The Prognostic Value of Pulmonary Hypertension in Patients with Mitral Regurgitation Undergoing Mitral Valve Transcatheter Edge-to-Edge Repair: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Outcomes
2.4. Statistical Analysisz
3. Results
3.1. Search Results and Study Quality Evaluation
3.2. Baseline Characteristics
3.3. Early and Late Outcomes and Sensitivity Analysis
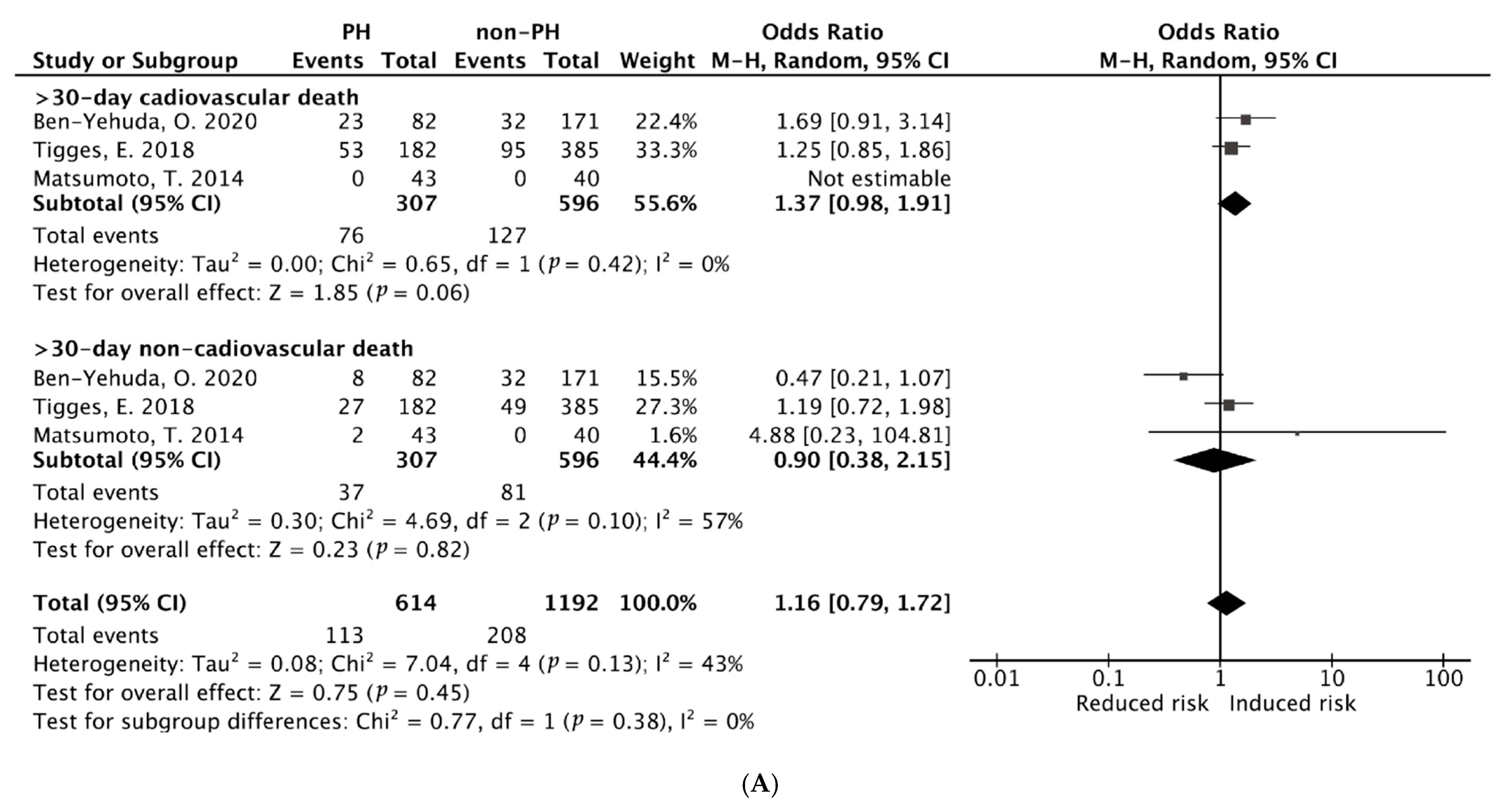
3.3.1. Pooled ORs of Early (30-Day) Cardiac and Non-Cardiac Mortality After M-TEER
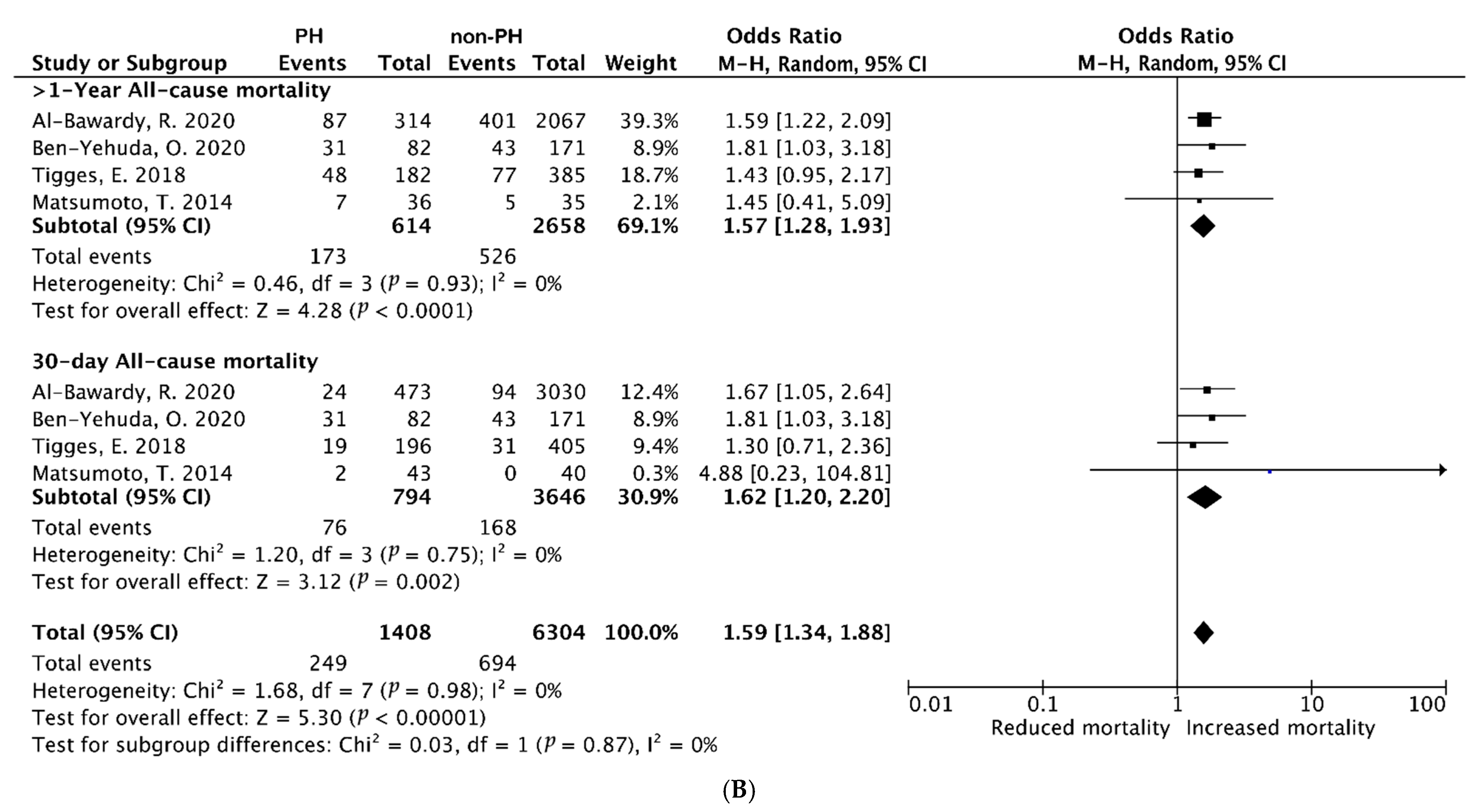
3.3.2. Pooled ORs of Early (30-Day) and Late (≥1-Year) All-Cause Mortality After PMVr
3.3.3. Echocardiographic Follow-Up: Pooled Estimated MD of SPAP After M-TEER Within 30 Days
3.3.4. Late (≥1-Year) HF Rehospitalization After TMVr (Univariate Analysis)
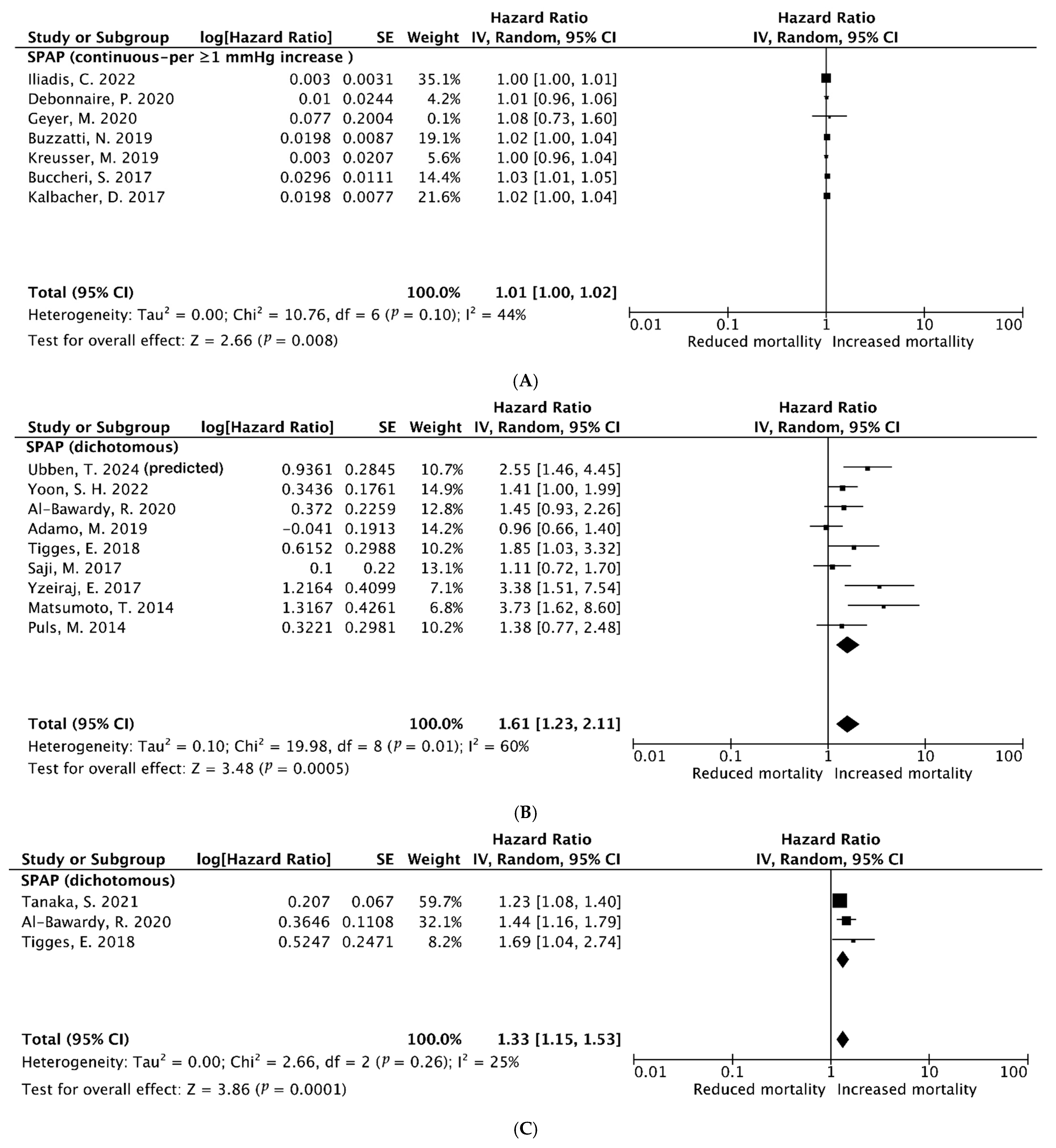
3.3.5. Late (≥1-Year) All-Cause Mortality After TMVr (Univariate Analysis and Multivariable Analysis)
3.3.6. Late (≥1-Year) Combined Outcome of HF Rehospitalization and All-Cause Mortality After M-TEER (Multivariable Analysis)
3.4. Publication Bias
4. Discussion
4.1. Principal Findings
4.2. Potential Mechanisms
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glower, D.D.; Kar, S.; Trento, A.; Lim, D.S.; Bajwa, T.; Quesada, R.; Whitlow, P.L.; Rinaldi, M.J.; Grayburn, P.; Mack, M.J.; et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: Results of the EVEREST II study. J. Am. Coll. Cardiol. 2014, 64, 172–181. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Cesnjevar, R.A.; Feyrer, R.; Walther, F.; Mahmoud, F.O.; Lindemann, Y.; von der Emde, J. High-risk mitral valve replacement in severe pulmonary hypertension—30 years experience. Eur. J. Cardiothorac. Surg. 1998, 13, 344–351, discussion 351–342. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 43, 3618–3731. [Google Scholar] [CrossRef]
- Cremer, P.C.; Guduguntla, V. Pulmonary Hypertension in Mitral Regurgitation: Silent, But Deadly. JACC Cardiovasc. Imaging 2024, 17, 1164–1167. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zahid, S.; Khan, M.U.; Kichloo, A.; Jamal, S.; Khan, A.M.; Ullah, W.; Sattar, Y.; Munir, M.B.; Balla, S. Comparison of In-Hospital Outcomes of Transcatheter Mitral Valve Repair in Patients with vs. Without Pulmonary Hypertension (From the National Inpatient Sample). Am. J. Cardiol. 2021, 153, 101–108. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Evans, C.F.; DeFilippi, C.R.; Hobbs, G.; Young, C.A.; Griffith, B.P.; Gammie, J.S. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: Implications for timing of surgery. J. Thorac. Cardiovasc. Surg. 2011, 142, 1439–1452. [Google Scholar] [CrossRef]
- Guha, A.; Amione-Guerra, J.; Park, M.H. Epidemiology of Pulmonary Hypertension in Left Heart Disease. Prog. Cardiovasc. Dis. 2016, 59, 3–10. [Google Scholar] [CrossRef]
- Franzen, O.; van der Heyden, J.; Baldus, S.; Schlüter, M.; Schillinger, W.; Butter, C.; Hoffmann, R.; Corti, R.; Pedrazzini, G.; Swaans, M.J.; et al. MitraClip® therapy in patients with end-stage systolic heart failure. Eur. J. Heart Fail. 2011, 13, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Palazzini, M.; Dardi, F.; Manes, A.; Bacchi Reggiani, M.L.; Gotti, E.; Rinaldi, A.; Albini, A.; Monti, E.; Galiè, N. Pulmonary hypertension due to left heart disease: Analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur. J. Heart Fail. 2018, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nakamura, M.; Yeow, W.L.; Hussaini, A.; Ram, V.; Makar, M.; Gurudevan, S.V.; Trento, A.; Siegel, R.J.; Kar, S. Impact of pulmonary hypertension on outcomes in patients with functional mitral regurgitation undergoing percutaneous edge-to-edge repair. Am. J. Cardiol. 2014, 114, 1735–1739. [Google Scholar] [CrossRef]
- Puls, M.; Tichelbäcker, T.; Bleckmann, A.; Hünlich, M.; von der Ehe, K.; Beuthner, B.E.; Rüter, K.; Beißbarth, T.; Seipelt, R.; Schöndube, F.; et al. Failure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClip. EuroIntervention 2014, 9, 1407–1417. [Google Scholar] [CrossRef]
- Buccheri, S.; Capodanno, D.; Barbanti, M.; Popolo Rubbio, A.; Di Salvo, M.E.; Scandura, S.; Mangiafico, S.; Ronsivalle, G.; Chiarandà, M.; Capranzano, P.; et al. A Risk Model for Prediction of 1-Year Mortality in Patients Undergoing MitraClip Implantation. Am. J. Cardiol. 2017, 119, 1443–1449. [Google Scholar] [CrossRef]
- Kalbacher, D.; Schäfer, U.; von Bardeleben, R.S.; Zuern, C.S.; Bekeredjian, R.; Ouarrak, T.; Sievert, H.; Nickenig, G.; Boekstegers, P.; Senges, J.; et al. Impact of tricuspid valve regurgitation in surgical high-risk patients undergoing MitraClip implantation: Results from the TRAMI registry. EuroIntervention 2017, 12, e1809–e1816. [Google Scholar] [CrossRef]
- Saji, M.; Katz, M.R.; Ailawadi, G.; Fowler, D.E.; Ragosta, M.; Lim, D.S. Predictive Value of Age-Adjusted Charlson Co-Morbidity Index for 1-, 3-, and 5-Year Mortality in Patients Requiring Transcatheter Mitral Valve Repair. Am. J. Cardiol. 2017, 120, 309–314. [Google Scholar] [CrossRef]
- Yzeiraj, E.; Bijuklic, K.; Tiburtius, C.; Witt, J.; Krause, K.; Steude, J.; Hansen, L.; Rieß, F.C.; Schofer, J. Tricuspid regurgitation is a predictor of mortality after percutaneous mitral valve edge-to-edge repair. EuroIntervention 2017, 12, e1817–e1824. [Google Scholar] [CrossRef]
- Adamo, M.; Grasso, C.; Capodanno, D.; Rubbio, A.P.; Scandura, S.; Giannini, C.; Fiorelli, F.; Fiorina, C.; Branca, L.; Brambilla, N.; et al. Five-year clinical outcomes after percutaneous edge-to-edge mitral valve repair: Insights from the multicenter GRASP-IT registry. Am. Heart J. 2019, 217, 32–41. [Google Scholar] [CrossRef]
- Buzzatti, N.; Denti, P.; Scarfò, I.S.; Giambuzzi, I.; Schiavi, D.; Ruggeri, S.; Castiglioni, A.; De Bonis, M.; La Canna, G.; Alfieri, O. Mid-term outcomes (up to 5 years) of percutaneous edge-to-edge mitral repair in the real-world according to regurgitation mechanism: A single-center experience. Catheter. Cardiovasc. Interv. 2019, 94, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kreusser, M.M.; Geis, N.A.; Berlin, N.; Greiner, S.; Pleger, S.T.; Bekeredjian, R.; Katus, H.A.; Raake, P.W. Invasive hemodynamics and cardiac biomarkers to predict outcomes after percutaneous edge-to-edge mitral valve repair in patients with severe heart failure. Clin. Res. Cardiol. 2019, 108, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, R.; Vemulapalli, S.; Thourani, V.H.; Mack, M.; Dai, D.; Stebbins, A.; Palacios, I.; Inglessis, I.; Sakhuja, R.; Ben-Assa, E.; et al. Association of Pulmonary Hypertension with Clinical Outcomes of Transcatheter Mitral Valve Repair. JAMA Cardiol. 2020, 5, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda, O.; Shahim, B.; Chen, S.; Liu, M.; Redfors, B.; Hahn, R.T.; Asch, F.M.; Weissman, N.J.; Medvedofsky, D.; Puri, R.; et al. Pulmonary Hypertension in Transcatheter Mitral Valve Repair for Secondary Mitral Regurgitation: The COAPT Trial. J. Am. Coll. Cardiol. 2020, 76, 2595–2606. [Google Scholar] [CrossRef]
- Debonnaire, P.; Heyning, C.M.V.; Haddad, M.E.; Coussement, P.; Paelinck, B.; de Ceuninck, M.; Timmermans, F.; De Bock, D.; Drieghe, B.; Dujardin, K.; et al. Left Ventricular End-Systolic Dimension and Outcome in Patients With Heart Failure Undergoing Percutaneous MitraClip Valve Repair for Secondary Mitral Regurgitation. Am. J. Cardiol. 2020, 126, 56–65. [Google Scholar] [CrossRef]
- Geyer, M.; Keller, K.; Born, S.; Bachmann, K.; Tamm, A.R.; Ruf, T.F.; Kreidel, F.; Hahad, O.; Ahoopai, M.; Hobohm, L.; et al. Predictors of short- and long-term outcomes of patients undergoing transcatheter mitral valve edge-to-edge repair. Catheter. Cardiovasc. Interv. 2020, 97, E390–E401. [Google Scholar] [CrossRef]
- Rashi, Y.; Haberman, D.; Tonchev, I.; Peretz, A.; Medvedovsky, A.T.; Gotsman, I.; Minha, S.; Poles, L.; Shimoni, S.; Goland, S.; et al. Pulmonary artery pressures and outcomes after MitraClip. ESC Heart Fail. 2020, 7, 4071–4079. [Google Scholar] [CrossRef]
- Tigges, E.; Blankenberg, S.; von Bardeleben, R.S.; Zürn, C.; Bekeredjian, R.; Ouarrak, T.; Sievert, H.; Nickenig, G.; Boekstegers, P.; Senges, J.; et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: Results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur. J. Heart Fail. 2018, 20, 585–594. [Google Scholar] [CrossRef]
- Iliadis, C.; Kalbacher, D.; Lurz, P.; Petrescu, A.M.; Orban, M.; Puscas, T.; Lupi, L.; Stazzoni, L.; Pires-Morais, G.; Koell, B.; et al. Left atrial volume index and outcome after transcatheter edge-to-edge valve repair for secondary mitral regurgitation. Eur. J. Heart Fail. 2022, 24, 1282–1292. [Google Scholar] [CrossRef]
- Yoon, S.H.; Makar, M.; Kar, S.; Koseki, K.; Oakley, L.; Sekhon, N.; Patel, D.; Chakravarty, T.; Nakamura, M.; Hamilton, M.; et al. Impact of Left Ventricular Global Longitudinal Strain on Outcomes After Transcatheter Edge-to-Edge Repair in Secondary Mitral Regurgitation. Am. J. Cardiol. 2022, 182, 69–76. [Google Scholar] [CrossRef]
- Tanaka, S.; Imamura, T.; Ushijima, R.; Fukuda, N.; Ueno, H.; Kinugawa, K. Risk Stratification of Percutaneous Edge-to-Edge Repair by MitraClip in Patients with Mitral Regurgitation. Int. Heart J. 2021, 62, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ubben, T.; Frerker, C.; Fujita, B.; Rosenkranz, S.; Pfister, R.; Baldus, S.; Alessandrini, H.; Kuck, K.H.; Willems, S.; Eitel, I.; et al. Association of pulmonary hypertension with the outcome in patients undergoing edge-to-edge mitral valve repair. Heart 2024, 110, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mandurino-Mirizzi, A.; Crimi, G.; Raineri, C.; Magrini, G.; Gazzoli, F.; Frassica, R.; Gritti, V.; Montalto, C.; Scelsi, L.; Turco, A.; et al. Haemodynamic impact of MitraClip in patients with functional mitral regurgitation and pulmonary hypertension. Eur. J. Clin. Investig. 2021, 51, e13676. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; D’Souza, S.; Sahni, T.; Yehya, A. Pulmonary hypertension secondary to valvular heart disease: A state-of-the-art review. Heart Fail. Rev. 2024, 29, 277–286. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Rossi, A.; Bergamini, C.; Benfari, G.; Maffeis, C.; Greco, C.; Drago, A.; Guazzi, M.; Ribichini, F.L.; Cicoira, M. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur. J. Heart Fail. 2020, 22, 499–506. [Google Scholar] [CrossRef]
- Neuss, M.; Schau, T.; Isotani, A.; Pilz, M.; Schöpp, M.; Butter, C. Elevated Mitral Valve Pressure Gradient After MitraClip Implantation Deteriorates Long-Term Outcome in Patients With Severe Mitral Regurgitation and Severe Heart Failure. JACC Cardiovasc. Interv. 2017, 10, 931–939. [Google Scholar] [CrossRef]
- Purga, S.L.; Karas, M.G.; Horn, E.M.; Torosoff, M.T. Contribution of the left atrial remodeling to the elevated pulmonary capillary wedge pressure in patients with WHO Group II pulmonary hypertension. J. Echocardiogr. 2019, 17, 187–196. [Google Scholar] [CrossRef]
- Ilonze, O.J.; Ebong, I.A.; Guglin, M.; Nair, A.; Rich, J.; McLaughlin, V.; Tedford, R.J.; Mazimba, S. Considerations in the Diagnosis and Management of Pulmonary Hypertension Associated with Left Heart Disease. JACC Heart Fail. 2024, 12, 1328–1342. [Google Scholar] [CrossRef]
- Testani, J.M.; St John Sutton, M.G.; Wiegers, S.E.; Khera, A.V.; Shannon, R.P.; Kirkpatrick, J.N. Accuracy of noninvasively determined pulmonary artery systolic pressure. Am. J. Cardiol. 2010, 105, 1192–1197. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, H.; Zhu, W.; Meng, X.; Lu, X. Evaluation of the hemodynamics and right ventricular function in pulmonary hypertension by echocardiography compared with right-sided heart catheterization. Exp. Ther. Med. 2017, 14, 3616–3622. [Google Scholar] [CrossRef]
- Baratto, C.; Caravita, S.; Vachiéry, J.L. Pulmonary Hypertension Associated with Left Heart Disease. Semin. Respir. Crit. Care Med. 2023, 44, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Bartko, P.E.; Heitzinger, G.; Pavo, N.; Heitzinger, M.; Spinka, G.; Prausmüller, S.; Arfsten, H.; Andreas, M.; Gabler, C.; Strunk, G.; et al. Burden, treatment use, and outcome of secondary mitral regurgitation across the spectrum of heart failure: Observational cohort study. Bmj 2021, 373, n1421. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.H.; Shah, S.J. Understanding the Pathobiology of Pulmonary Hypertension Due to Left Heart Disease. Circ. Res. 2022, 130, 1382–1403. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.; Colucci, W.S.; Givertz, M.M. Endothelin mediates increased pulmonary vascular tone in patients with heart failure: Demonstration by direct intrapulmonary infusion of sitaxsentan. Circulation 2002, 106, 1618–1621. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Hoendermis, E.S.; Liu, L.C.; Hummel, Y.M.; van der Meer, P.; de Boer, R.A.; Berger, R.M.; van Veldhuisen, D.J.; Voors, A.A. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur. Heart J. 2015, 36, 2565–2573. [Google Scholar] [CrossRef]
- Vachiéry, J.L.; Delcroix, M.; Al-Hiti, H.; Efficace, M.; Hutyra, M.; Lack, G.; Papadakis, K.; Rubin, L.J. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur. Respir. J. 2018, 51, 1701886. [Google Scholar] [CrossRef]
- Jiang, G.; Li, B.; Zhang, G.; Xu, E.; Liu, Y.; Xu, Z. Effects of sildenafil on prognosis in patients with pulmonary hypertension after left-sided valvular surgery. Heart Lung Circ. 2014, 23, 680–685. [Google Scholar] [CrossRef]
- Ram, E.; Sternik, L.; Klempfner, R.; Eldar, M.; Goldenberg, I.; Peled, Y.; Raanani, E.; Kogan, A. Sildenafil for Pulmonary Hypertension in the Early Postoperative Period After Mitral Valve Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1648–1656. [Google Scholar] [CrossRef]
- Bonow, R.O.; Carabello, B.; de Leon, A.C., Jr.; Edmunds, L.H., Jr.; Fedderly, B.J.; Freed, M.D.; Gaasch, W.H.; McKay, C.R.; Nishimura, R.A.; O’Gara, P.T.; et al. Guidelines for the management of patients with valvular heart disease: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998, 98, 1949–1984. [Google Scholar]
- Maisano, F.; Viganò, G.; Blasio, A.; Colombo, A.; Calabrese, C.; Alfieri, O. Surgical isolated edge-to-edge mitral valve repair without annuloplasty: Clinical proof of the principle for an endovascular approach. EuroIntervention 2006, 2, 181–186. [Google Scholar] [PubMed]
- Tempe, D.K.; Hasija, S.; Datt, V.; Tomar, A.S.; Virmani, S.; Banerjee, A.; Pande, B. Evaluation and Comparison of Early Hemodynamic Changes After Elective Mitral Valve Replacement in Patients with Severe and Mild Pulmonary Arterial Hypertension. J. Cardiothorac. Vasc. Anesth. 2009, 23, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Grill, D.E.; Borlaug, B.A. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Heart Fail. 2013, 1, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, R.R.; Kolwalkar, J.P.; Krishnajirao, S.P.; Narayan, M. A novel approach for the treatment of dysphagia lusoria. Eur. J. Cardiothorac. Surg. 2013, 43, 434–436, Erratum in Eur. J. Cardiothorac. Surg. 2019, 55, 1242. [Google Scholar] [CrossRef][Green Version]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Anker, S.D.; Friede, T.; von Bardeleben, R.S.; Butler, J.; Khan, M.S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure: Baseline characteristics of patients in the RESHAPE-HF2 trial and comparison to COAPT and MITRA-FR trials. Eur. J. Heart Fail. 2024, 26, 1608–1615. [Google Scholar] [CrossRef]


| A. Pooled Characteristics of the Patients in 6 Cohort Studies | |||||||||||||
| First Author | Year | Region | Study Design | Sample Size | Baseline Characteristics | ||||||||
| Age ± SD | Male (%) | No. of PH | MR Etiology (%) | SPAP ± SD | mPAP ± SD | Concomitant Lung/Cardiac Diseases | Follow-Up (Year) | Study Quality | |||||
| Ubben, T. [32] | 2024 | Germany | Retrospective cohort | 449 | 76.0 ± 8.4 | 61.7 | Yes: 200 | PMR: 27.6 | 51.0 ± 14.0 (SPAPe) | 25.0 ± 7.0 | COPD (18.9) Stroke (10.6) Hypertension (79.9) AF (70.6) CAD (63.9) | 2.8 | 7/8 |
| No: 249 | FMR: 61.0 | ||||||||||||
| Al-Bawardy, R. [23] | 2020 | United States | Retrospective cohort | 4071 | 81.0 ± 8.0 | 57.7 | Yes: 2968 | PMR: 73.4 | 48.4 ± 17.1 (overall) | 31.4 ± 11.1 (overall) | COPD (11.9) MI (24.7) Stroke (10.0) Hypertension (85.3) AF (62.0) CAD (6.9) | 3.5 | 8/8 |
| No: 1103 | FMR: 26.6 | ||||||||||||
| Ben-Yehuda, O. [24] | 2020 | United States & Canada | Prospective cohort | 253 | 72.9 ± 10.8 | 69.6 | Yes: 82 | PMR: 0.0 | 59.1 ± 8.8 | NA | COPD (22.3) MI (53.8) Stroke (0.3) Hypertension (80.4) AF (56.0) CAD (53.8) | 2 | 8/8 |
| No: 171 | FMR: 100.0 | ||||||||||||
| Rashi, Y [27]. | 2020 | Israel | Retrospective cohort | 177 | 74.8 ± 10.0 | 58.0 | Yes: 59 | PMR: 28.8 | 70.8 ± 9.2 | NA | COPD (23.7) HF (98.3) AF (45.9) CAD (57.6) | 1 | 8/8 |
| No: 118 | FMR: 71.2 | ||||||||||||
| Tigges, E. [28] | 2018 | Germany | Retrospective cohort | 643 | 75.7 ± 8.4 | 59.8 | Yes: 216 | PMR: 31.4 | 61.1 ± 8.2 | NA | COPD (23.6) MI (26.4) HF (21.8) Hypertension (78.7) CAD (76.4) | 1 | 7/8 |
| No: 427 | FMR: 68.6 | ||||||||||||
| Matsumoto, T. [14] | 2014 | United States | Retrospective cohort | 91 | 63.5 ± 9.0 | 61.5 | Yes: 48 | PMR: 0.0 | 63.5 ± 9.0 | NA | COPD (18.7) HF (29.7) MI (31.9) Hypertension (80.2) AF (18.7) CAD (62.6) | 3 | 8/8 |
| No: 43 | FMR: 100.0 | ||||||||||||
| B. Pooled characteristics of the patients in other 13 studies. | |||||||||||||
| First Author | Year | Region | Study Design | Sample Size | Baseline Charateristics | ||||||||
| All-Cause Death, n (%) | Age ± SD | Male (%) | Concomitant Lung/Cardiac Diseases (%) | MR Etiology (%) | Follow-Up (Year) | Study Quality | |||||||
| Yoon, S. H. [30] | 2022 | United States & Netherlands | Retrospective cohort | 380 | 2-year: 131 (31.0) | 71.0 ± 13.0 | 61.1 | COPD (6.1) MI (29.2) Stroke (7.1) Hypertension (82.9) AF (48.9) | DMR: 0 | 3 | 7/8 | ||
| FMR: 100 | |||||||||||||
| Iliadis, C. [29] | 2022 | Germany | Retrospective cohort | 1074 | 2-year: 289 (36.0) | 75.0 ± 5.0 | 66.0 | COPD (18) MI (39) Stroke (9) AF (59) | DMR: 0 | 5 | 6/8 | ||
| FMR: 100 | |||||||||||||
| Tanaka, S. [31] | 2021 | Japan | Retrospective cohort | 25 | 1-year: 4 (15.4) | 87.4 ± 11.0 | 56.0 | AF (36) | DMR: 16.0 | 1 | 6/8 | ||
| FMR: 84.0 | |||||||||||||
| Debonnaire, P. [25] | 2020 | Belgium | Prospective cohort | 107 | 1.5-year: 49 (19.0) | 73.0 ± 10.0 | 70.1 | CAD (77) MI (61) Stroke (12) AF (44) | DMR: 0 | 3 | 7/8 | ||
| FMR: 100 | |||||||||||||
| Geyer, M. [26] | 2020 | Germany | Retrospective cohort | 461 | 1.5-year: 112 (24.3) | 78.6 ± 7.3 | 53.0 | COPD (12.9) MI (22.9) Stroke (10.6) Hypertension (81.9) AF (48.9) CAD (60.9) | DMR: 42.6 | 1 | 8/8 | ||
| FMR: 57.4 | |||||||||||||
| Kreusser, M. [22] | 2019 | Germany | Retrospective cohort | 174 | year:31 (17.8) | 75.2 ± 11.9 | 69.5 | COPD (19) Stroke (13.8) Hypertension (56.9) AF (36.2) CAD (57.5) | DMR:20.1 | 1 | 7/8 | ||
| FMR: 79.9 | |||||||||||||
| Adamo, M. [20] | 2019 | Italy | Prospective cohort | 304 | Year: (15.1) 3-year: (35.5) 5-year: (47.3) | 72.0 ± 10.0 | 63.8 | COPD (21.7) HF (65.8) Hypertension (67.4) AF (41.2) CAD (54.3) | DMR:22.5 | 5 | 7/8 | ||
| FMR: 77.5 | |||||||||||||
| Buzzatti, N. [21] | 2019 | Italy | Prospective cohort | 339 | 5-year: (53.5) | 72.0 ± 10.3 | 74.0 | COPD (30.9) HF (11.9) Hypertension (81.9) AF (39.7) CAD (57.5) | DMR:27.5 | 5 | 7/8 | ||
| FMR: 68.6 | |||||||||||||
| Kalbacher, D. [17] | 2017 | Germany | Retrospective cohort | 766 | year:154 (20.1) | 75.3 ± 8.5 | 61.0 | COPD (26.4) MI (26.4) Stroke (3.8) Hypertension (79.8) AF (55.7) HF (62.3) | DMR:29.8 | 1 | 8/8 | ||
| FMR: 70.2 | |||||||||||||
| Buccheri, S. [16] | 2017 | Italy | Prospective cohort | 311 | Year: (16.5) | 72.6 ± 9.9 | 59.8 | COPD (21.9) Stroke (10.6) Hypertension (78.1) AF (40.5) | DMR:22.2 | 1 | 7/8 | ||
| FMR: 77.8 | |||||||||||||
| Saji, M. [18] | 2017 | United States | Retrospective cohort | 222 | Year: 36 (15.1) 3-year: 38 (35.5) 5-year:33 (47.3) | 77. ± 11.1 | 49.5 | COPD (31.9) MI (31.5) Stroke (16.2) Hypertension (77.0) AF (62.1) CAD (62.1) | DMR: 69.4 | 5 | 7/8 | ||
| FMR: 30.6 | |||||||||||||
| Yzeiraj, E. [19] | 2017 | Germany | Retrospective cohort | 139 | 1.5-Year: 35 (25.2) | 76.4 ± 7.5 | 61.2 | COPD (17.3) MI (45.3) Stroke (10.1) Hypertension (81.9) AF (67.6) CAD (61.2) | DMR: 15.8 | 2 | 7/8 | ||
| FMR: 84.2 | |||||||||||||
| Puls, M. [15] | 2014 | Germany | Retrospective cohort | 150 | 1-Year:(21.5) | 74.4 ± 9.3 | 63.0 | COPD (22.0) Stroke (8.0) Hypertension (81.9) AF (66.0) HF (45.0) | DMR: 35.0 | 1 | 7/8 | ||
| FMR: 65.0 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shancuoji; Liao, Y.; Li, J.; Chen, M. The Prognostic Value of Pulmonary Hypertension in Patients with Mitral Regurgitation Undergoing Mitral Valve Transcatheter Edge-to-Edge Repair: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 852. https://doi.org/10.3390/diagnostics15070852
Shancuoji, Liao Y, Li J, Chen M. The Prognostic Value of Pulmonary Hypertension in Patients with Mitral Regurgitation Undergoing Mitral Valve Transcatheter Edge-to-Edge Repair: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(7):852. https://doi.org/10.3390/diagnostics15070852
Chicago/Turabian StyleShancuoji, Yanbiao Liao, Junli Li, and Mao Chen. 2025. "The Prognostic Value of Pulmonary Hypertension in Patients with Mitral Regurgitation Undergoing Mitral Valve Transcatheter Edge-to-Edge Repair: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 7: 852. https://doi.org/10.3390/diagnostics15070852
APA StyleShancuoji, Liao, Y., Li, J., & Chen, M. (2025). The Prognostic Value of Pulmonary Hypertension in Patients with Mitral Regurgitation Undergoing Mitral Valve Transcatheter Edge-to-Edge Repair: A Systematic Review and Meta-Analysis. Diagnostics, 15(7), 852. https://doi.org/10.3390/diagnostics15070852







