Osteoporosis Evaluation by Radiofrequency Echographic Multispectrometry (REMS) in Primary Healthcare
Abstract
1. Introduction
2. Material and Methods
2.1. Exams and Equipment
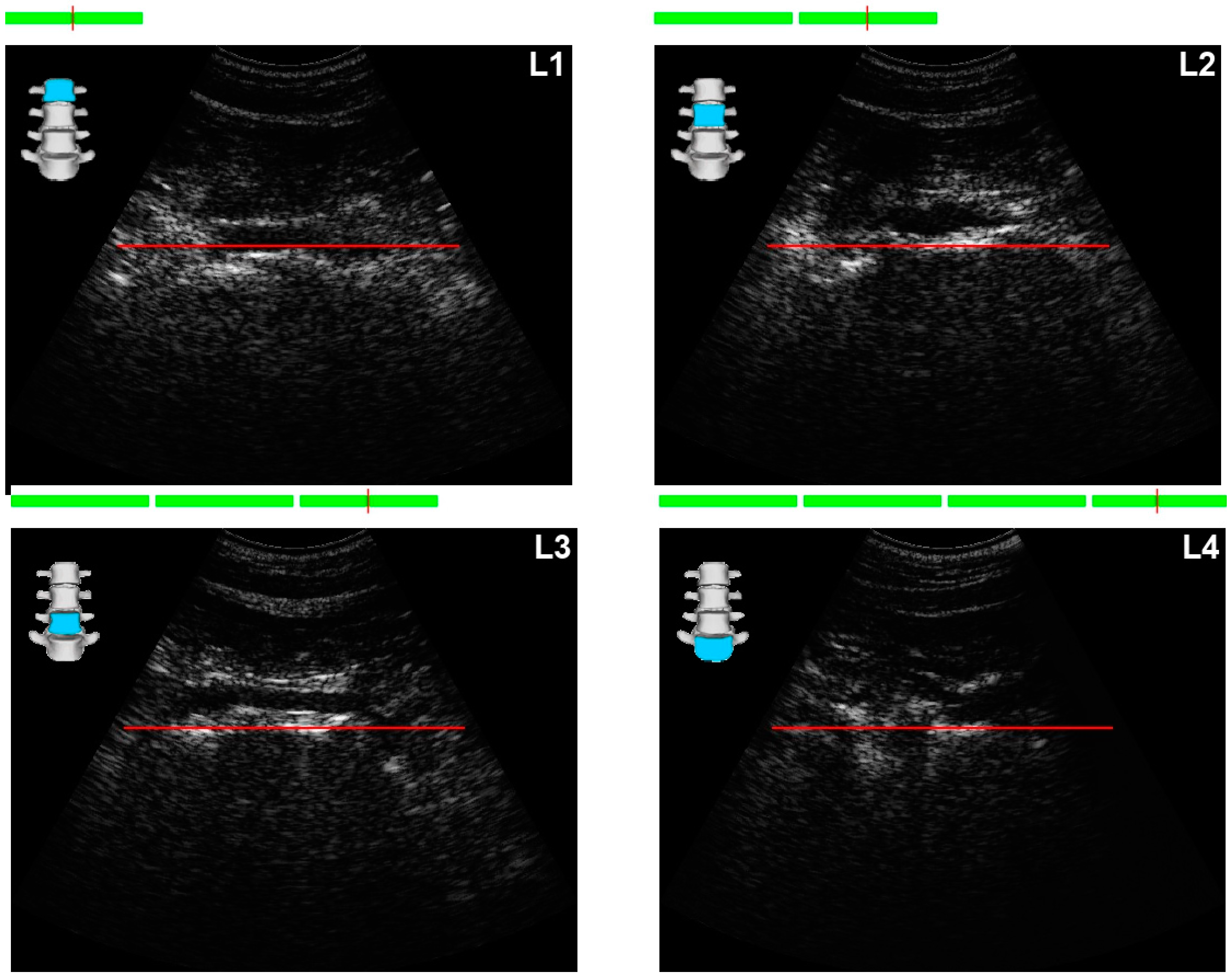
Positioning the Probe
- -
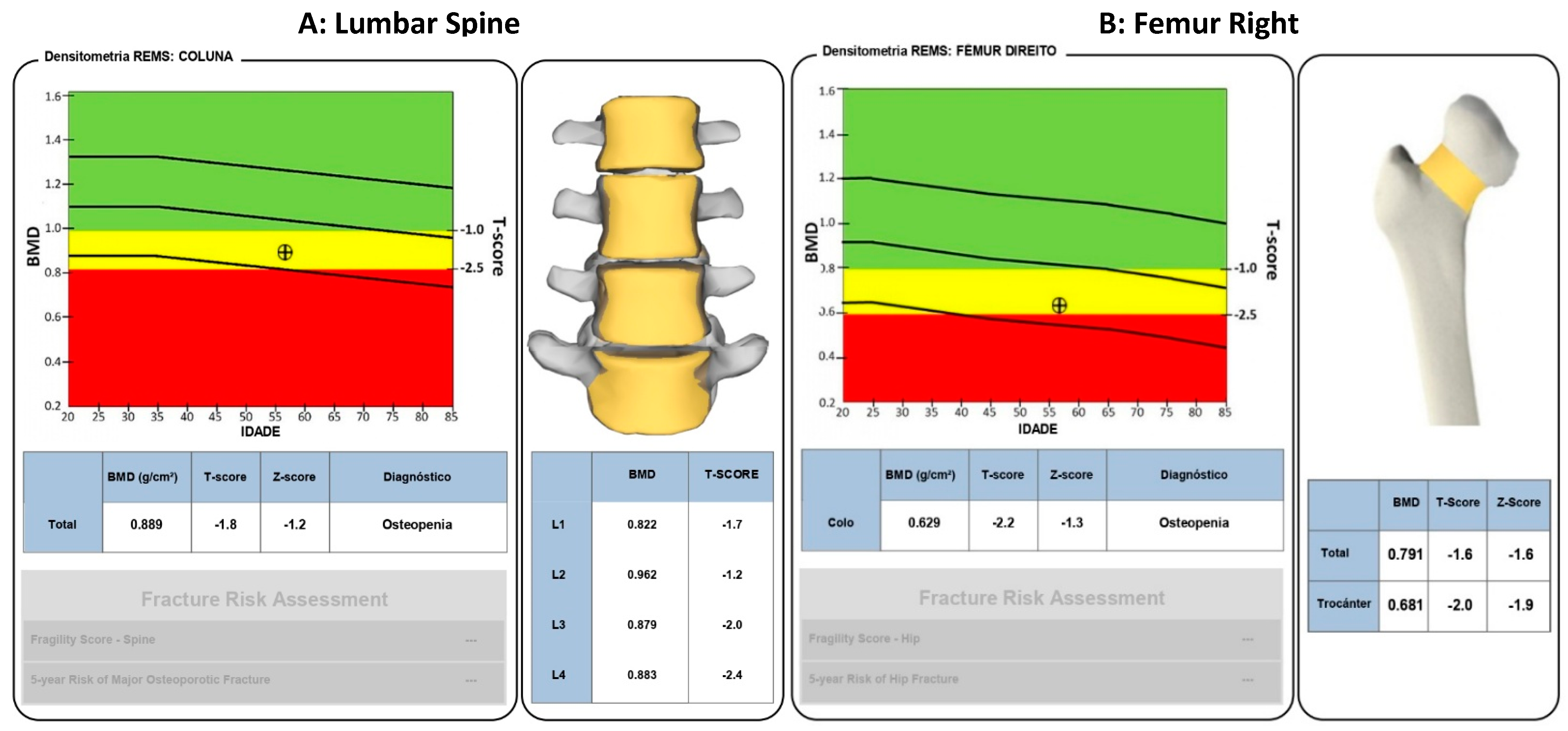
- T-Score ≥ −1 (normal);
- -
- T-Score between −1 and −2.5 (osteopenia);
- -
- T-Score ≤ −2.5 (osteoporosis) [17].
2.2. Statistical Analysis
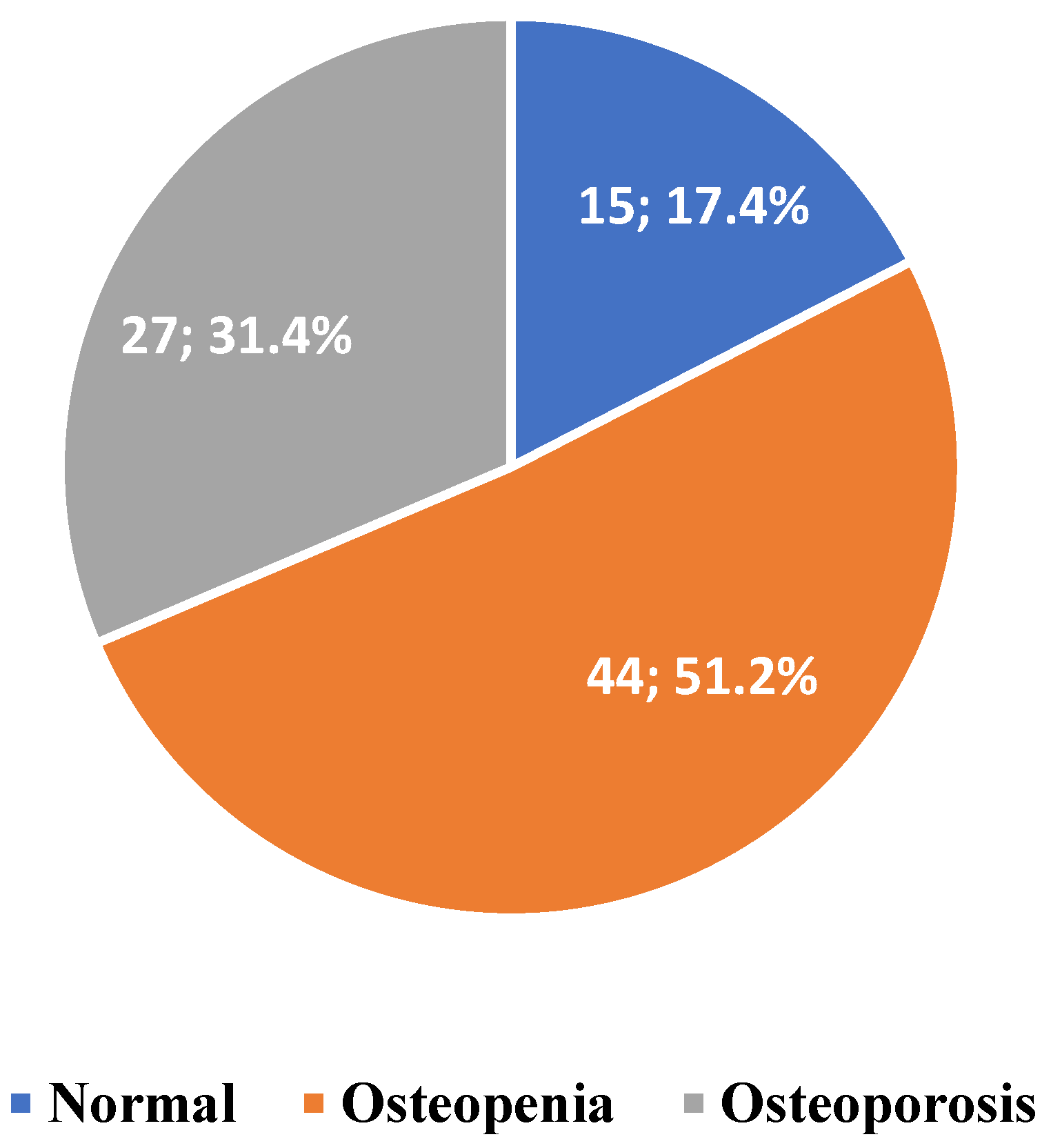
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Bone Mineral Density. |
| SD | Standard Deviation. |
| DXA | Dual Energy X-ray Absorptiometry Bone Densitometry. |
| BMI | Body Mass Index. |
| WHO | World Health Organisation. |
| REMS | Radiofrequency Echographic Multispectrometry. |
| RF | Radiofrequency. |
References
- Biscaia, A.R.; Heleno, L.C.V. The reform of primary health care in Portugal: Portuguese, modern and innovative. Sci. Collect. Health 2017, 22, 701–711. [Google Scholar] [CrossRef]
- World Health Organisation: Primary Health Care. Available online: https://www.who.int/world-health-day/world-health-day-2019/fact-sheets/details/primary-health-care (accessed on 6 December 2023).
- Lapão, L.V.; Pisco, L. The reform of primary health care in Portugal, 2005-2018: The future and the challenges of maturity. Cad. De Saude Publica 2019, 35, e00042418. [Google Scholar] [CrossRef]
- OCDE/European Observatory on Health Systems and Policies. Portugal: Country Health Profile 2021; State of Health in the EU: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2021. [Google Scholar]
- Ramalho, L.; Crespo, N. Access to Primary Health Care in Portugal: Conditions and Strategies; University Institute of Lisbon: Lisboa, Portugal, 2021; Available online: https://repositorio.iscte-iul.pt/bitstream/10071/23510/1/master_luisa_guerreiro_ramalho.pdf (accessed on 20 December 2023).
- Pinheiro, C.; Ramos, N.; Guerra, J. The Management of Chronic Diseases: Implications for Practice in Primary Health Care, 1st ed.; Open University: Lisbon, Portugal, 2008; Available online: https://repositorioaberto.uab.pt/handle/10400.2/1233 (accessed on 20 December 2023).
- Kabir, A.; Karim, M.N.; Islam, R.M.; Romero, L.; Billah, B. Health system readiness for non-communicable diseases at the primary care level: A systematic review. BMJ Open 2022, 12, e060387. [Google Scholar] [CrossRef]
- Singer, A.J.; Sharma, A.; Deignan, C.; Borgermans, L. Closing the gap in osteoporosis management: The critical role of primary care in bone health. In Current Medical Research and Opinion; Taylor and Francis Ltd.: Oxfordshire, UK, 2023; Volume 39, pp. 387–398. [Google Scholar] [CrossRef]
- Rodrigues, A.; Canhão, H.; Marques, A.; Ambrósio, C.; Borges, J.; Coelho, P.; Costa, L. Portuguese recommendations for the prevention, diagnosis and management of primaty osteoporosis—2018 update. Port. Rheumatol. Acta Port. Soc. Rheumatol. 2018, 43, 10–31. [Google Scholar]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update Executive Summary. Endocr. Pract. 2020, 26, 564–570. [Google Scholar] [CrossRef]
- Rose, D.; Preethi, T.; Prakasam, K. A Review on Osteoporosis. Int. J. All Res. Educ. Sci. Methods (IJARESM) 2022, 10, 2455–6211. [Google Scholar]
- Ajaj, A.; Elmaggoze, S.; Abofila, M.; Azab, A. Osteoporosis and Osteopaenia in Aged People: Insight into Aetiology Osteoporosis and Osteopaenia in Aged People: Insight into Aetiology, Risk Factors, Clinical Symptoms, Diagnosis, Complications, Prevention, and Treatment. Biotechnol. Bioprocess. 2023, 4. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; Mccloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch Osteóporos 2021, 16, 82. [Google Scholar] [CrossRef]
- Willers, C.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; Mccloskey, E.V.; Borgström, F.; Kanis, J.A. Osteoporosis in Europe: A compendium of country-specific reports. Arch. Osteoporos. 2021, 17, 1–129. [Google Scholar] [CrossRef]
- Falaschi, P.; Marsh, D. Orthogeriatrics: The Management of Older Patients with Fragility Fractures, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/33347100/ (accessed on 20 December 2023).
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- El Maghraoui, A.; Roux, C. DXA scanning in clinical practice. QJM Int. J. Med. 2008, 101, 605–617. [Google Scholar] [CrossRef]
- Choksi, P.; Jepsen, K.J.; Clines, G.A. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol. 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, P.; Tałałaj, M.; Życińska, K.; Zgliczyński, W.S.; Wierzba, W. Current applications and selected technical details of dual-energy x-ray absorptiometry. Med Sci. Monit. 2021, 27, e930839-1–e930839-15. [Google Scholar] [CrossRef]
- Kennel, K.A.; Sfeir, J.G.; Drake, M.T. Optimizing DXA to assess skeletal health: Key concepts for clinicians. J. Clin. Endocrinol. Metab. 2020, 105, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.; Begum, A.; Harraz, M.; Alawi, H.; Bamagos, S.; Yaghmour, A.; Hafiz, L. Dual-Energy X-Ray Absorptiometry (DEXA) Scan Versus Computed Tomography for Bone Density Assessment. Cureus 2021, 22, e13261. [Google Scholar] [CrossRef]
- Hsieh, C.I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [CrossRef]
- Messina, C.; Fusco, S.; Gazzotti, S.; Albano, D.; Bonaccorsi, G.; Guglielmi, G.; Bazzocchi, A. DXA beyond bone mineral density and the REMS technique: New insights for current radiologists practice. Radiol. Medica 2024, 129, 1224–1240. [Google Scholar] [CrossRef]
- Chen, M.; Gerges, M.; Raynor, W.Y.; Park, P.S.U.; Nguyen, E.; Chan, D.H.; Gholamrezanezhad, A. State of the Art Imaging of Osteoporosis. Semin. Nucl. Med. 2024, 54, 415–426. [Google Scholar] [CrossRef]
- Diez-Perez, A.; Brandi, M.L.; Al-Daghri, N.; Branco, J.C.; Bruyère, O.; Cavalli, L.; Cooper, C.; Cortet, B.; Dawson-Hughes, B.; Dimai, H.P.; et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art—Outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin. Exp. Res. 2019, 31, 1375–1389. [Google Scholar] [CrossRef]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogués, X.; Ovejero Crespo, D.; Quarta, E.; Brandi, M.L. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Reginster, J.Y.; Al-Daghri, N.; Bruyere, O.; Burlet, N.; Campusano, C.; Cooper, C.; Perez, A.D.; Halbout, P.; Ghi, T.; et al. Radiofrequency echographic multi spectrometry (REMS) in the diagnosis and management of osteoporosis: State of the art. Aging Clin. Exp. Res. 2024, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska-Płaza, A.; Wroński, J.; Płaza, M.; Sudoł-Szopińska, I.; Głuszko, P. Diagnostic agreement between radiofrequency echographic multispectrometry and dual-energy X-ray absorptiometry in the assessment of osteoporosis in a Polish group of patients. Pol. Arch. Intern. Med. 2021, 131, 840–847. [Google Scholar] [CrossRef]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Amorim, D.M.R.; Sakane, E.N.; Maeda, S.S.; Lazaretti Castro, M. New technology REMS for bone evaluation compared to DXA in adult women for the osteoporosis diagnosis: A real-life experience. Arch. Osteoporos. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Caffarelli, C.; Al Refaie, A.; Mondillo, C.; Manasse, G.; Versienti, A.; Tomai Pitinca, M.D.; Conticini, E.; Frediani, B.; Gonnelli, S. The Advantages of Radiofrequency Echographic MultiSpectrometry in the Evaluation of Bone Mineral Density in a Population with Osteoarthritis at the Lumbar Spine. Diagnostics 2024, 14, 523. [Google Scholar] [CrossRef]
- 33McCloskey, E.; Rathi, J.; Heijmans, S.; Blagden, M.; Cortet, B.; Czerwinski, E.; Hadji, P.; Payer, J.; Palmer, K.; Stad, R.; et al. The osteoporosis treatment gap in patients at risk of fracture in European primary care: A multi-country cross-sectional observational study. Osteoporos. Int. 2021, 32, 251–259. [Google Scholar] [CrossRef]
- Miller, K.L.; Mccoy, K.; Richards, C.; Seaman, A.; Solimeo, S.L. Engagement in Primary Prevention Program among Rural Veterans With Osteoporosis Risk. JBMR Plus 2022, 6, e10682. [Google Scholar] [CrossRef]
- Chow, C.L.J.; Shum, J.S.; Hui, K.T.P.; Lin, A.F.C.; Chu, E.C.P. Optimizing Primary Healthcare in Hong Kong: Strategies for the Successful Integration of Radiology Services. Cureus 2023, 15, e37022. [Google Scholar] [CrossRef]
- Borsoi, L.; Armeni, P.; Brandi, M.L. Cost-minimization analysis to support the HTA of Radiofrequency Echographic Multi Spectrometry (REMS) in the diagnosis of osteoporosis. Glob. Reg. Health Technol. Assess. 2023, 10, 1–11. [Google Scholar] [CrossRef]
- Föger-Samwald, U.; Kerschan-Schindl, K.; Butylina, M.; Pietschmann, P. Age Related Osteoporosis: Targeting Cellular Senescence. Int. J. Mol. Sci. 2022, 23, 2701. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.E.; Meijer, O.C.; Winter, E.M. The multi-faceted nature of age-associated osteoporosis. Bone Rep. 2024, 20, 101750. [Google Scholar] [CrossRef]
- Oliveira, D.; Oliveira, G.; Silva, D.; Pivetta, N.; Antunes, M.; Júnior, J.; Cavaglieri, C. Prevalence of osteoporosis and its associated factors in older adult’s users of the Primary Health Care. Rev. Bras. De Atividade Física Saúde 2019, 23, 1–6. [Google Scholar] [CrossRef]
- Rinonapoli, G.; Pace, V.; Ruggiero, C.; Ceccarini, P.; Bisaccia, M.; Meccariello, L.; Caraffa, A. Obesity and bone: A complex relationship. Int. J. Mol. Sci. 2021, 22, 13662. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, Y.; Liu, Y.; Peng, Y. Analysis of related factors of primary and recurrent fractures in postmenopausal osteoporotic patients: A Retrospective Study. Res. Sq. 2022. [CrossRef]
- Khu, A.; Sumardi, M. A REMS Scan-Based Report on Relation Between Body Mass Index and Osteoporosis in Urban Population of Medan at Royal Prima Hospital. Maj. Kedokt. Bdg. 2020, 52, 22–27. [Google Scholar] [CrossRef]
- Hejazi, J.; Davoodi, A.; Khosravi, M.; Sedaghat, M.; Abedi, V.; Hosseinverdi, S.; Ehrampoush, E.; Homayounfar, R.; Shojaie, L. Nutrition and osteoporosis prevention and treatment. Biomed. Res. Ther. 2020, 7, 3709–3720. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, H.; Kong, Y. Association of vitamins with bone mineral density and osteoporosis measured by dual-energy x-ray absorptiometry: A cross-sectional study. BMC Musculoskelet. Disord. 2024, 25, 69. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Papadopoulou, S.K.; Detopoulou, P.; Tsoumana, D.; Giaginis, C.; Kondyli, F.S.; Lymperaki, E.; Pritsa, A. Vitamin D and Calcium in Osteoporosis, and the Role of Bone Turnover Markers: A Narrative Review of Recent Data from RCTs. Diseases 2023, 11, 29. [Google Scholar] [CrossRef]
- Bae, S.; Lee, S.; Park, H.; Ju, Y.; Min, S.K.; Cho, J.; Kim, H.; Ha, Y.C.; Rhee, Y.; Kim, Y.P.; et al. Position Statement: Exercise Guidelines for Osteoporosis Management and Fall Prevention in Osteoporosis Patients. J. Bone Metab. 2023, 30, 149–165. [Google Scholar] [CrossRef]
- Brooke-Wavell, K.; Skelton, D.A.; Barker, K.L.; Clark, E.M.; De Biase, S.; Arnold, S.; Paskins, Z.; Robinson, K.R.; Lewis, R.M.; Tobias, J.H.; et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br. J. Sports Med. 2022, 56, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, A.; Luisa, B.M.; Carla, C.; Ernesto, C.; Francesco, C.; Marco, D.P.; Angelo, F.; Davide, G.; Francesca, G.; Stefano, G.; et al. Bone health status evaluation in men by means of REMS technology. Aging Clin. Exp. Res. 2024, 36, 74. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.V. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. J. Endocrinol. Investig. 2023, 47, 501–511. [Google Scholar] [CrossRef]
- Zhuang, H.; Wang, P.; Li, Y.; Lin, J.; Yao, X.; Xu, H. Analysis of Related Factors of Brittle Hip Fracture in Postmenopausal Women with Osteoporosis. Orthop. Surg. 2020, 12, 194–198. [Google Scholar] [CrossRef]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; De Vita, M.; Catapano, S.; Gonnelli, S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine. BMC Musculoskelet. Disord. 2022, 23, 469. [Google Scholar] [CrossRef]
- Ramirez Zegarra, R.; Degennaro, V.; Brandi, M.L.; Cagninelli, G.; Casciaro, S.; Celora, G.; Conversano, F.; Lombardi, F.A.; Pisani, P.; Ghi, T. Longitudinal changes of the femoral bone mineral density from first to third trimester of pregnancy: Bone health assessment by means of non-ionizing REMS technology. Aging Clin. Exp. Res. 2024, 36, 31. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J. Radiol. 2013, 5, 398. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics 2023, 13, 1666. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.V.; Harvey, N.C.; Lorentzon, M.; Prieto-Alhambra, D.; Abrahamsen, B.; Adachi, J.D.; Borgström, F.; Bruyere, O.; Carey, J.J.; et al. Is it time to consider population screening for fracture risk in postmenopausal women? A position paper from the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group. Arch. Osteoporos. 2022, 17, 87. [Google Scholar] [CrossRef]
- Rinaldi, C.; Bortoluzzi, S.; Airoldi, C.; Leigheb, F.; Nicolini, D.; Russotto, S.; Vanhaecht, K.; Panella, M. The Early Detection of Osteoporosis in a Cohort of Healthcare Workers: Is There Room for a Screening Program. Res. Public Health 2021, 18, 1368. [Google Scholar] [CrossRef]
- Wang, M.; Seibel, M.J. Approach to the Patient With Bone Fracture: Making the First Fracture the Last. J. Clin. Endocrinol. Metab. 2023, 108, 3345–3352. [Google Scholar] [CrossRef]
- Salminen, H.; Piispanen, P.; Toth-Pal, E. Primary care physicians’ views on osteoporosis management: A qualitative study. Arch. Osteoporos. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.T.; Grotzke, M.P.; Rosenblum, Y.; Nelson, R.E.; Lafleur, J.; Miller, K.L.; Ma, J.; Cannon, G.W. The Bone Health Team: A team-based approach to improving osteoporosis care for primary care patients. J. Prim. Care Community Health 2017, 8, 135–140. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology. EFRS Patient Safety in Medical Imaging: A joint paper of the European Society of Radiology (ESR) and the European Federation of Radiographer Societies (EFRS). Insights Into Imaging 2019, 25, e26–e38. [Google Scholar] [CrossRef]
- European Federation of Radiographer Societies. EFRS White Paper on the Future of the Profession: Radiographer Education, Research, and Practice: 2021–2031. 2021. Available online: https://www.um.edu.mt/library/oar/bitstream/123456789/106471/1/Radiographer_education_research_and_practice.pdf (accessed on 20 December 2023).
- Nguyen, V.H. Osteoporosis prevention and osteoporosis exercise in community-based public health programs. Osteoporos. Sarcopenia 2017, 3, 18–31. [Google Scholar] [CrossRef]
- Calikyan, A.; Silverberg, J.; McLeod, K.M. Osteoporosis Screening Disparities among Ethnic and Racial Minorities: A Systematic Review. J. Osteoporos. 2023, 2023, 1277319. [Google Scholar] [CrossRef]
- Caffarelli, C.; Al Refaie, A.; De Vita, M.; Tomai Pitinca, M.D.; Goracci, A.; Fagiolini, A.; Gonnelli, S. Radiofrequency echographic multispectrometry (REMS): An innovative technique for the assessment of bone status in young women with anorexia nervosa. Eat. Weight Disord. 2022, 27, 3207–3213. [Google Scholar] [CrossRef]

| n | % | ||
|---|---|---|---|
| Sex | Male | 11 | 12.8 |
| Female | 75 | 87.2 | |
| Age | Mean = 62.60 SD ± 12.33 | ||
| If female | I’ve never been pregnant | 1 | 1.3 |
| Menopause | 57 | 76.0 | |
| I’m not menopausal | 17 | 22.7 | |
| Menopause age | Mean = 48.89 SD ± 6.71 | ||
| Race | Caucasian | 86 | 100.0 |
| BMI | Underweight | 1 | 1.2 |
| Normal weight | 32 | 37.2 | |
| Overweight | 43 | 50.0 | |
| Obesity | 10 | 10.7 | |
| Does osteosynthesis material exist in the pelvis or spine? | No | 82 | 95.3 |
| Yes | 4 | 4.7 | |
| Have you been diagnosed with osteoporosis, which was confirmed by your doctor? | No | 66 | 76.7 |
| Yes | 20 | 23.3 | |
| Had a bone fracture | No | 61 | 70.9 |
| Yes | 19 | 22.1 | |
| No answer | 6 | 7.0 | |
| Alcohol habits | No | 77 | 89.5 |
| Yes, 1 to 2 times a week | 5 | 5.8 | |
| Yes, 3 to 5 times a week | 2 | 2.3 | |
| Yes, every day | 2 | 2.3 | |
| Smoking habits | No | 83 | 96.5 |
| Yes, 1 to 2 times a week | 2 | 2.3 | |
| Physical activity | No | 41 | 47.7 |
| Yes, 1 to 3 h per week | 36 | 41.9 | |
| Yes, 3 to 6 h per week | 8 | 9.3 | |
| Yes, more than 6 h a week | 1 | 1.2 | |
| n | % | ||
|---|---|---|---|
| Exposed to the sun between 10 a.m. and 4 p.m.? | No | 59 | 68.6 |
| Yes, less than 1 h a day | 16 | 18.6 | |
| Yes, 1 to 3 h a day | 6 | 7.0 | |
| Yes, more than 3 h a day | 5 | 5.8 | |
| Do you eat foods rich in vitamin D? | Never | 5 | 5.8 |
| 1 to 2 times a week | 68 | 79.1 | |
| 3 to 5 times a week | 11 | 12.8 | |
| More than 5 times a week | 2 | 2.3 | |
| Do you usually eat foods rich in vitamin K? | Never | 2 | 2.3 |
| 1 to 2 times a week | 50 | 58.1 | |
| 3 to 5 times a week | 25 | 29.1 | |
| More than 5 times a week | 9 | 10.5 | |
| Do you usually eat foods rich in calcium? | 1 to 2 times a week | 38 | 44.2 |
| 3 to 5 times a week | 31 | 36.0 | |
| More than 5 times a week | 17 | 19.8 | |
| Do you often eat foods rich in phosphorus? | Never | 1 | 1.2 |
| 1 to 2 times a week | 42 | 48.8 | |
| 3 to 5 times a week | 31 | 36.0 | |
| More than 5 times a week | 12 | 14.0 | |
| Do you eat foods rich in magnesium? | Never | 13 | 15.1 |
| 1 to 2 times a week | 52 | 60.5 | |
| 3 to 5 times a week | 17 | 19.8 | |
| More than 5 times a week | 4 | 4.7 | |
| Take vitamin D supplements? | No | 71 | 82.6 |
| Yes | 15 | 17.4 | |
| Take vitamin K supplements? | No | 84 | 97.7 |
| Yes | 2 | 2.3 | |
| Take calcium supplements? | No | 72 | 83.7 |
| Yes | 14 | 16.3 | |
| Take magnesium supplements? | No | 70 | 81.4 |
| Yes | 16 | 18.6 | |
| Thyroid hormones? | No | 82 | 95.3 |
| Yes | 4 | 4.7 |
| Age | Anova Test (p-Value) * | |||
|---|---|---|---|---|
| Normal X ± s | Osteopenia X ± s | Osteoporosis X ± s | ||
| Spinal diagnosis | 50.40 ± 11.82 | 62.70 ± 10.88 | 69.22 ± 9.72 | 0.000 ** |
| Diagnosis of the femur | 50.00 ± 11.29 | 61.19 ± 9.17 | 72.33 ± 7.56 | 0.000 *** |
| Spinal BMD Results | (p-Value) * | |||||
|---|---|---|---|---|---|---|
| Normal n (%) | Osteopenia n (%) | Osteoporosis n (%) | Total n (%) | |||
| Has a diagnosis of osteoporosis confirmed by m doctor | No | 15 (100%) | 33 (75.0%) | 18 (66.7%) | 66 (76.7%) | 0.046 |
| Yes | 0 (0.0%) | 11 (25.0%) | 9 (33.3%) | 20 (23.3%) | ||
| Total | 15 (100%) | 44 (100%) | 27 (100%) | 86 (100%) | ||
| Had a bone fracture | No | 10 (71.4%) | 35 (83.3%) | 16 (66.7%) | 61 (76.3%) | 0.278 |
| Yes | 4 (28.6%) | 7 (16.7%) | 8 (33.3%) | 19 (23.8%) | ||
| Total | 14 (100%) | 42 (100%) | 24 (100%) | 80 (100%) | ||
| BMD Results in the Femur | (p-Value) * | |||||
|---|---|---|---|---|---|---|
| Normal n (%) | Osteopenia n (%) | Osteoporosis n (%) | Total n (%) | |||
| Has a confirmed diagnosis of osteoporosis | No | 19 (100%) | 29 (78.4%) | 18 (60.0%) | 66 (76.7%) | 0.005 |
| Yes | 0 (0.0%) | 8 (21.6%) | 12 (40.0%) | 20 (23.3%) | ||
| Total | 19 (100%) | 37 (100%) | 30 (100%) | 86 (100%) | ||
| Had a bone fracture | No | 14 (77.8%) | 29 (82.9%) | 18 (66.7%) | 61 (76.3%) | 0.327 |
| Yes | 4 (22.2%) | 6 (17.1%) | 9 (33.3%) | 19 (23.8%) | ||
| Total | 18 (100%) | 35 (100%) | 27 (100%) | 80 (100%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.; Santos, R. Osteoporosis Evaluation by Radiofrequency Echographic Multispectrometry (REMS) in Primary Healthcare. Diagnostics 2025, 15, 808. https://doi.org/10.3390/diagnostics15070808
Vieira A, Santos R. Osteoporosis Evaluation by Radiofrequency Echographic Multispectrometry (REMS) in Primary Healthcare. Diagnostics. 2025; 15(7):808. https://doi.org/10.3390/diagnostics15070808
Chicago/Turabian StyleVieira, Ana, and Rute Santos. 2025. "Osteoporosis Evaluation by Radiofrequency Echographic Multispectrometry (REMS) in Primary Healthcare" Diagnostics 15, no. 7: 808. https://doi.org/10.3390/diagnostics15070808
APA StyleVieira, A., & Santos, R. (2025). Osteoporosis Evaluation by Radiofrequency Echographic Multispectrometry (REMS) in Primary Healthcare. Diagnostics, 15(7), 808. https://doi.org/10.3390/diagnostics15070808






