEG frequency classification models, accounting for age-related changes and individual variability in peak alpha frequency to enhance diagnostic accuracy.
1.4. Machine Learning for EEG-Based Diagnosis
Artificial neural networks (ANNs) are machine learning models that process inputs through multiple layers to generate outputs based on activation functions. They are widely used in LD detection by identifying patterns without making assumptions about the dataset. The k-means algorithm groups data points into clusters by determining centroids, while support vector machines (SVMs) classify data by finding an optimal hyperplane in an N-dimensional space.
Research in Ref. [
1] applied ANNs, k-means, and fuzzy logic classifiers to EEG data, achieving accuracies of up to 89.6%. Similarly, Ref. [
29] utilized a multilayer perceptron (MLP) for dyslexia detection through brain wave analysis, reporting 86% accuracy. They combined ANN, SVM, and principal component analysis (PCA) to analyze event-related potentials (ERPs) with 78% accuracy. Other studies have achieved higher accuracy rates using ANNs on fMRI and DTI scans (94.8%), SVM on eye-tracking data (95.6%), handwriting analysis (77.6%), and psychometric tests (99%) [
30,
31,
32,
33].
1.5. Proposed ANN Framework for EEG-Based Neuroinflammation Detection
This study leverages artificial neural networks (ANNs) to classify EEG data, distinguishing between neurotypical and neuroinflammatory states. The proposed model is trained on a dataset comprising EEG recordings from children diagnosed with LDs, incorporating z-scored quantitative EEG (QEEG) band power data. The ANN framework includes the following:
Input Features: Theta, alpha, and gamma band power from 14 EEG channels.
Model Architecture: Multilayer perceptron with dropout layers to prevent overfitting.
Performance Metrics: Accuracy, F1-score, sensitivity, and specificity.
The trained ANN achieves an accuracy of 98.5%, outperforming traditional diagnostic methods [
1,
32,
34,
35]. Recent advancements in deep learning algorithms further enhance EEG classification accuracy. Convolutional neural networks (CNNs) and long short-term memory (LSTM) networks have shown promise in analyzing temporal dependencies in EEG signals. The incorporation of ensemble learning approaches, which combine multiple models for improved prediction, continues to refine the reliability of EEG-based neuroinflammation detection.
Convolutional neural networks (CNNs) have emerged as a powerful tool for EEG analysis, particularly due to their ability to automatically detect spatial and temporal patterns in brain activity. Unlike artificial neural networks (ANNs), which rely on manually extracted features such as spectral power, CNNs can process raw EEG signals or time-frequency representations (e.g., spectrograms, wavelet transforms), making them highly effective for complex classification tasks. CNN architectures typically consist of convolutional layers, which extract local patterns in frequency and time, followed by pooling layers that reduce dimensionality while preserving crucial features. The final fully connected layers perform classification based on the learned EEG representations. CNNs are particularly useful for identifying neurophysiological markers in EEG data, such as detecting neuroinflammatory patterns in learning disabilities (LDs). In this study, ANNs were applied to frequency bands to capture distinct EEG features associated with LD-related neural alterations. If not used directly, CNNs remain a promising avenue for future research, offering the potential to enhance EEG-based neuroinflammation detection through automated feature extraction and deep learning-based classification.
Research Objectives
This study aims to investigate the reliability of EEG biomarkers in detecting neuroinflammation in individuals with learning disabilities. It examines the characteristic EEG band power patterns, including theta, alpha, and gamma, that are associated with neuroinflammatory states in individuals with learning disabilities. Additionally, the study evaluates the accuracy of artificial neural networks (ANNs) in classifying EEG-based neuroinflammatory markers related to learning disabilities. Furthermore, it explores the potential of machine learning models to enhance the precision of EEG-based neuroinflammation detection compared to traditional diagnostic methods.
The present study utilized electroencephalography (EEG) to investigate the neuroinflammatory markers associated with learning disabilities (LDs). A total of 200 participants were recruited, including 100 children diagnosed with LDs and 100 typically developing children as a control group. EEG signals were recorded using a 14-channel setup, ensuring comprehensive coverage of cortical activity. The frequency band data, specifically focusing on theta (4–8 Hz), alpha (8–13 Hz), and gamma (30–100 Hz) power, were extracted and analyzed to identify characteristic neurophysiological patterns indicative of neuroinflammation.
To enhance diagnostic precision, an artificial neural network (ANN) model was employed for the classification of EEG-derived features. The ANN was trained on the processed EEG data, leveraging a supervised learning approach with cross-validation techniques to optimize performance and prevent overfitting. The results demonstrated a high classification accuracy, indicating the efficacy of machine learning models in distinguishing neurotypical individuals from those exhibiting neuroinflammatory states linked to LDs.
These findings underscore the potential of EEG-based biomarkers as a non-invasive and objective tool for detecting neuroinflammation in individuals with LDs. By integrating neurophysiological and computational methodologies, this study contributes to advancing precision diagnostics in neurodevelopmental research and provides a foundation for future investigations in relation to targeted interventions for LDs.
3. Results
This study aimed to develop a robust machine learning algorithm for classifying learning disabilities (LDs), specifically dyslexia, and evaluated its effectiveness through comprehensive analysis and direct feedback from families with dyslexic children. The dataset comprised a substantial 10,040 EEG recording sessions collected from 200 participants, who were evenly divided between those diagnosed with LDs and typically developing children, allowing for balanced and reliable model training.
The artificial neural networks (ANNs) utilized in this study demonstrated remarkable classification performance, underscoring their potential as powerful tools in neurodevelopmental diagnostics. The proposed preprocessing techniques, combined with the carefully designed neural network architecture, achieved a remarkable classification accuracy of 98.5%, accompanied by a minimal loss of 0.06. These results were further substantiated by rigorous validation through k-fold cross-validation, which maintained a high confidence level with a 95% confidence interval (CI). The achieved accuracy and low loss rate highlight a significant advancement in the detection of LD biomarkers using EEG data, pushing the boundaries of current diagnostic methodologies.
Moreover, the study explored the impact of preprocessing techniques, such as minimum–maximum scaling, on model performance. While this technique introduced a slight reduction in accuracy, from 98.5% to 98.33%, the model retained robust performance metrics, including an F1 score of 0.983 and an increased loss of 0.08 (as detailed in
Table 1 and
Table 2). These findings indicate that while preprocessing can slightly alter accuracy, the overall diagnostic capability of the ANN remains consistently high (
Supplementary File S1).
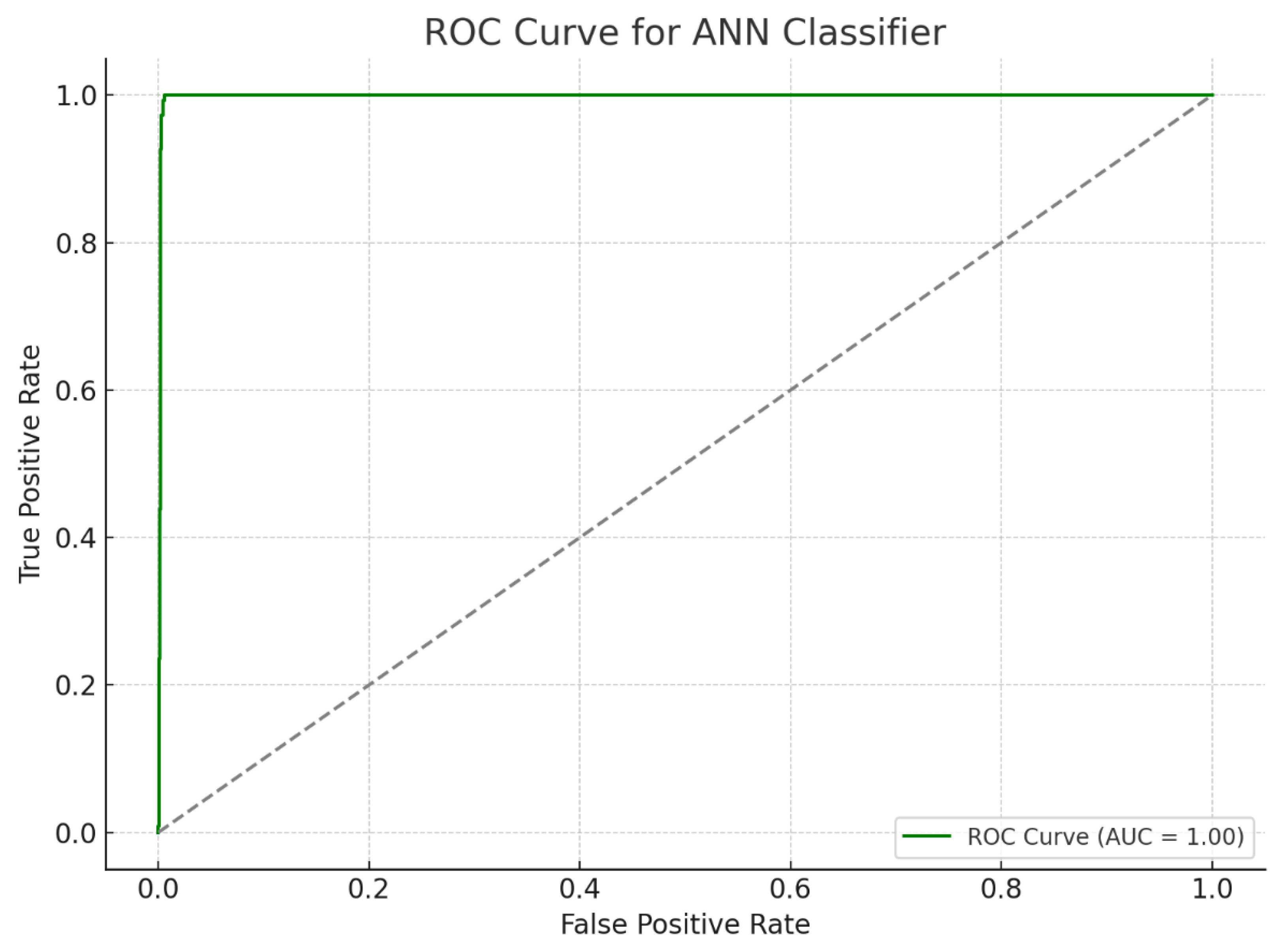
The receiver operating characteristic (ROC) curve, a critical metric for evaluating the sensitivity and specificity of diagnostic models, further demonstrated the ANN model’s efficacy. The area under the ROC curve (AUC) illustrated the model’s strong discriminatory power between dyslexic and typically developing children, affirming its clinical utility. The high sensitivity and specificity values confirm the ANN model’s capability to accurately distinguish between LD and non-LD cases, providing a reliable tool for early diagnosis and intervention planning (
Figure 4 and
Figure 5).
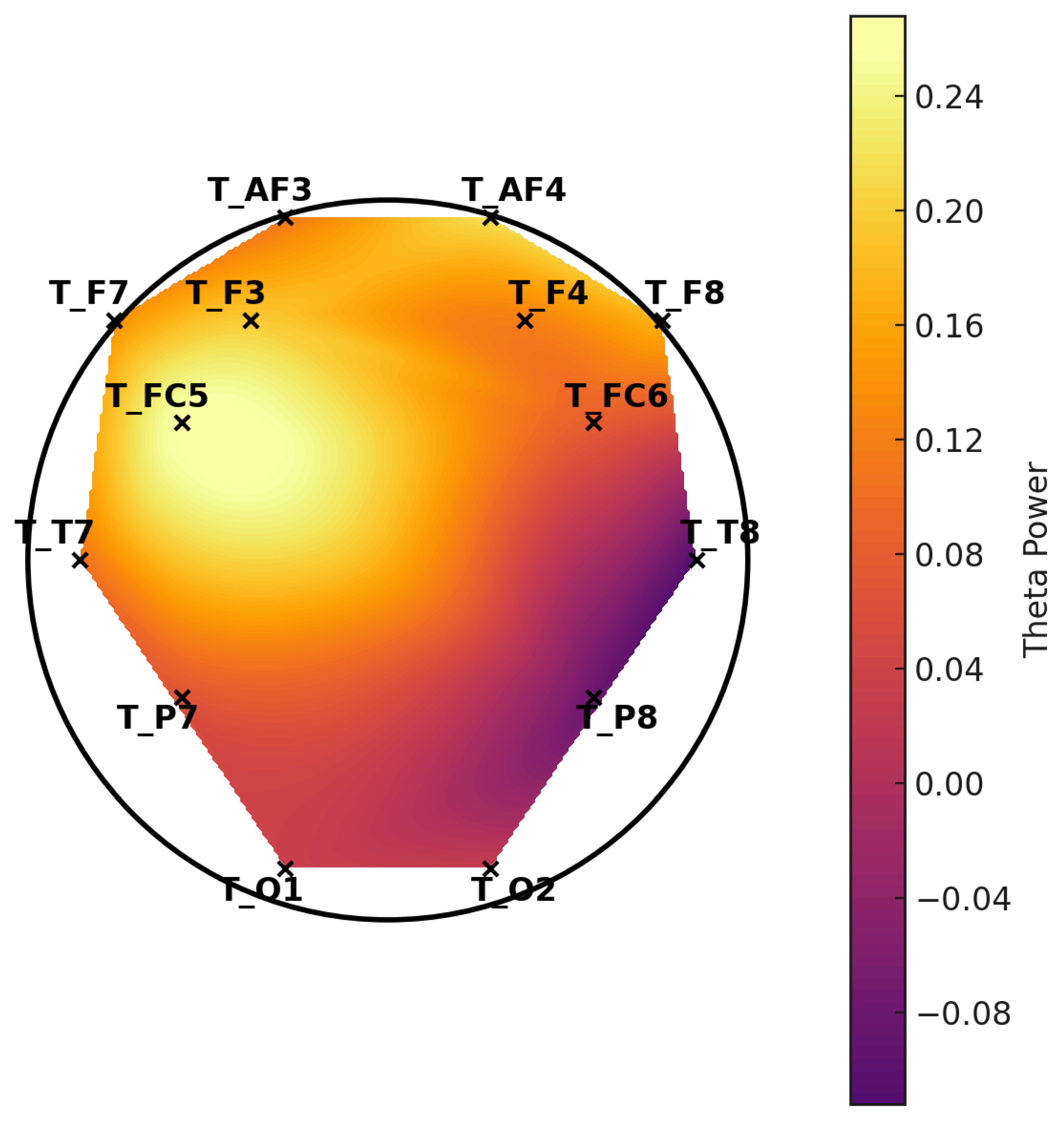
While increased beta and gamma power were observed in participants with LDs, it is important to note that these frequency bands are highly susceptible to EMG contamination. Given that EMOTIV EPOC-X does not provide independent EMG recordings or ICA-based correction, these findings should be interpreted with caution.
4. Discussion
The present study utilized electroencephalography (EEG) to examine potential associations between EEG alterations and neuroinflammation in children with learning disabilities (LDs). By integrating the Z-score normalization of 14-channel quantitative EEG (QEEG) data, we aimed to enhance classification accuracy in distinguishing children with LDs from typically developing controls. A total of 200 participants were recruited, including 100 children diagnosed with LDs and 100 typically developing children as a control group. EEG signals were recorded using a 14-channel setup, ensuring comprehensive coverage of cortical activity. The frequency band data, specifically focusing on theta (4–8 Hz), alpha (8–13 Hz), and gamma (30–100 Hz) power, were extracted and analyzed to identify characteristic neurophysiological patterns. While these EEG alterations may be associated with neuroinflammatory processes, further validation is required through multimodal biomarker analysis and longitudinal studies.
To improve diagnostic precision, an artificial neural network (ANN) model was employed for the classification of EEG-derived features. The ANN was trained on the processed QEEG data using a supervised learning approach with cross-validation techniques to optimize performance and prevent overfitting. The results demonstrated a classification accuracy of 98.5%, confirming the effectiveness of machine learning models in differentiating children with LDs from typically developing peers. However, this high classification accuracy should be interpreted with caution, as EEG alterations may stem from various neurodevelopmental factors beyond neuroinflammation, such as cognitive processing differences, developmental changes, and potential EMG contamination in higher-frequency bands. The novelty of this research lies in integrating ANN modeling with QEEG data and embedding the trained model into a mobile application, which enhances the accessibility of EEG-based assessments for clinical and research applications. While this study demonstrates that ANNs can achieve high precision in detecting EEG-based patterns related to LDs, further research is needed to validate these findings in larger and more diverse populations.
From an epigenetic perspective, LDs have been associated with alterations in gene expression due to environmental influences, including maternal stress, immune dysfunction, and neuroinflammation. Epigenetic modifications such as DNA methylation and histone modifications can influence neurodevelopmental pathways critical for cognitive processing. Studies suggest that maternal immune activation can trigger epigenetic changes, leading to altered neural connectivity and lateralization patterns in individuals with LDs [
33]. Our findings align with these perspectives, as QEEG data revealed distinct neural activation differences between children with LDs and typically developing children. The correlation patterns of theta, beta, and gamma signals between hemispheres suggest disruptions in left-hemisphere function, which is typically dominant for language processing. However, these findings do not establish a direct causal link between neuroinflammation and LDs, highlighting the need for future multimodal research.
Regarding neuroinflammation, accumulating evidence suggests that immune-mediated neuroinflammatory processes may play a role in neurodevelopmental disorders, including LDs. Chronic neuroinflammation, characterized by elevated pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), has been implicated in synaptic pruning disruptions and imbalances in excitatory and inhibitory neural activity [
7]. In our study, children with LDs exhibited lower correlations in alpha power at the right hemisphere with beta-1, beta-2, and gamma band activity at both hemispheres, suggesting potential disruptions in neural connectivity. These findings, however, do not definitively confirm that neuroinflammation is the primary cause of these EEG alterations, as alternative explanations such as cognitive compensation strategies, developmental variability, and methodological factors may also contribute. To strengthen the interpretation of these findings, future research should integrate inflammatory biomarker analysis (e.g., cytokine profiling) and neuroimaging modalities (e.g., fMRI, PET scans) to validate the potential role of neuroinflammation in LDs.
The high correlation among QEEG features necessitated careful preprocessing techniques to minimize noise and potential outliers. Despite these challenges, ANN models demonstrated exceptional classification performance, exceeding the accuracy rates reported in previous ML-based dyslexia detection studies. For instance, [
1] achieved 89.6% accuracy using ANNs with EEG data, while [
29] employed a multilayer perceptron, detecting dyslexia with 85% accuracy using resting-state EEG. Similarly, analyzed event-related potential (ERP) signals, achieving 78% accuracy with ANNs. More recently, the researchers in [
21] utilized convolutional neural networks (CNNs) with MRI scans, reaching 84.6% accuracy. These findings indicate that machine learning models can play a significant role in EEG-based classification tasks, but their generalizability must be further assessed.
To facilitate practical implementation, the trained ANN model was converted into a TensorFlow Lite (TFLITE) model and embedded into an Android and iOS mobile application. This advancement enables high-accuracy LD detection through a two-minute resting-state QEEG measurement, collected via a mobile app module (
Figure 6 and
Figure 7). The integration of ML-based neurophysiological analysis within a mobile platform represents a groundbreaking step toward accessible, non-invasive, and early detection of LDs. However, further research should examine the long-term reliability and reproducibility of EEG-based classification methods in real-world clinical settings.
Future Directions
Given the findings and limitations of this study, future research should (1) adopt individualized EEG frequency analysis, particularly for alpha peak frequency, to enhance the diagnostic accuracy of QEEG-based assessments; (2) integrate multimodal biomarkers, such as inflammatory cytokine profiling, fMRI, and PET imaging, to confirm the potential role of neuroinflammation in LDs; (3) use EEG systems with raw data access to enable independent component analysis (ICA) and advanced artifact rejection methods, improving the differentiation between neural signals and muscle-related noise; (4) implement EMG detection algorithms to ensure that changes in EEG power, particularly in beta and gamma bands, are not due to muscle artifacts or stress-induced tension; and (5) conduct longitudinal studies to track how EEG changes evolve over time in children with LDs, providing deeper insights into the neurodevelopmental trajectory of these disorders.
By incorporating these methodological improvements, future studies can enhance the specificity, accuracy, and clinical applicability of EEG-based biomarkers for LDs.
5. Conclusions
This study highlights the potential of EEG-based biomarkers in detecting neuroinflammation linked to learning disabilities. However, the findings should be interpreted with caution, given the limitations of fixed EEG band definitions and the need for additional validation through individualized EEG frequency analysis. The EEG-based assessment of neuroinflammation presents a transformative approach to diagnosing and managing neurological disorders, particularly learning disabilities (LDs). By leveraging advanced artificial neural network (ANN) modeling, our findings highlight the significant diagnostic accuracy achievable through neurophysiological biomarkers, reinforcing their role in precision medicine. These insights not only support the growing integration of EEG-based diagnostics into clinical practice but also pave the way for novel, non-invasive, and cost-effective screening tools.
As neuroimaging and machine learning technologies continue to evolve, their synergy will further refine early detection methodologies, allowing for timely interventions that can mitigate the long-term impact of neurodevelopmental disorders. Future research should focus on large-scale, longitudinal studies that incorporate multimodal biomarker integration, combining EEG with other neuroimaging techniques, genetic profiling, and biochemical assays. Such a holistic approach will enhance the robustness of diagnostic frameworks and inform personalized therapeutic strategies, ultimately improving patient outcomes.
The integration of artificial intelligence-driven models in neurological assessments holds immense potential for revolutionizing early diagnosis, intervention, and treatment monitoring. By addressing existing challenges such as data variability, model generalizability, and real-world applicability, the field can move towards developing standardized, clinically validated protocols. With continued advancements in computational neuroscience, EEG-based assessments could become a cornerstone of next-generation neurodiagnostics, contributing to a more precise and individualized approach to brain health management.
Study Limitations
One limitation of this study is the effect of brain maturation. As children develop, significant neurological changes occur, which may impact QEEG measurements over the six-month study period.