Wrist Function Test and Its Use to Assess Treatment Efficacy in Ischemic Stroke Survivors—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Eligibility Criteria
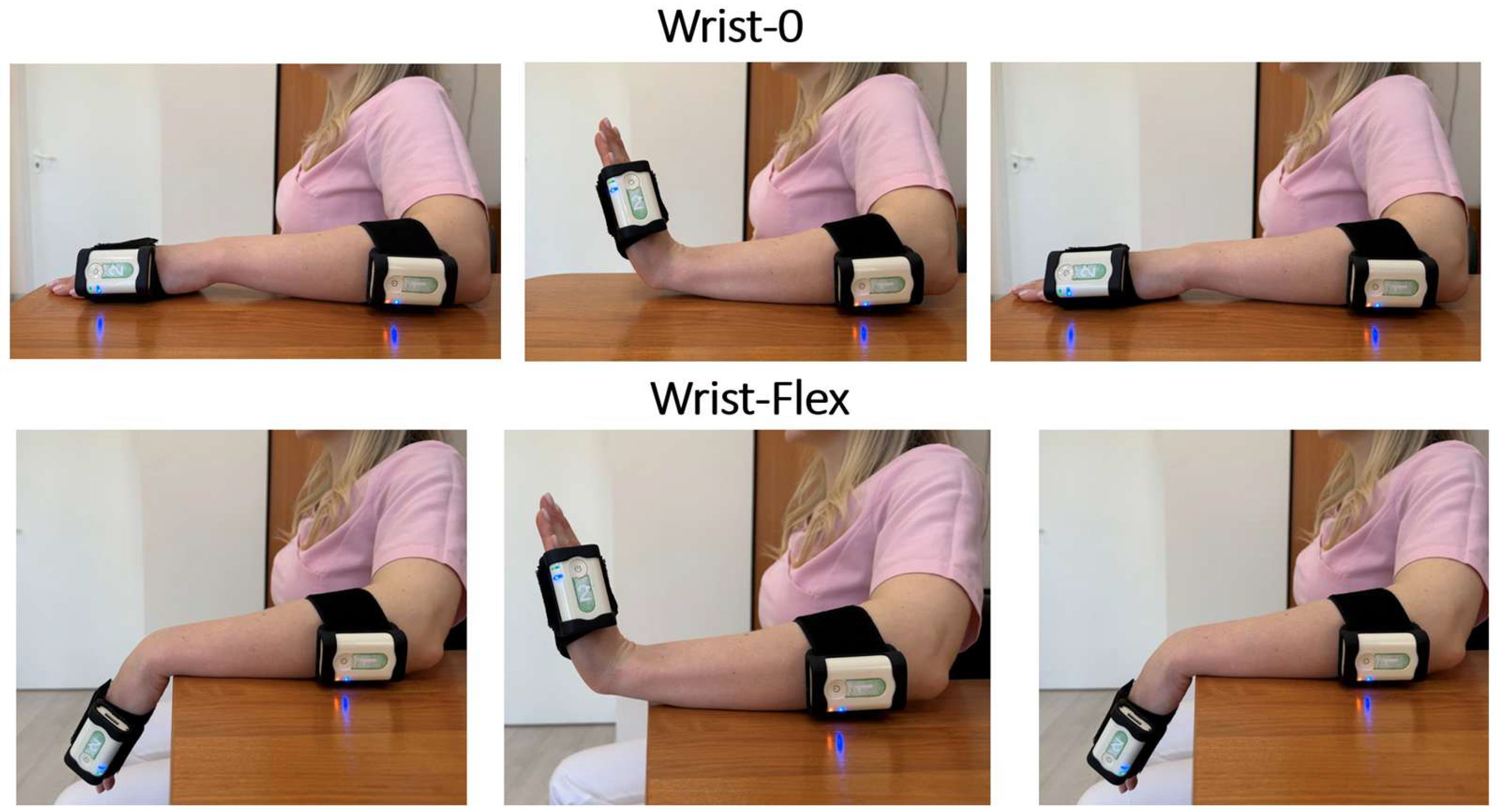
2.3. Instrumental Technique for Function Assessment
2.4. FES Treatment Session
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FES | Functional Electrical Stimulation |
| IMU | Inertial Measurement Unit |
| MRC | Medical Research Council |
| FMA-UE | Fugl-Meyer Assessment for Upper Extremity |
| ARAT | Action Research Arm Test |
| EEG | Electroencephalography |
| ICF | International Classification of Functioning |
References
- Truelsen, T.; Piechowski-Jóźwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Newell, A.; Rosenbloom, P.S. Mechanisms of skill acquisition and the law of practice. In Cognitive Skills and Their Acquisition; Anderson, J.R., Ed.; Erlbaum: Hillsdale, NJ, USA, 1981; pp. 1–55. [Google Scholar]
- Duncan, P.W. Synthesis of intervention trials to improve motor recovery following stroke. Top. Stroke Rehabil. 1997, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Popovich, M.R.; Thrasher, T.A. Neuroprosthetics. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 1056–1065. [Google Scholar] [CrossRef]
- Nguyen, R.; Masani, K.; Micera, S.; Morari, M.; Popovic, M.R. Spatially distributed sequential stimulation reduces fatigue in paralyzed triceps surae muscles: A case study. Artif. Organs 2011, 35, 1174–1180. [Google Scholar] [CrossRef]
- Meadmore, K.L.; Exell, T.A.; Hallewell, E.; Hughes, A.M.; Freeman, C.T.; Kutlu, M.; Benson, V.; Rogers, E.; Burridge, J.H. The application of precisely controlled functional electrical stimulation to the shoulder, elbow, and wrist for upper limb stroke rehabilitation: A feasibility study. J. Neuroeng. Rehabil. 2014, 11, 105. [Google Scholar] [CrossRef]
- Cuesta-Gomez, A.; Molina-Rueda, F.; Carratala-Tejada, M.; Imatz-Ojanguren, E.; Torricelli, D.; Miangolarra-Page, J. The use of functional electrical stimulation on the upper limb and interscapular muscles of patients with stroke for the improvement of reaching movements: A feasibility study. Front. Neurol. 2017, 8, 186. [Google Scholar] [CrossRef]
- Hara, Y. Rehabilitation with functional electrical stimulation in stroke patients. Int. J. Phys. Med. Rehabil. 2013, 1, 147. [Google Scholar] [CrossRef]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 40. [Google Scholar] [CrossRef]
- Mateo, S.; Revol, P.; Fourtassi, M.; Rossetti, Y.; Collet, C.; Rode, G. Kinematic characteristics of tenodesis grasp in C6 quadriplegia. Spinal Cord 2013, 51, 144–149. [Google Scholar] [CrossRef]
- Su, F.C.; Chou, Y.L.; Yang, C.S.; Lin, G.T.; An, K.N. Movement of finger joints induced by synergistic wrist motion. Clin. Biomech. 2005, 20, 491–497. [Google Scholar] [CrossRef]
- Moser, N.; O’Malley, M.K.; Erwin, A. Importance of Wrist Movement Direction in Performing Activities of Daily Living Efficiently. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3174–3177. [Google Scholar] [CrossRef]
- Nadeem, M.; Loss, J.G.; Li, Z.M.; Seitz, W.H., Jr. Ulnar Extension Coupling in Functional Wrist Kinematics During Hand Activities of Daily Living. J. Hand Surg. 2022, 47, 187.e1–187.e13. [Google Scholar] [CrossRef]
- Renner, C.I.; Bungert-Kahl, P.; Hummelsheim, H. Change of strength and rate of rise of tension relate to functional arm recovery after stroke. Arch. Phys. Med. Rehabil. 2009, 90, 1548–1556. [Google Scholar] [CrossRef]
- Thompson-Butel, A.G.; Lin, G.; Shiner, C.T.; McNulty, P.A. Comparison of three tools to measure improvements in upper-limb function with poststroke therapy. Neurorehabilit. Neural Repair 2015, 29, 341–348. [Google Scholar] [CrossRef]
- Vanmechelen, I.; Haberfehlner, H.; De Vleeschhauwer, J.; Van Wonterghem, E.; Feys, H.; Desloovere, K.; Aerts, J.M.; Monbaliu, E. Assessment of movement disorders using wearable sensors during upper limb tasks: A scoping review. Front. Robot. AI 2023, 9, 1068413. [Google Scholar] [CrossRef]
- Santisteban, L.; Térémetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Corazza, S.; Mündermann, L.; Gambaretto, E.; Ferrigno, G.; Andriacchi, T.P. Markerless Motion Capture through Visual Hull, Articulated ICP and Subject Specific Model Generation. Int. J. Comput. Vis. 2010, 87, 156–169. [Google Scholar] [CrossRef]
- Sethi, A.; Patterson, T.; McGuirk, T.; Patten, C.; Richards, L.G.; Stergiou, N. Temporal structure of variability decreases in upper extremity movements post stroke. Clin. Biomech. 2013, 28, 134–139. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Tung, L.-C.; Yang, J.-F.; Chen, Y.-C.; Yeh, C.-Y.; Wang, C.-H. Electromyographic Analyses of Global Synkinesis in the Paretic Upper Limb After Stroke. Phys. Ther. 2005, 85, 755–765. [Google Scholar] [CrossRef]
- LaStayo, P.C.; Wheeler, D.L. Reliability of passive wrist flexion and extension goniometric measurements: A multicenter study. Phys. Ther. 1994, 74, 162–176. [Google Scholar] [CrossRef]
- Reissner, L.; Fischer, G.; List, R.; Taylor, W.R.; Giovanoli, P.; Calcagni, M. Minimal detectable difference of the finger and wrist range of motion: Comparison of goniometry and 3D motion analysis. J. Orthop. Surg. Res. 2019, 14, 173. [Google Scholar] [CrossRef]
- Costa, V.; Ramírez, Ó.; Otero, A.; Muñoz-García, D.; Uribarri, S.; Raya, R. Validity and reliability of inertial sensors for elbow and wrist range of motion assessment. PeerJ 2020, 8, e9687. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; de Sousa Ribeiro, R.; Mokni, M.; Weikert, T.; Pohl, J.; Schwarz, A.; Held, J.P.O.; Sauerzopf, L.; Kühnis, B.; Gavagnin, E.; et al. Upper limb movement quality measures: Comparing IMUs and optical motion capture in stroke patients performing a drinking task. Front. Digit. Health 2024, 6, 1359776. [Google Scholar] [CrossRef]
- Cahill-Rowley, K.; Rose, J. Temporal-spatial reach parameters derived from inertial sensors: Comparison to 3D marker-based motion capture. J. Biomech. 2017, 52, 11–16. [Google Scholar] [CrossRef] [PubMed]
- McHugh, B.P.; Morton, A.M.; Akhbari, B.; Molino, J.; Crisco, J.J. Accuracy of an electrogoniometer relative to optical motion tracking for quantifying wrist range of motion. J. Med. Eng. Technol. 2020, 44, 49–54. [Google Scholar] [CrossRef]
- Akhbari, B.; Morton, A.M.; Moore, D.C.; Weiss, A.C.; Wolfe, S.W.; Crisco, J.J. Accuracy of biplane videoradiography for quantifying dynamic wrist kinematics. J. Biomech. 2019, 92, 120–125. [Google Scholar] [CrossRef]
- Thies, S.B.; Tresadern, P.A.; Kenney, L.P.; Smith, J.; Howard, D.; Goulermas, J.Y.; Smith, C.; Rigby, J. Movement variability in stroke patients and controls performing two upper limb functional tasks: A new assessment methodology. J. Neuroeng. Rehabil. 2009, 6, 2. [Google Scholar] [CrossRef]
- Ada, L.; Canning, C.G.; Low, S.L. Stroke patients have selective muscle weakness in shortened range. Brain 2003, 126 Pt 3, 724–731. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabilit. Neural Repair 2012, 26, 76–84. [Google Scholar] [CrossRef]
- Noorkõiv, M.; Rodgers, H.; Price, C.I. Accelerometer measurement of upper extremity movement after stroke: A systematic review of clinical studies. J. Neuroeng. Rehabil. 2014, 11, 144. [Google Scholar] [CrossRef]
- Tretriluxana, J.; Gordon, J.; Winstein, C.J. Manual asymmetries in grasp pre-shaping and transport-grasp coordination. Exp. Brain Res. 2008, 188, 305–315. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ishii, D.; Yamamoto, S.; Okamoto, Y.; Wakatabi, M.; Kohno, Y. Asymmetry of Interhemispheric Connectivity during Rapid Movements of Right and Left Hands: A TMS-EEG Study. J. Mot. Behav. 2022, 54, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.A.; Santos, E.G.R.; Monteiro, L.C.P.; Santos-Lobato, B.L.; Pinto, G.H.L.; Belgamo, A.; Cabral, A.S.; de Athayde Costa ESilva, A.; Callegari, B.; Souza, G.S. The hand tremor spectrum is modified by the inertial sensor mass during lightweight wearable and smartphone-based assessment in healthy young subjects. Sci. Rep. 2022, 12, 16808. [Google Scholar] [CrossRef]
- Wirth, M.A.; Fischer, G.; Verdú, J.; Reissner, L.; Balocco, S.; Calcagni, M. Comparison of a New Inertial Sensor Based System with an Optoelectronic Motion Capture System for Motion Analysis of Healthy Human Wrist Joints. Sensors 2019, 19, 5297. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Lee, W.H.; Seo, H.G.; Smuck, M.W.; Kim, S. Evaluation of Motion Segment Size as a New Sensor-based Functional Outcome Measure in Stroke Rehabilitation. J. Int. Med. Res. 2022, 50, 3000605221122750. [Google Scholar] [CrossRef]
- Adeel, M.; Peng, C.W.; Lee, I.J.; Lin, B.S. Prediction of Spasticity through Upper Limb Active Range of Motion in Stroke Survivors: A Generalized Estimating Equation Model. Bioengineering 2023, 10, 1273. [Google Scholar] [CrossRef]
- Shin, H.; Watkins, Z.; Hu, X. Exploration of Hand Grasp Patterns Elicitable Through Non-Invasive Proximal Nerve Stimulation. Sci. Rep. 2017, 7, 16595. [Google Scholar] [CrossRef]
- Haaland, K.Y.; Harrington, D.L. Hemispheric asymmetry of movement. Curr. Opin. Neurobiol. 1996, 6, 796–800. [Google Scholar] [CrossRef]
- Sunderland, A.; Bowers, M.P.; Sluman, S.M.; Wilcock, D.J.; Ardron, M.E. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke 1999, 30, 949–955. [Google Scholar] [CrossRef]
- Seo, N.J.; Rymer, W.Z.; Kamper, D.G. Delays in grip initiation and termination in persons with stroke: Effects of arm support and active muscle stretch exercise. J. Neurophysiol. 2009, 101, 3108–3115. [Google Scholar] [CrossRef]
- Metrot, J.; Froger, J.; Hauret, I.; Mottet, D.; van Dokkum, L.; Laffont, I. Motor recovery of the ipsilesional upper limb in subacute stroke. Arch. Phys. Med. Rehabil. 2013, 94, 2283–2290. [Google Scholar] [CrossRef]
- Schwerz de Lucena, D.; Rowe, J.; Chan, V.; Reinkensmeyer, D.J. Magnetically Counting Hand Movements: Validation of a Calibration-Free Algorithm and Application to Testing the Threshold Hypothesis of Real-World Hand Use after Stroke. Sensors 2021, 21, 1502. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Hillier, S.L.; Ridding, M.C.; Miles, T.S. Impairments in precision grip correlate with functional measures in adult hemiplegia. Clin. Neurophysiol. 2006, 117, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Merlau, B.; Cormier, C.; Alaux, A.; Morin, M.; Montané, E.; Amarantini, D.; Gasq, D. Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap. Sensors 2023, 23, 7427. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Pepper, M.G.; Yan, Y.; Phillips, M.; Sakel, M. Low Cost Inertial Sensors for the Motion Tracking and Orientation Estimation of Human Upper Limbs in Neurological Rehabilitation. IEEE Access 2020, 8, 54254–54268. [Google Scholar] [CrossRef]


| Group/ Parameter | Time Point | Wrist MRC | ARAT | Fugle Maer | ICF d440 | |
|---|---|---|---|---|---|---|
| Flexion | Extension | |||||
| Control | Before | 3.0 [2.0; 3.0] | 3.0 [2.0; 3.0] | 28.0 [10.75; 32.0] | 86.0 [74.75; 95.5] | 2.0 [2.0; 3.0] |
| After | 3.0 [2.0; 3.0] | 3.0 [2.0; 3.0] | 32.0 [15.25; 42.0] * | 96.0 [81.25; 101.5] * | 2.0 [1.0; 3.0] * | |
| FES | Before | 3.0 [2.0; 3.0] | 3.0 [2.0; 3.0] | 24.0 [11.0; 32.5] | 90.0 [80.02; 99.66] | 2.0 [1.5; 3.0] |
| After | 3.0 [2.0; 3.0] | 3.0 [2.2; 3.0] | 38.0 [22.5; 40.5] * | 102.0 [89.035; 108.83] * | 2.0 [1.0; 2.0] * | |
| Test/Parameter | Amplitude (Degrees) | Phase (%) | Cycle (s) | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Wrist-0 | 79 [67; 86] * | 80 [74; 86] * | 41 [38; 45] * | 46 [39; 48] | 1.78 [1.53; 2.46] | 1.85 [1.4; 2.06] * |
| Wrist-Flex | 137 [123; 156] | 138 [124; 158] | 47 [42; 58] | 43 [39; 53] | 2.27 [1.7; 2.57] | 2.07 [1.74; 2.67] |
| Group/Parameter | Time Point | Test | Amplitude (Degrees) | Phase (%) | Cycle (s) |
|---|---|---|---|---|---|
| Control | Before | Wrist-0 | 29.0 [8.5; 45.5] *& | 51.5 [20.0; 57.5] | 2.08 [0.71; 3.02] & |
| Wrist-Flex | 70.0 [47.5; 87.0] * | 51.0 [45.5; 56.5] | 2.72 [2.31; 3.725] * | ||
| After | Wrist-0 | 26.0 [17.5; 53.75] *& | 48.0 [38.0; 57.25] | 1.96 [1.1525; 2.6925] | |
| Wrist-Flex | 73.0 [44.5; 90.75] * | 52.0 [47.5; 56.5] | 2.63 [1.9425; 3.12] # | ||
| FES | Before | Wrist-0 | 21.0 [0.0; 27.0] *& | 50.0 [0.0; 64.0] | 2.135 [0.0; 2.84] |
| Wrist-Flex | 49.5 [39.0; 65.0] * | 48.5 [39.5; 58.0] | 2.45 [1.84; 4.015] | ||
| After | Wrist-0 | 28.5 [21.0; 32.5] #*& | 53.5 [47.0; 66.5] * | 2.365 [1.94; 3.185] | |
| Wrist-Flex | 59.5 [49.0; 73.5] #* | 54.5 [45.0; 59.0] | 3.215 [2.11; 4.19] * | ||
| Healthy | Wrist-0 | 79 [68.75; 86.0] & | 41 [38.0; 44.75] & | 1.78 [1.54; 2.4475] | |
| Wrist-Flex | 137 [123.0; 154.5] | 47 [42.25; 56.75] | 2.27 [1.79; 2.545] | ||
| Group/Parameter | Time Point | Test | Amplitude (Degrees) | Phase (%) | Cycle (s) |
|---|---|---|---|---|---|
| Control | Before | Wrist-0 | 70.0 [65.0; 78.0] *& | 48.0 [44.5; 50.5] * | 1.93 [1.75; 2.6] & |
| Wrist-Flex | 125.0 [112.0; 135.0] * | 49.0 [45.5; 51.0] | 2.415 [2.04; 3.005] | ||
| After | Wrist-0 | 70.0 [66.25; 78.75] & | 47.0 [41.5; 51.5] * | 1.67 [1.53; 2.0025] & | |
| Wrist-Flex | 130.0 [120.0; 135.75] | 50.0 [46.25; 53.75] | 2.05; [1.77; 2.2675] | ||
| FES | Before | Wrist-0 | 64.0 [50.0; 68.5] *& | 45.0 [39.25; 51.0] | 1.95 [1.8425; 2.1975] & |
| Wrist-Flex | 114.0 [101.75; 119.75] * | 47.0 [42.25; 48.75] | 2.53 [1.915; 3.375] | ||
| After | Wrist-0 | 56.0 [50.5; 67.25] *&@ | 47.0 [42.75; 49.75] | 2.05 [1.75; 2.7475] &@ | |
| Wrist-Flex | 110.0 [99.75; 121.75] *@ | 51.0 [46.25; 55.75] | 3.06 [2.09; 3.215] @ | ||
| Healthy | Wrist-0 | 79 [68.75; 86.0] & | 41 [38.0; 44.75] & | 1.78 [1.54; 2.4475] | |
| Wrist-Flex | 137 [123.0; 154.5] | 47 [42.25; 56.75] | 2.27 [1.79; 2.545] | ||
| Test | Group | Before and After (Cohen’s d) | Compared to Healthy Group Before Treatment (Cohen’s d) | Compared to Healthy Group After Treatment (Cohen’s d) |
|---|---|---|---|---|
| Amplitude (degree) | ||||
| Wrist-0 | FES | 0.602 & | −4.754 * | −4.37 * |
| Control | 0.164 | −3.461 * | −2.855 * | |
| Wrist-Flex | FES | 0.517 | −3.437 * | −3.443 * |
| Control | 0.101 | −2.87 * | −2.569 * | |
| Phase of maximum extension (%) | ||||
| Wrist-0 | FES | 0.381 | 0.09 | −0.486 |
| Control | 0.044 | 0.097 | 0.042 | |
| Wrist-Flex | FES | 0.677 & | 0.306 | −0.438 |
| Control | 0.083 | 0.003 | −0.079 | |
| Cycle duration (t) | ||||
| Wrist-0 | FES | 0.1 | −0.284 | −0.642 |
| Control | 0.211 | 0.012 | −0.242 | |
| Wrist-Flex | FES | 0.313 | −0.485 | −1.27 # |
| Control | −0.248 | −0.888 # | −0.523 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skvortsov, D.; Lobunko, D.; Lobunko, A.; Belonovskaya, N.; Ivanova, G. Wrist Function Test and Its Use to Assess Treatment Efficacy in Ischemic Stroke Survivors—A Pilot Study. Diagnostics 2025, 15, 840. https://doi.org/10.3390/diagnostics15070840
Skvortsov D, Lobunko D, Lobunko A, Belonovskaya N, Ivanova G. Wrist Function Test and Its Use to Assess Treatment Efficacy in Ischemic Stroke Survivors—A Pilot Study. Diagnostics. 2025; 15(7):840. https://doi.org/10.3390/diagnostics15070840
Chicago/Turabian StyleSkvortsov, Dmitry, Danila Lobunko, Anna Lobunko, Nina Belonovskaya, and Galina Ivanova. 2025. "Wrist Function Test and Its Use to Assess Treatment Efficacy in Ischemic Stroke Survivors—A Pilot Study" Diagnostics 15, no. 7: 840. https://doi.org/10.3390/diagnostics15070840
APA StyleSkvortsov, D., Lobunko, D., Lobunko, A., Belonovskaya, N., & Ivanova, G. (2025). Wrist Function Test and Its Use to Assess Treatment Efficacy in Ischemic Stroke Survivors—A Pilot Study. Diagnostics, 15(7), 840. https://doi.org/10.3390/diagnostics15070840






