Postoperative Delirium and Cognitive Dysfunction After Cardiac Surgery: The Role of Inflammation and Clinical Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Inclusion/Exclusion Criteria
- Pre-existing Neurological and Psychiatric Conditions: History of neurodegenerative diseases (e.g., Parkinson’s disease or Alzheimer’s disease), cerebrovascular disease (e.g., stroke or transient ischemic attack), pre-existing diagnosis of dementia or cognitive impairment, or major psychiatric disorders (e.g., schizophrenia, bipolar disorder, or major depressive disorder) requiring ongoing psychotropic medication.
- Active Systemic Illness: Active sepsis, acute or chronic inflammatory diseases requiring immunosuppressive therapy (e.g., rheumatoid arthritis or inflammatory bowel disease), end-stage renal disease requiring dialysis, Child-Pugh Class C liver cirrhosis, or pre-existing hematological malignancies or severe coagulopathies. Furthermore, patients who exhibited elevated inflammatory marker values before surgery were not included in the study.
- Sensory and Substance Use Disorders: Severe vision or hearing impairment that would preclude accurate cognitive assessment (vision impairment uncorrectable to 20/200 in the better eye or hearing impairment requiring hearing aids and still unable to understand conversational speech) or history of chronic alcohol abuse or dependence.
- Perioperative Physiological Instability: Intraoperative mean arterial pressure (MAP) variations greater than 20% of baseline or MAP < 50 mmHg during cardiopulmonary bypass (CPB), preoperative or intraoperative hematocrit <20%, intraoperative hypothermia below 32 °C, or postoperative benzodiazepine administration.
- Pre-existing Cognitive Impairment (Based on Screening): Preoperative cognitive impairment as indicated by a score below 23 points on the Mini-Mental State Exam (MMSE) or below 4 points on the Mini-Cog test. This stricter Mini-Cog cutoff (scores of 3 or less usually indicate dementia) was chosen to conservatively exclude even mild pre-existing cognitive deficits that could confound POCD assessment [25].
2.3. Surgical Procedures and Anesthesia
2.4. Postoperative Care and Biomarker Measurements
2.5. Postoperative Delirium and Postoperative Cognitive Dysfunction (POD/POCD) Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Postoperative Inflammatory Marker Changes in the Entire Cohort
3.3. Comparison of Clinical, Surgical, and Biomarker Variables Between Patients With and Without POD/POCD
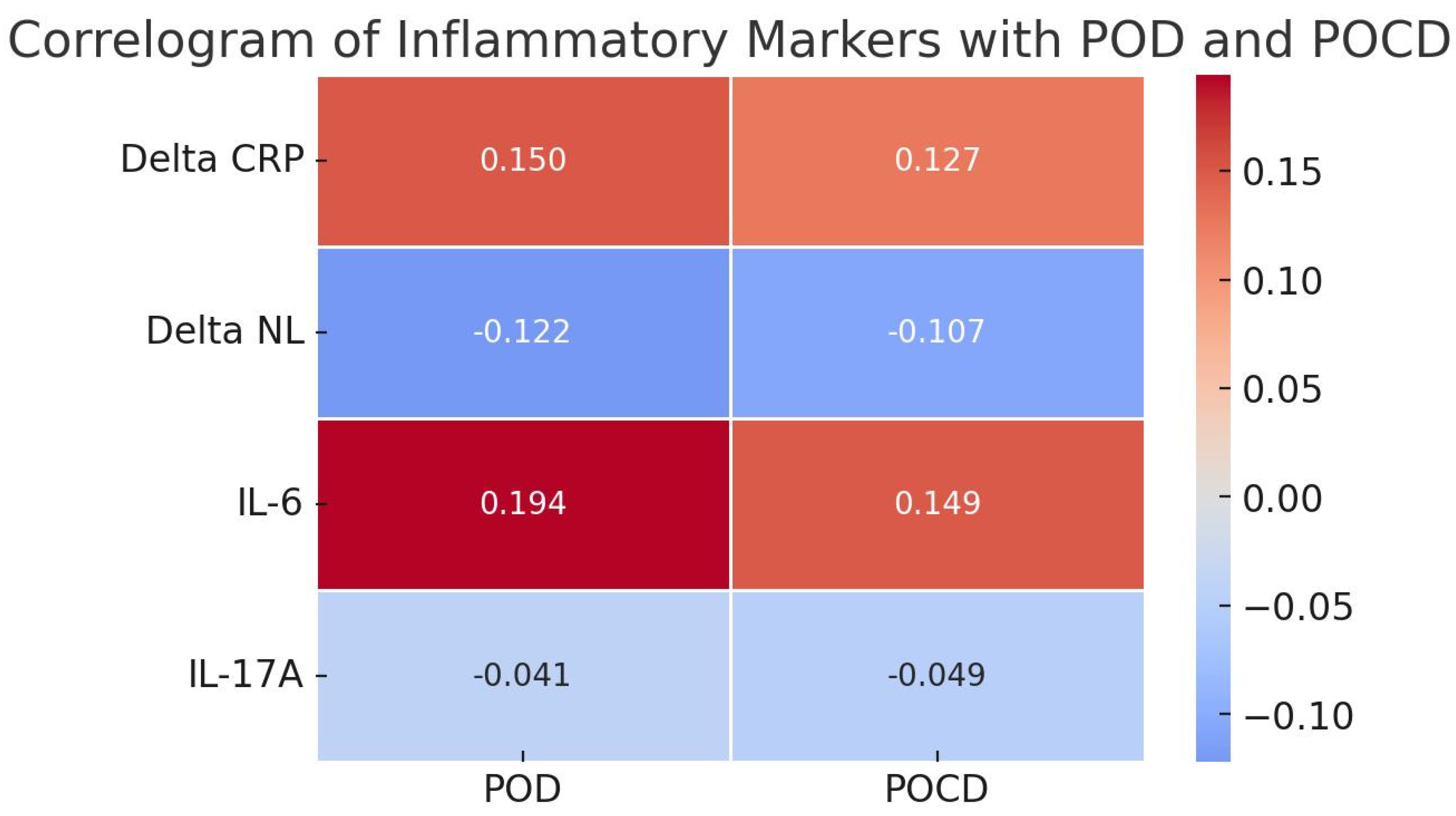
3.4. Neuropsychological Evaluation
4. Discussion
- Validation of IL-6 and NLR predictive accuracy: Prospective, multi-center studies to rigorously evaluate the predictive accuracy of postoperative IL-6 levels and NLR, both alone and in combination with clinical risk scores, for POD and POCD development.
- Randomized controlled trials to explore the impact of intervention strategies to minimize modifiable risk factors, such as mechanical ventilation duration, optimize hemodynamic management to reduce vasopressor requirements, and refine transfusion protocols to reduce blood product exposure on POD/POCD incidence.
- Long-term follow-up studies to characterize the cognitive trajectories of patients identified as high risk based on postoperative inflammatory markers and clinical factors and to assess the impact of interventions on long-term cognitive outcomes.
- Further mechanistic studies to elucidate the specific roles of IL-6, NLR, and other inflammatory pathways in the neuroinflammation underlying POD and POCD following cardiac surgery, potentially using preclinical models and advanced biomarker analyses.
- Studies expanding the investigation to include patients with a broader range of preoperative inflammatory marker values, particularly NLR, to fully define their predictive utility and determine if baseline inflammation is a key modifier of postoperative risk. The interpretation of our findings should consider several limitations. As a single-center study with a relatively modest sample size (N = 88), our results may not be fully generalizable to broader cardiac surgery populations. The exclusion of patients with elevated preoperative NLR values (≥3.4) to minimize confounding might have limited the spectrum of inflammatory risk captured in our cohort and potentially underestimated the full predictive value of NLR. We used cognitive screening tools (MMSE and MoCA) for POCD assessment rather than a more comprehensive neuropsychological battery, which may have limited the sensitivity to detect subtle cognitive deficits. Furthermore, while we identified associations between inflammatory markers and POD/POCD, our observational design cannot establish causality. Finally, we did not directly assess preoperative glycemic control, which could be a relevant confounding factor, particularly given the known interaction between diabetes, age, and cognitive risk.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabol, F.; Bilý, B.; Artemiou, P.; Kolesár, A.; Török, P.; Bilecová-Rabajdová, M.; Kolarčík, P.; Luczy, J. Incidence and Risk Factors of Delirium in Patients after Cardiac Surgery: Modifiable and Non-Modifiable Factors. Cor Vasa 2015, 57, e168–e175. [Google Scholar] [CrossRef]
- Meagher, D.J.; Morandi, A.; Inouye, S.K.; Ely, W.; Adamis, D.; Maclullich, A.J.; Rudolph, J.L.; Neufeld, K.; Leonard, M.; Bellelli, G.; et al. Concordance between DSM-IV and DSM-5 Criteria for Delirium Diagnosis in a Pooled Database of 768 Prospectively Evaluated Patients Using the Delirium Rating Scale-Revised-98. BMC Med. 2014, 12, 164. [Google Scholar] [CrossRef]
- Maxwell, M.; Michael, M.; McDonagh, D.L. Central Nervous System Risk Assessment: Preventing Postoperative Brain Injury. In Perioperative Medicine; Elsevier: Amsterdam, The Netherlands, 2022; pp. 57–66. ISBN 978-0-323-56724-4. [Google Scholar]
- Mattimore, D.; Fischl, A.; Christophides, A.; Cuenca, J.; Davidson, S.; Jin, Z.; Bergese, S. Delirium after Cardiac Surgery—A Narrative Review. Brain Sci. 2023, 13, 1682. [Google Scholar] [CrossRef]
- Yokoyama, C.; Yoshitnai, K.; Ogata, S.; Fukushima, S.; Matsuda, H. Effect of Postoperative Delirium after Cardiovascular Surgery on 5-Year Mortality. JA Clin. Rep. 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Gillio, A.; Lindroth, H.; Ortiz, D.; Holler, E.; Azar, J.; Boustani, M.; Zarzaur, B. Major Surgery and Long Term Cognitive Outcomes: The Effect of Postoperative Delirium on Dementia in the Year Following Discharge. J. Surg. Res. 2022, 270, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Regueira, P.; Albuquerque, E.; Baldeiras, I.; Cardoso, A.L.; Santana, I.; Cerejeira, J. Estimates of Geriatric Delirium Frequency in Noncardiac Surgeries and Its Evaluation Across the Years: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 613–620.e9. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Gao, L.; Wang, Y.; Wang, J. Incidence and Associated Factors of Delirium after Orthopedic Surgery in Elderly Patients: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2021, 33, 1493–1506. [Google Scholar] [CrossRef]
- Hua, Y.; Chen, S.; Xiong, X.; Lin, C.; Li, D.; Tu, P. Risk Factors for Postoperative Delirium in Elderly Urological Patients: A Meta-Analysis. Medicine 2022, 101, e30696. [Google Scholar] [CrossRef]
- Sircar, K.; Yagdiran, A.; Bredow, J.; Annecke, T.; Eysel, P.; Scheyerer, M.J. The Influence of Orthopedic Surgery on the Incidence of Post-Operative Delirium in Geriatric Patients: Results of a Prospective Observational Study. J. Clin. Orthop. Trauma 2022, 33, 102000. [Google Scholar] [CrossRef]
- Majewski, P.; Zegan-Barańska, M.; Karolak, I.; Kaim, K.; Żukowski, M.; Kotfis, K. Current Evidence Regarding Biomarkers Used to Aid Postoperative Delirium Diagnosis in the Field of Cardiac Surgery—Review. Medicina 2020, 56, 493. [Google Scholar] [CrossRef]
- Salameh, A.; Dhein, S.; Dähnert, I.; Klein, N. Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass. Int. J. Mol. Sci. 2016, 17, 1945. [Google Scholar] [CrossRef]
- Indja, B.; Fanning, J.P.; Maller, J.J.; Fraser, J.F.; Bannon, P.G.; Vallely, M.; Grieve, S.M. Neural Network Imaging to Characterize Brain Injury in Cardiac Procedures: The Emerging Utility of Connectomics. Br. J. Anaesth. 2017, 118, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, H.; Miao, Z.; Shao, S.; Shi, H.; Dong, C. Dynamic Changes in the Systemic Inflammation Response Index Predict the Outcome of Resectable Gastric Cancer Patients. Front. Oncol. 2021, 11, 577043. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Y.; Zhang, F.; Ma, Y.; Lou, J.; Li, H.; Liu, Y.; Mi, W.; Cao, J. Systemic Immune-Inflammation Index Predicts Postoperative Delirium in Elderly Patients after Surgery: A Retrospective Cohort Study. BMC Geriatr. 2022, 22, 730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Xu, L. The Potential Risk Factors of Postoperative Cognitive Dysfunction for Endovascular Therapy in Acute Ischemic Stroke with General Anesthesia. Open Med. 2024, 19, 20241085. [Google Scholar] [CrossRef]
- Zhao, B.; Zhai, W.; Ren, M.; Zhang, Z.; Han, J. Systemic Immune Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI) as Predictors of Postoperative Delirium in Patients Undergoing off-Pump Coronary Artery Bypass Grafting (OPCABG) with Cerebral Infarction. BMC Surg. 2024, 24, 338. [Google Scholar] [CrossRef]
- Li, G.; Yu, J.; Jiang, S.; Wu, K.; Xu, Y.; Lu, X.; Wang, Y.; Lin, J.; Yang, X.; Li, Z.; et al. Systemic Immune-Inflammation Index Was Significantly Associated with All-Cause and Cardiovascular-Specific Mortalities in Patients Receiving Peritoneal Dialysis. J. Inflamm. Res. 2023, 16, 3871–3878. [Google Scholar] [CrossRef]
- Sarejloo, S.; Shojaei, N.; Lucke-Wold, B.; Zelmanovich, R.; Khanzadeh, S. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Delirium in Critically Ill Patients: A Systematic Review and Meta-Analysis. BMC Anesth. 2023, 23, 58. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-Lymphocyte Ratio, Past, Present and Future Perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Firment, J.; Hulin, I. Zahorec Index or Neutrophil-to-Lymphocyte Ratio, Valid Biomarker of Inflammation and Immune Response to Infection, Cancer and Surgery. Bratisl. Lek. Listy 2024, 125, 75–83. [Google Scholar] [CrossRef]
- Qiu, Y.; Mo, C.; Xu, S.; Chen, L.; Ye, W.; Kang, Y.; Chen, G.; Zhu, T. Research Progress on Perioperative Blood-Brain Barrier Damage and Its Potential Mechanism. Front. Cell Dev. Biol. 2023, 11, 1174043. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Weiss, R.M.; Wei, S.-G. Brain Interleukin-17A Contributes to Neuroinflammation and Cardiac Dysfunction in Rats with Myocardial Infarction. Front. Neurosci. 2022, 16, 1032434. [Google Scholar] [CrossRef]
- Waisman, A.; Hauptmann, J.; Regen, T. The Role of IL-17 in CNS Diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef]
- Riley McCarten, J.; Anderson, P.; Kuskowski, M.A.; McPherson, S.E.; Borson, S.; Dysken, M.W. Finding Dementia in Primary Care: The Results of a Clinical Demonstration Project. J. Am. Geriatr. Soc. 2012, 60, 210–217. [Google Scholar] [CrossRef]
- Gong, Y.; Liang, S.; Zeng, L.; Ni, Y.; Zhou, S.; Yuan, X. Effects of Blood Sample Handling Procedures on Measurable Interleukin 6 in Plasma and Serum. Clin. Lab. Anal. 2019, 33, e22924. [Google Scholar] [CrossRef]
- Verberk, I.M.; Nossent, E.J.; Bontkes, H.J.; Teunissen, C.E. Pre-Analytical Sample Handling Effects on Blood Cytokine Levels: Quality Control of A COVID-19 Biobank. Biomark. Med. 2021, 15, 987–997. [Google Scholar] [CrossRef]
- Figueiredo, S.; Devezas, M. Bridging the Gaps in Understanding POD and POCD: A Thorough Examination of Genetic and Clinical Biomarkers. Perioper. Care Oper. Room Manag. 2024, 35, 100401. [Google Scholar] [CrossRef]
- Suraarunsumrit, P.; Srinonprasert, V.; Kongmalai, T.; Suratewat, S.; Chaikledkaew, U.; Rattanasiri, S.; McKay, G.; Attia, J.; Thakkinstian, A. Outcomes Associated with Postoperative Cognitive Dysfunction: A Systematic Review and Meta-Analysis. Age Ageing 2024, 53, afae160. [Google Scholar] [CrossRef]
- Cao, S.; Chen, D.-; Yang, L.; Zhu, T. Effects of an Abnormal Mini-Mental State Examination Score on Postoperative Outcomes in Geriatric Surgical Patients: A Meta-Analysis. BMC Anesth. 2019, 19, 74. [Google Scholar] [CrossRef]
- Danquah, M.O.; Yan, E.; Lee, J.W.; Philip, K.; Saripella, A.; Alhamdah, Y.; He, D.; Englesakis, M.; Chung, F. The Utility of the Montreal Cognitive Assessment (MoCA) in Detecting Cognitive Impairment in Surgical Populations—A Systematic Review and Meta-Analysis. J. Clin. Anesth. 2024, 97, 111551. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yuan, X.; Guo, J.; Gao, X. Influence of Education Level on MMSE and MoCA Scores of Elderly Inpatients. Appl. Neuropsychol. Adult 2023, 30, 414–418. [Google Scholar] [CrossRef]
- Borda, M.G.; Reyes-Ortiz, C.; Pérez-Zepeda, M.U.; Patino-Hernandez, D.; Gómez-Arteaga, C.; Cano-Gutiérrez, C.A. Educational Level and Its Association with the Domains of the Montreal Cognitive Assessment Test. Aging Ment. Health 2019, 23, 1300–1306. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, Q.; Qian, S.; Lin, X.; Yan, F.; Sun, L.; Xiao, S.; Wang, J.; Fang, Y.; Li, X. Determining Appropriate Screening Tools and Cutoffs for Cognitive Impairment in the Chinese Elderly. Front. Psychiatry 2021, 12, 773281. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, X.; Ding, S.; Feng, X.; Wu, F.; Wu, Y. Associations between Postoperative Cognitive Dysfunction, Serum Interleukin-6 and Postoperative Delirium among Patients after Coronary Artery Bypass Grafting: A Mediation Analysis. Nurs. Crit. Care 2024, 29, 1245–1252. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, H.-P.; Wu, T.-T. Postoperative C-Reactive Protein Predicts Postoperative Delirium in Colorectal Cancer Following Surgery. Clin. Interv. Aging 2023, 18, 559–570. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; He, Y.; Wang, Z.; Zhang, M. White-Cell Derived Inflammatory Biomarkers in Prediction of Postoperative Delirium in Elderly Patients Undergoing Surgery for Lower Limb Fracture Under Non-General Anaesthesia. Clin. Interv. Aging 2022, 17, 383–392. [Google Scholar] [CrossRef]
- Lu, W.; Lin, S.; Wang, C.; Jin, P.; Bian, J. The Potential Value of Systemic Inflammation Response Index on Delirium After Hip Arthroplasty Surgery in Older Patients: A Retrospective Study. Int. J. Gen. Med. 2023, 16, 5355–5362. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Wang, X. The Neutrophil-Lymphocyte Ratio Is Associated with Postoperative Mortality of Cardiac Surgery. J. Thorac. Dis. 2020, 13, 67. [Google Scholar] [CrossRef]
- Vu, T.; Smith, J.A. An Update on Postoperative Cognitive Dysfunction Following Cardiac Surgery. Front. Psychiatry 2022, 13, 884907. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, J.; Zheng, K.; Zhang, H. Research Progress of Postoperative Cognitive Dysfunction in Cardiac Surgery under Cardiopulmonary Bypass. Ibrain 2023, 10, 290–304. [Google Scholar] [CrossRef]
- Lim, H.A.; Kang, J.K.; Kim, H.W.; Song, H.; Lim, J.Y. The Neutrophil-to-Lymphocyte Ratio as a Predictor of Postoperative Outcomes in Patients Undergoing Coronary Artery Bypass Grafting. J. Chest Surg. 2023, 56, 99–107. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wang, H.; Wang, S.; Bi, H.; Liu, J.; Jin, X. Correlation Analysis of the Pindicator of PNI,SII of Reoperative Immunological and Postoperative Delirium in Patients Undergoing Thoracoscopic Surgery. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Ottens, T.H.; Dieleman, J.M.; Sauër, A.-M.C.; Peelen, L.M.; Nierich, A.P.; De Groot, W.J.; Nathoe, H.M.; Buijsrogge, M.P.; Kalkman, C.J.; Van Dijk, D. Effects of Dexamethasone on Cognitive Decline after Cardiac Surgery: A Randomized Clinical Trial. Anesthesiology 2014, 121, 492–500. [Google Scholar] [CrossRef]
- MacKenzie, E.; McCulloch, J.; O’Kean, M.; Pickard, J.; Harper, A. Cerebral Circulation and Norepinephrine: Relevance of the Blood-Brain Barrier. Am. J. Physiol.-Leg. Content 1976, 231, 483–488. [Google Scholar] [CrossRef]
- Zhu, S.-H.; Ji, M.-H.; Gao, D.-P.; Li, W.-Y.; Yang, J.-J. Association between Perioperative Blood Transfusion and Early Postoperative Cognitive Dysfunction in Aged Patients Following Total Hip Replacement Surgery. Upsala J. Med. Sci. 2014, 119, 262–267. [Google Scholar] [CrossRef]
- Garraud, O.; Tariket, S.; Sut, C.; Haddad, A.; Aloui, C.; Chakroun, T.; Laradi, S.; Cognasse, F. Transfusion as an Inflammation Hit: Knowns and Unknowns. Front. Immunol. 2016, 7, 534. [Google Scholar] [CrossRef]
- Firdous, S.M.; Khan, S.A.; Maity, A. Oxidative Stress–Mediated Neuroinflammation in Alzheimer’s Disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8189–8209. [Google Scholar] [CrossRef]
- Kotfis, K.; Ślozowska, J.; Listewnik, M.; Szylińska, A.; Rotter, I. The Impact of Acute Kidney Injury in the Perioperative Period on the Incidence of Postoperative Delirium in Patients Undergoing Coronary Artery Bypass Grafting—Observational Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 1440. [Google Scholar] [CrossRef]
- Franco, Á.D.O.; Starosta, R.T.; Roriz-Cruz, M. The Specific Impact of Uremic Toxins upon Cognitive Domains: A Review. Braz. J. Nephrol. 2019, 41, 103–111. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Ling, J.; Wu, Y.; Yang, P.; Liu, X.; Liu, J.; Zhang, D.; Yin, X.; Yu, P.; et al. The Association between Diabetes Mellitus and Postoperative Cognitive Dysfunction: A Systematic Review and Meta-Analysis. Int. J. Surg. 2024, 111, 2633–2650. [Google Scholar] [CrossRef]
| Variable | Total (N = 88) |
|---|---|
| Gender (Female), n (%) | 16 (18.18%) |
| Age Group, n (%) | |
| ≤55 years | 21 (18.57%) |
| 55 to 65 years | 23 (28.57%) |
| 65 to 75 years | 38 (45.71%) |
| ≥75 years | 6 (7.15%) |
| Height, cm | 171.06 ± 9.22 |
| Weight, kg | 81.35 ± 15.71 |
| BMI, kg/m2 | 27.56 ± 4.47 |
| BMI Group, n (%) | |
| ≤18.5 kg/m2 | 0 (0%) |
| 18.5 to 24 kg/m2 | 22 (25%) |
| ≥24 kg/m2 | 66 (75%) |
| Medical Condition, n (%) | |
| Coronary heart disease, n (%) | 32 (36.36%) |
| Diabetes mellitus, n (%) | 23 (26.13%) |
| Transfusion | 49 (55.68%) |
| Smokers | 19 (21.59%) |
| POD | 17 (19.31%) |
| POCD | 22 (25%) |
| Surgical approaches, n (%) | |
| Valve surgery | 56 (63.63%) |
| Coronary surgery | 32 (36.37%) |
| CPB time, min | 114.95 ± 34.17 |
| Vasopressor support | 19.35 ± 9.80 |
| EF% | 46.68 ± 5.95 |
| Mean arterial pressure | 89.18 ± 9.31 |
| Variable | GROUP 1. POD/POCD Group | GROUP 2. No POD/POCD Group | p-Value | Cohen’s d | OR | 95% Confidence Intervals | Fisher’s Exact Test p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age (average) | 66 ± 8.99 | 60 ± 11.75 | 0.004 | −0.726 | ||||
| Gender/M | 16 | 55 | 0.523 | 0.386 | 0.386 | 0.2 | 2.2 | 0.53 |
| Weight (average) | 78.04 ± 10.75 | 82.48 ± 16.96 | 0.244 | 0.289 | ||||
| CPB time/min | 135.3 ± 43.9 | 107.65 ± 26.89 | p < 0.001 | −0.916 | ||||
| MV/hours (average) | 27 ± 5.07 | 14.89 ± 4.40 | p = 0.004 | −0.726 | ||||
| Diabetes mellitus | 7 (31.81%) | 16 (24.24%) | 0.484 | 0.377 | 0.5 | 4.2 | 0.57 | |
| Postoperative atrial fibrillation | 4 (18.18%) | 10 (15.15%) | p = 0.001 | 1.618). | 0.3 | 4.4 | 0.74 | |
| NA/hours (average) | 26.61 ± 11.80 | 17.06 ± 7.88 | p < 0.001 | −1.019 | ||||
| CRP mg/dL at 24 h | 78.26 ± 24.46 | 74.32 ± 21.02 | p = 0.469 | |||||
| CRP mg/dL at 48 h | 217.28 ± 70.82 | 177.31 ± 61.556 | p = 0.020 | −0.581 | ||||
| NLR preop | 2.91 ± 1.42 | 2.64 ± 1.26 | p = 0.395 | |||||
| NLR at 24 h | 21.4 ± 14.59 | 15.52 ± 8.75 | p = 0.038 | −0.519 | ||||
| NLR at 48 h | 14.98 ± 8.66 | 8.81 ± 4.23 | p = 0.013 | −0.623 | ||||
| IL-6 pg/mL preoperative values | 17.04 | 15.63 | p = 0.301 | |||||
| IL-6 pg/mL at 48 h | 196.94 ± 131.99 | 160.79 ± 102.97 | p = 0.013 | −0.623 | ||||
| IL-17-A pg/mL preoperative values | 2.08 | 4.79 | p = 0.500 | |||||
| IL-17-A pg/mL at 48 h | 5.16 | 12.08 | p = 0.500 | |||||
| SII preoperative values | 490.3 ± 312.83 | 457.83 ± 229.04 | p = 0.630 | |||||
| SII at 24 h | 2341.9 ± 1280.82 | 2309 ± 1521.93 | p = 0.844 | |||||
| SIRI preoperative values | 1.42 ± 0.97 | 1.36 ± 1.29 | p = 0.879 | |||||
| SIRI at 24 h | 12.97 ± 7.93 | 15.52 ± 9.20 | p = 0.936 | |||||
| SIRId (dynamic) | 12.49 ± 10.71 | 12.83 ± 10.42 | p = 0.959 | |||||
| Blood transfusion | 18 (81%) | 30 (45.45%) | p = <0.001 | 2.028 | 2.028 | 2.0 | 28.18 | 0.001 |
| Smokers | 4 (18.18%) | 14 (21.21%) | p = 0.881 | 0.088 | 0.3 | 3.4 | 1 | |
| EF %(average) | 43.2% ± 1.76% | 47.72% ± 1.11% | p = 0.072 | |||||
| VEMS 70% | 10 (45%) | 12(18.18%) | p = 0.015 | |||||
| Creatine kinase IU/L (average) | 991.19 ± 876.96 | 566.07 ± 436.71 | p = 0.023 | −0.570 | ||||
| Lactate dehydrogenase U/L (average) | 429.61 ± 139.14 | 331.36 ± 98.76 | p < 0.001 | −0.981 | ||||
| Preoperative creatinine md/dL | 1.57 ± 1.11 | 0.97 ± 0.45 | p < 0.001 | −0.868 | ||||
| Postoperative creatinine mg/dL | 1.95 ± 1.43 | 1.16 ± 0.53 | p < 0.001 | −0.933 | ||||
| Glucose/24 h mg/dL | 160.09 ± 34.05 | 153.43 ± 35.18 | p = 0441 | |||||
| Model | Deviance | AIC | BIC | df | ΔΧ2 | p | McFadden R2 | Nagelkerke R2 | Tjur R2 | Cox & Snell R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| M0 | 97.805 | 99.805 | 102.259 | 85 | 0.000 | 0.000 | ||||
| M1 | 1.392 × 10−7 | 50.000 | 111.359 | 61 | 97.805 | <0.001 | 1.000 | 1.000 | 1.000 | 0.679 |
| Wald Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | Estimate | Standard Error | Odds Ratio | z | Wald Statistic | df | p | |
| M0 | (Intercept) | −1.068 | 0.247 | 0.344 | −4.321 | 18.669 | 1 | <0.001 |
| M1 | (Intercept) | −3607.479 | 556,892.801 | 0.000 | −0.006 | 4.196 × 10−5 | 1 | 0.995 |
| Age | 27.864 | 4333.550 | 1.263 × 1012 | 0.006 | 4.134 × 10−5 | 1 | 0.995 | |
| Gender (M) | 65.211 | 27,909.801 | 2.092 × 1028 | 0.002 | 5.459 × 10−6 | 1 | 0.998 | |
| Provenience (urban) | −158.730 | 46,243.628 | 1.160 × 10−69 | −0.003 | 1.178 × 10−5 | 1 | 0.997 | |
| Weight | 3.478 | 691.307 | 32.407 | 0.005 | 2.532 × 10−5 | 1 | 0.996 | |
| CPB time/min | 6.803 | 921.187 | 900.333 | 0.007 | 5.454 × 10−5 | 1 | 0.994 | |
| Mechanical ventilation/hours | 23.699 | 5717.932 | 1.960 × 1010 | 0.004 | 1.718 × 10−5 | 1 | 0.997 | |
| Diabetes mellitus | −7.708 | 21,123.056 | 4.493 × 10−4 | −3.649 × 10−4 | 1.332 × 10−7 | 1 | 1.000 | |
| C-reactive protein (CRP) at 24 h postoperatively | −3.284 | 528.525 | 0.037 | −0.006 | 3.861 × 10−5 | 1 | 0.995 | |
| C-reactive protein (CRP) at 48 h postoperatively | 3.237 | 408.577 | 25.457 | 0.008 | 6.277 × 10−5 | 1 | 0.994 | |
| Preoperative neutrophil-to-lymphocyte ratio | −117.037 | 15,992.441 | 1.484 × 10−51 | −0.007 | 5.356 × 10−5 | 1 | 0.994 | |
| Neutrophil-to-lymphocyte ratio (NLR) at 24 h postoperatively | 7.251 | 1147.312 | 1409.735 | 0.006 | 3.994 × 10−5 | 1 | 0.995 | |
| Neutrophil-to-lymphocyte ratio (NLR) at 48 h postoperatively | −5.994 | 2647.201 | 0.002 | −0.002 | 5.126 × 10−6 | 1 | 0.998 | |
| IL-6 at 48 h | 1.890 | 259.199 | 6.617 | 0.007 | 5.315 × 10−5 | 1 | 0.994 | |
| IL-17 at 48 h | −8.928 | 1184.888 | 1.326 × 10−4 | −0.008 | 5.678 × 10−5 | 1 | 0.994 | |
| Smokers | 242.676 | 33,565.572 | 2.472 × 10105 | 0.007 | 5.227 × 10−5 | 1 | 0.994 | |
| Preoperative creatinine | −63.177 | 151,667.152 | 3.654 × 10−28 | −4.165 × 10−4 | 1.735 × 10−7 | 1 | 1.000 | |
| Postoperative creatinine | 122.408 | 142,604.008 | 1.450 × 1053 | 8.584 × 10−4 | 7.368 × 10−7 | 1 | 0.999 | |
| Lactate dehydrogenase | 0.295 | 55.022 | 1.343 | 0.005 | 2.878 × 10−5 | 1 | 0.996 | |
| Noradrenaline/hours | 4.365 | 1304.360 | 78.626 | 0.003 | 1.120 × 10−5 | 1 | 0.997 | |
| Glucose/24 h | −2.568 | 362.526 | 0.077 | −0.007 | 5.018 × 10−5 | 1 | 0.994 | |
| Preoperative SII value | −0.648 | 104.127 | 0.523 | −0.006 | 3.870 × 10−5 | 1 | 0.995 | |
| Postoperative SII value | −0.063 | 10.644 | 0.939 | −0.006 | 3.537 × 10−5 | 1 | 0.995 | |
| Preoperative SIRI | 112.435 | 15,629.056 | 6.760 × 1048 | 0.007 | 5.175 × 10−5 | 1 | 0.994 | |
| Postoperative SIRI | 10.446 | 1489.364 | 34,415.173 | 0.007 | 4.919 × 10−5 | 1 | 0.994 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staicu, R.-E.; Vernic, C.; Ciurescu, S.; Lascu, A.; Aburel, O.-M.; Deutsch, P.; Rosca, E.C. Postoperative Delirium and Cognitive Dysfunction After Cardiac Surgery: The Role of Inflammation and Clinical Risk Factors. Diagnostics 2025, 15, 844. https://doi.org/10.3390/diagnostics15070844
Staicu R-E, Vernic C, Ciurescu S, Lascu A, Aburel O-M, Deutsch P, Rosca EC. Postoperative Delirium and Cognitive Dysfunction After Cardiac Surgery: The Role of Inflammation and Clinical Risk Factors. Diagnostics. 2025; 15(7):844. https://doi.org/10.3390/diagnostics15070844
Chicago/Turabian StyleStaicu, Raluca-Elisabeta, Corina Vernic, Sebastian Ciurescu, Ana Lascu, Oana-Maria Aburel, Petru Deutsch, and Elena Cecilia Rosca. 2025. "Postoperative Delirium and Cognitive Dysfunction After Cardiac Surgery: The Role of Inflammation and Clinical Risk Factors" Diagnostics 15, no. 7: 844. https://doi.org/10.3390/diagnostics15070844
APA StyleStaicu, R.-E., Vernic, C., Ciurescu, S., Lascu, A., Aburel, O.-M., Deutsch, P., & Rosca, E. C. (2025). Postoperative Delirium and Cognitive Dysfunction After Cardiac Surgery: The Role of Inflammation and Clinical Risk Factors. Diagnostics, 15(7), 844. https://doi.org/10.3390/diagnostics15070844






