Adverse Childhood Experiences (ACEs) in Specific Vulnerable Developmental Periods Can Increase the Likelihood of Chronic Pain in Adulthood—Results from a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Procedure
2.2. Measures
2.2.1. Maltreatment and Abuse Chronology of Exposure (MACE) Scale
2.2.2. Essener Trauma Inventory (ETI)
2.2.3. Brief Symptom Inventory (BSI-18)
2.2.4. Pain Assessment
2.3. Statistical Procedure
3. Results
| CP (n = 813) | No CP (n = 1764) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | χ2/t-Value | ϕ/d | p | |
| Sex | 2.54 | 0.03 | 0.11 | ||||
| Male | 334 | 41.1% | 788 | 44.7% | |||
| Female | 440 | 54.1% | 903 | 51.2% | |||
| Missing data | 39 | 4.8% | 73 | 4.1% | |||
| Mean age (SD) | 47.3 | (16.3) | 41.2 | (15.8) | |||
| 18–30 years | 151 | 18.6% | 546 | 31.0% | 65.94 | 0.17 ** | <0.001 |
| 30–50 years | 267 | 32.8% | 595 | 33.7% | |||
| 50–70 years | 271 | 33.3% | 420 | 23.8% | |||
| >70 years | 67 | 8.2% | 76 | 4.3% | |||
| Missing data | 57 | 7.0% | 127 | 7.2% | |||
| Relationship status | 24.67 | 0.10 * | <0.001 | ||||
| Married/long-term relationship | 529 | 65.1% | 444 | 25.2% | |||
| Single | 154 | 18.9% | 1111 | 63.0% | |||
| Divorced | 71 | 8.7% | 97 | 5.5% | |||
| Widowed | 17 | 2.1% | 16 | 0.9% | |||
| Missing data | 42 | 5.2% | 96 | 5.4% | |||
| Level of education | 48.02 | 0.14 * | <0.001 | ||||
| School not finished | 14 | 1.7% | 29 | 1.6% | |||
| Compulsory school | 88 | 10.8% | 115 | 6.5% | |||
| Compulsory school and apprenticeship | 325 | 40.0% | 568 | 32.2% | |||
| Higher education | 202 | 24.8% | 509 | 28.9% | |||
| University degree | 99 | 12.2% | 363 | 20.6% | |||
| Missing data | 85 | 10.5% | 180 | 10.2% | |||
| Living situation | 16.54 | 0.08 * | 0.002 | ||||
| Living alone | 158 | 19.4% | 336 | 19.0% | |||
| Living with partner/family | 479 | 58.9% | 989 | 56.1% | |||
| Living with family of origin | 38 | 4.7% | 135 | 7.7% | |||
| Living in shared apartment | 41 | 5.0% | 147 | 8.3% | |||
| Missing data | 97 | 11.9% | 157 | 8.8% | |||
| Living environment | 1.69 | 0.03 | 0.19 | ||||
| Urban region | 306 | 37.6% | 710 | 40.2% | |||
| Rural region | 436 | 53.6% | 900 | 51.0% | |||
| Missing data | 71 | 8.7% | 154 | 8.7% | |||
| Parenthood | 326 | 40.1% | 637 | 36.1% | 6.08 | 0.05 | 0.014 |
| Missing data | 125 | 15.4% | 239 | 13.5% | |||
| Disability | 83 | 10.2% | 64 | 3.6% | 47.25 | 0.14 * | <0.001 |
| Missing data | 53 | 6.5% | 75 | 4.3% | |||
3.1. Prevalence of CP and Pain Impairment
3.2. Dose-Dependent Relationship of ACEs with CP
3.3. Type-Dependent Relationship of ACEs with CP
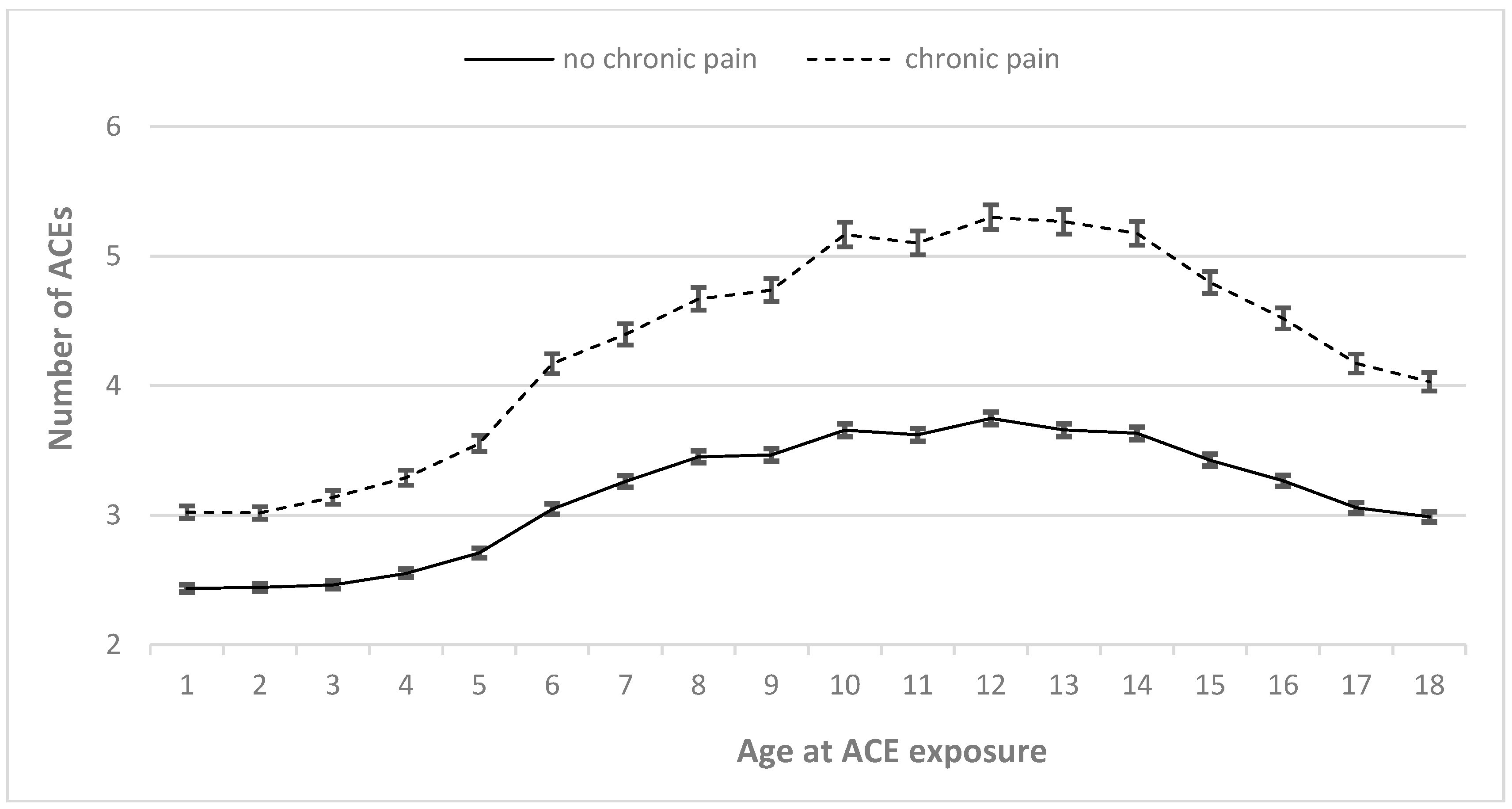
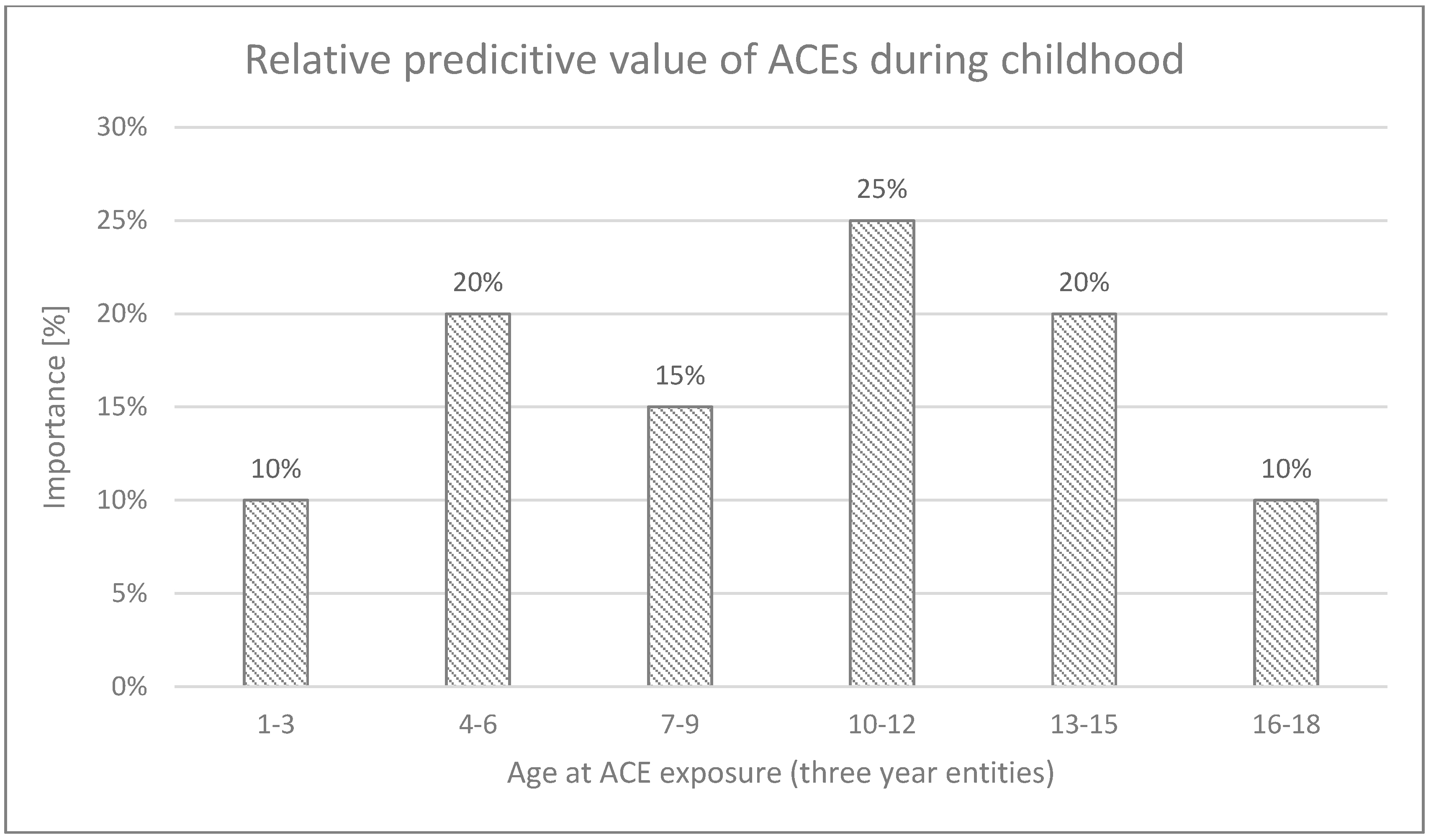
3.4. Timing-Dependent Relationship of ACEs with CP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, W.; Schmutzer, G.; Hilbert, A.; Brahler, E.; Henningsen, P. Prevalence of Chronic Disabling Noncancer Pain and Associated Demographic and Medical Variables: A Cross-Sectional Survey in the General German Population. Clin. J. Pain 2015, 31, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J Pain Off. J. Am. Pain Soc. 2016, 17, T70–T92. [Google Scholar] [CrossRef]
- Lumley, M.A.; Cohen, J.L.; Borszcz, G.S.; Cano, A.; Radcliffe, A.M.; Porter, L.S.; Schubiner, H.; Keefe, F.J. Pain and emotion: A biopsychosocial review of recent research. J. Clin. Psychol. 2011, 67, 942–968. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Aaron, R.V.; Ravyts, S.G.; Carnahan, N.D.; Bhattiprolu, K.; Harte, N.; McCaulley, C.C.; Vitalicia, L.; Rogers, A.B.; Wegener, S.T.; Dudeney, J. Prevalence of Depression and Anxiety Among Adults With Chronic Pain: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e250268. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Tripp, D.A. Pain Catastrophizing: Controversies, Misconceptions and Future Directions. J. Pain 2024, 25, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Dalechek, D.E.; Caes, L.; McIntosh, G.; Whittaker, A.C. Anxiety, history of childhood adversity, and experiencing chronic pain in adulthood: A systematic literature review and meta-analysis. Eur. J. Pain 2024, 28, 867–885. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Violence Prevention 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef] [PubMed]
- Riedl, D.; Lampe, A.; Exenberger, S.; Nolte, T.; Trawöger, I.; Beck, T. Prevalence of adverse childhood experiences (ACEs) and associated physical and mental health problems amongst hospital patients: Results from a cross-sectional study. Gen. Hosp. Psychiatry 2020. ePub ahead of print. [Google Scholar] [CrossRef]
- Van Houdenhove, B.; Luyten, P.; Egle, U.T. The role of childhood trauma in chronic pain and fatigue. In Trauma and Physical Health-Understanding the Effects of Extreme Stress and of Psychological Harm; Banyard, V.L., Edwards, V.J., Kendall-Tackett, K., Eds.; Routledge: London, UK, 2009. [Google Scholar]
- Davis, D.A.; Luecken, L.J.; Zautra, A.J. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin. J. Pain 2005, 21, 398–405. [Google Scholar]
- Lampe, A.; Doering, S.; Rumpold, G.; Solder, E.; Krismer, M.; Kantner-Rumplmair, W.; Schubert, C.; Sollner, W. Chronic pain syndromes and their relation to childhood abuse and stressful life events. J. Psychosom. Res. 2003, 54, 361–367. [Google Scholar]
- Anda, R.; Tietjen, G.; Schulman, E.; Felitti, V.; Croft, J. Adverse childhood experiences and frequent headaches in adults. Headache 2010, 50, 1473–1481. [Google Scholar] [CrossRef]
- You, D.S.; Albu, S.; Lisenbardt, H.; Meagher, M.W. Cumulative Childhood Adversity as a Risk Factor for Common Chronic Pain Conditions in Young Adults. Pain Med. 2019, 20, 486–494. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Keshavan, M.S.; Clark, D.B.; Casey, B.J.; Giedd, J.N.; Boring, A.M.; Frustaci, K.; Ryan, N.D. A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol. Psychiatry 1999, 45, 1271–1284. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Weissman, D.; Bitrán, D. Childhood Adversity and Neural Development: A Systematic Review. Annu. Rev. Dev. Psychol. 2019, 1, 277–312. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Anda, R.F.; Felitti, V.J.; Bremner, J.D.; Walker, J.D.; Whitfield, C.; Perry, B.D.; Dube, S.R.; Giles, W.H. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Berens, A.E.; Jensen, S.K.G.; Nelson, C.A., 3rd. Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 2017, 15, 135. [Google Scholar] [CrossRef]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef]
- Andersen, S.L.; Tomada, A.; Vincow, E.S.; Valente, E.; Polcari, A.; Teicher, M.H. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 2008, 20, 292–301. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 2013, 170, 1114–1133. [Google Scholar] [CrossRef]
- Andersen, S.L.; Teicher, M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008, 31, 183–191. [Google Scholar] [CrossRef]
- Schalinski, I.; Teicher, M.H. Type and timing of childhood maltreatment and severity of shutdown dissociation in patients with schizophrenia spectrum disorder. PLoS ONE 2015, 10, e0127151. [Google Scholar] [CrossRef]
- Fujisawa, T.X.; Shimada, K.; Takiguchi, S.; Mizushima, S.; Kosaka, H.; Teicher, M.H.; Tomoda, A. Type and timing of childhood maltreatment and reduced visual cortex volume in children and adolescents with reactive attachment disorder. NeuroImage Clin. 2018, 20, 216–221. [Google Scholar] [CrossRef]
- Tomoda, A.; Nishitani, S.; Takiguchi, S.; Fujisawa, T.X.; Sugiyama, T.; Teicher, M.H. The neurobiological effects of childhood maltreatment on brain structure, function, and attachment. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 1–30. [Google Scholar] [CrossRef]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F., 3rd; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef]
- Knudsen, E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef]
- Schaefer, J.D.; Cheng, T.W.; Dunn, E.C. Sensitive periods in development and risk for psychiatric disorders and related endpoints: A systematic review of child maltreatment findings. Lancet Psychiatry 2022, 9, 978–991. [Google Scholar] [CrossRef]
- Campbell, K.A. The neurobiology of childhood trauma, from early physical pain onwards: As relevant as ever in today’s fractured world. Eur. J. Psychotraumatology 2022, 13, 2131969. [Google Scholar] [CrossRef]
- Isele, D.; Teicher, M.H.; Ruf-Leuschner, M.; Elbert, T.; Kolassa, I.-T.; Schury, K.; Schauer, M. [KERF—An instrument for measuring adverse childhood experiences: Construction and psychometric evaluation of the German MACE (Maltreatment and Abuse Chronology of Exposure) Scale]. Z. Fur Klin. Psychol. Und Psychother. 2014, 43, 121–130. [Google Scholar] [CrossRef]
- Teicher, M.H.; Parigger, A. The ’Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS ONE 2015, 10, e0117423. [Google Scholar] [CrossRef]
- Tagay, S.; Erim, Y.; Stoelk, B.; Möllering, A.; Mewes, R.; Senf, W. The Essen Trauma-Inventory (ETI)–A screening instrument of identification of traumatic events and posttraumatic disorders. ZPPM 2007, 1, 75–89. [Google Scholar]
- Tagay, S.; Senf, W. The Essen Trauma-Inventory (ETI)-Manual; Hogrefe: Göttingen, Germany, 2014. [Google Scholar]
- Franke, G.H.; Jaeger, S.; Glaesmer, H.; Barkmann, C.; Petrowski, K.; Braehler, E. Psychometric analysis of the Brief Symptom Inventory 18 (BSI-18) in a representative German sample. BMC Med. Res. Methodol. 2017, 17, 14. [Google Scholar]
- Franke, G.H. Mini-Symptom-Checklist (BSI-18). Manual; Hogrefe: Göttingen, Germany, 2017. [Google Scholar]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2010. [Google Scholar]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar]
- Dunn, E.C.; McLaughlin, K.A.; Slopen, N.; Rosand, J.; Smoller, J.W. Developmental timing of child maltreatment and symptoms of depression and suicidal ideation in young adulthood: Results from the National Longitudinal Study of Adolescent Health. Depress. Anxiety 2013, 30, 955–964. [Google Scholar] [CrossRef]
- Kaplow, J.B.; Widom, C.S. Age of onset of child maltreatment predicts long-term mental health outcomes. J. Abnorm. Psychol. 2007, 116, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; McEwen, B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012, 106, 29–39. [Google Scholar] [CrossRef]
- Barboza Solis, C.; Kelly-Irving, M.; Fantin, R.; Darnaudery, M.; Torrisani, J.; Lang, T.; Delpierre, C. Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proc. Natl. Acad. Sci. USA 2015, 112, E738–E746. [Google Scholar] [CrossRef]
- Cecil, C.A.; Zhang, Y.; Nolte, T. Childhood maltreatment and DNA methylation: A systematic review. Neurosci. Biobehav. Rev. 2020, 112, 392–409. [Google Scholar] [CrossRef]
- Meaney, M.J.; Szyf, M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialog. Clin. Neurosci. 2005, 7, 103–123. [Google Scholar]
- Alvarez, P.; Green, P.G.; Levine, J.D. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol. Psychiatry 2013, 74, 688–695. [Google Scholar] [CrossRef]
- Green, P.G.; Chen, X.; Alvarez, P.; Ferrari, L.F.; Levine, J.D. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain 2011, 152, 2549–2556. [Google Scholar] [CrossRef]
- Schwaller, F.; Fitzgerald, M. The consequences of pain in early life: Injury-induced plasticity in developing pain pathways. Eur. J. Neurosci. 2014, 39, 344–352. [Google Scholar] [CrossRef]
- McCrory, E.J.; Viding, E. The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev. Psychopathol. 2015, 27, 493–505. [Google Scholar] [CrossRef] [PubMed]
- McCrory, E.J.; Gerin, M.I.; Viding, E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry-the contribution of functional brain imaging. J. Child Psychol. Psychiatry 2017, 58, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Egloff, N.; Hirschi, A.; von Kanel, R. Traumatization and chronic pain: A further model of interaction. J. Pain Res. 2013, 6, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Samson, J.A. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry Allied Discip. 2016, 57, 241–266. [Google Scholar] [CrossRef]
- Luby, J.L.; Belden, A.; Harms, M.P.; Tillman, R.; Barch, D.M. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. USA 2016, 113, 5742–5747. [Google Scholar] [CrossRef]
- Tottenham, N.; Sheridan, M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 2009, 3, 68. [Google Scholar] [CrossRef]
- Khasar, S.G.; Dina, O.A.; Green, P.G.; Levine, J.D. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J. Pain Off. J. Am. Pain Soc. 2009, 10, 1073–1077. [Google Scholar] [CrossRef]
- Heim, C.M.; Mayberg, H.S.; Mletzko, T.; Nemeroff, C.B.; Pruessner, J.C. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am. J. Psychiatry 2013, 170, 616–623. [Google Scholar] [CrossRef]
- Alexander, P.C. Childhood trauma, attachment, and abuse by multiple partners. Psychol. Trauma Theory Res. Pract. Policy 2009, 1, 78–88. [Google Scholar]
- McWilliams, L.A.; Cox, B.J.; Enns, M.W. Impact of adult attachment styles on pain and disability associated with arthritis in a nationally representative sample. Clin. J. Pain 2000, 16, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.A.; Carriere, J.S.; Kao, M.J.; Rico, T.; Darnall, B.D.; Mackey, S.C. Social Disruption Mediates the Relationship Between Perceived Injustice and Anger in Chronic Pain: A Collaborative Health Outcomes Information Registry Study. Ann. Behav. Med. Publ. Soc. Behav. Med. 2016, 50, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.A.; Ziadni, M.S.; Trost, Z.; Darnall, B.D.; Mackey, S.C. Pain catastrophizing, perceived injustice, and pain intensity impair life satisfaction through differential patterns of physical and psychological disruption. Scand. J. Pain 2017, 17, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Bissell, D.A.; Ziadni, M.S.; Sturgeon, J.A. Perceived injustice in chronic pain: An examination through the lens of predictive processing. Pain Manag. 2018, 8, 129–138. [Google Scholar] [CrossRef]
- Hird, E.J.; Charalambous, C.; El-Deredy, W.; Jones, A.K.; Talmi, D. Boundary effects of expectation in human pain perception. Sci. Rep. 2019, 9, 9443. [Google Scholar] [CrossRef]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 5747–5756. [Google Scholar] [CrossRef]
- Leichsenring, F.; Steinert, C.; Beutel, M.E.; Feix, L.; Gündel, H.; Hermann, A.; Karabatsiakis, A.; Knaevelsrud, C.; König, H.H.; Kolassa, I.T.; et al. Trauma-focused psychodynamic therapy and STAIR Narrative Therapy of post-traumatic stress disorder related to childhood maltreatment: Trial protocol of a multicentre randomised controlled trial assessing psychological, neurobiological and health economic outcomes (ENHANCE). BMJ Open 2020, 10, e040123. [Google Scholar] [CrossRef]
- Morris, A.S.; Hays-Grudo, J.; Zapata, M.I.; Treat, A.; Kerr, K.L. Adverse and Protective Childhood Experiences and Parenting Attitudes: The Role of Cumulative Protection in Understanding Resilience. Advers. Resil. Sci. 2021, 2, 181–192. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Hostinar, C.E. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: Developmental and experiential determinants. Soc. Neurosci. 2015, 10, 479–488. [Google Scholar] [CrossRef]
- Taubner, S.; Fonagy, P.; Bateman, A. Mentalisierungsbasierte Theraoie; Hogrefe: Göttingen, Germany, 2019; Volume 1. [Google Scholar]
- Bateman, A.; Fonagy, P. Handbook of Mentalizing in Mental Health Practice; American Psychiatric Publishing Inc.: Arlington, TX, USA, 2020. [Google Scholar]
- Wagner-Skacel, J.; Riedl, D.; Kampling, H.; Lampe, A. Mentalization and dissociation after adverse childhood experiences. Sci. Rep. 2022, 12, 6809. [Google Scholar] [CrossRef]
- Riedl, D.; Rothmund, M.S.; Grote, V.; Fischer, M.J.; Kampling, H.; Kruse, J.; Nolte, T.; Labek, K.; Lampe, A. Mentalizing and epistemic trust as critical success factors in psychosomatic rehabilitation: Results of a single center longitudinal observational study. Front. Psychiatry 2023, 14, 1150422. [Google Scholar] [CrossRef]
- Kampling, H.; Kruse, J.; Lampe, A.; Nolte, T.; Hettich, N.; Brähler, E.; Sachser, C.; Fegert, J.M.; Gingelmaier, S.; Fonagy, P.; et al. Epistemic trust and personality functioning mediate the association between adverse childhood experiences and posttraumatic stress disorder and complex posttraumatic stress disorder in adulthood. Front. Psychiatry 2022, 13, 919191. [Google Scholar] [CrossRef]
- Lüdemann, J.; Rabung, S.; Andreas, S. Systematic Review on Mentalization as Key Factor in Psychotherapy. Int. J. Environ. Res. Public Health 2021, 18, 9161. [Google Scholar] [CrossRef] [PubMed]
- Lampe, A.; Riedl, D.; Kampling, H.; Nolte, T.; Kirchhoff, C.; Grote, V.; Fischer, M.J.; Kruse, J. Improvements of complex post-traumatic stress disorder symptoms during a multimodal psychodynamic inpatient rehabilitation treatment-results of an observational single-centre pilot study. Eur. J. Psychotraumatology 2024, 15, 2333221. [Google Scholar] [CrossRef]
- Ballespí, S.; Vives, J.; Alonso, N.; Sharp, C.; Ramírez, M.S.; Fonagy, P.; Barrantes-Vidal, N. To know or not to know? Mentalization as protection from somatic complaints. PLoS ONE 2019, 14, e0215308. [Google Scholar] [CrossRef]
- Kasper, L.A.; Pfeifer, A.C.; Volkert, J.; Schiltenwolf, M.; Taubner, S. “Den Schmerz mentalisieren”–Implementierung eines mentalisierungsbasierten Manuals für die therapeutische Begleitung von Schmerzpatient:innen. Der Schmerz 2023, 38, 118–124. [Google Scholar] [CrossRef]
- Chou, P.L.; Rau, K.M.; Yu, T.W.; Huang, T.L.; Sun, J.L.; Wang, S.Y.; Lin, C.C. Patient-clinician relationship seems to affect adherence to analgesic use in cancer patients: A cross sectional study in a Taiwanese population. Int. J. Qual. Health Care 2017, 29, 935–940. [Google Scholar] [CrossRef]
- Fuertes, J.N.; Anand, P.; Haggerty, G.; Kestenbaum, M.; Rosenblum, G.C. The physician-patient working alliance and patient psychological attachment, adherence, outcome expectations, and satisfaction in a sample of rheumatology patients. Behav. Med. 2015, 41, 60–68. [Google Scholar] [CrossRef]
- Dorflinger, L.; Kerns, R.D.; Auerbach, S.M. Providers’ roles in enhancing patients’ adherence to pain self management. Transl. Behav. Med. 2013, 3, 39–46. [Google Scholar] [CrossRef]
- Nicola, M.; Correia, H.; Ditchburn, G.; Drummond, P.D. Defining pain-validation: The importance of validation in reducing the stresses of chronic pain. Front. Pain Res. 2022, 3, 884335. [Google Scholar] [CrossRef]
- Baldwin, J.R.; Reuben, A.; Newbury, J.B.; Danese, A. Agreement Between Prospective and Retrospective Measures of Childhood Maltreatment: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Hardt, J.; Rutter, M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. J. Child Psychol. Psychiatry 2004, 45, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Moffitt, T.E.; Caspi, A.; Belsky, D.W.; Harrington, H.; Schroeder, F.; Hogan, S.; Ramrakha, S.; Poulton, R.; Danese, A. Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J. Child Psychol. Psychiatry Allied Discip. 2016, 57, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, D.; Kirchhoff, C.; Egle, U.T.; Nolte, T.; Tschuggnall, M.; Rumpold, G.; Kantner-Rumplmair, W.; Grote, V.; Fischer, M.J.; Lampe, A. Adverse Childhood Experiences (ACEs) in Specific Vulnerable Developmental Periods Can Increase the Likelihood of Chronic Pain in Adulthood—Results from a Cross-Sectional Study. Diagnostics 2025, 15, 839. https://doi.org/10.3390/diagnostics15070839
Riedl D, Kirchhoff C, Egle UT, Nolte T, Tschuggnall M, Rumpold G, Kantner-Rumplmair W, Grote V, Fischer MJ, Lampe A. Adverse Childhood Experiences (ACEs) in Specific Vulnerable Developmental Periods Can Increase the Likelihood of Chronic Pain in Adulthood—Results from a Cross-Sectional Study. Diagnostics. 2025; 15(7):839. https://doi.org/10.3390/diagnostics15070839
Chicago/Turabian StyleRiedl, David, Christina Kirchhoff, Ulrich T. Egle, Tobias Nolte, Michael Tschuggnall, Gerhard Rumpold, Wilhelm Kantner-Rumplmair, Vincent Grote, Michael J. Fischer, and Astrid Lampe. 2025. "Adverse Childhood Experiences (ACEs) in Specific Vulnerable Developmental Periods Can Increase the Likelihood of Chronic Pain in Adulthood—Results from a Cross-Sectional Study" Diagnostics 15, no. 7: 839. https://doi.org/10.3390/diagnostics15070839
APA StyleRiedl, D., Kirchhoff, C., Egle, U. T., Nolte, T., Tschuggnall, M., Rumpold, G., Kantner-Rumplmair, W., Grote, V., Fischer, M. J., & Lampe, A. (2025). Adverse Childhood Experiences (ACEs) in Specific Vulnerable Developmental Periods Can Increase the Likelihood of Chronic Pain in Adulthood—Results from a Cross-Sectional Study. Diagnostics, 15(7), 839. https://doi.org/10.3390/diagnostics15070839







