The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
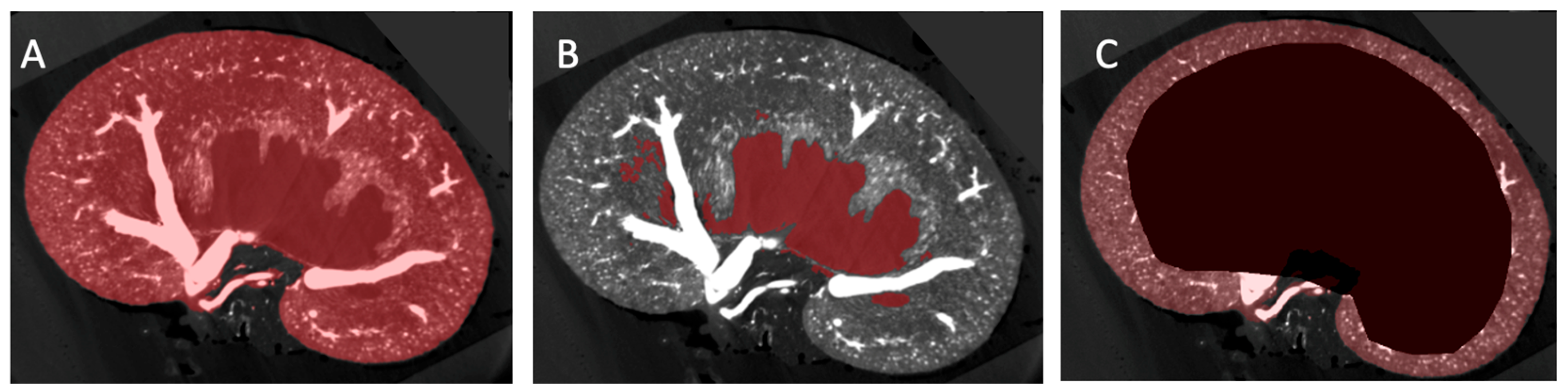
2.2. Image Segmentation and Vascular Density Measurement
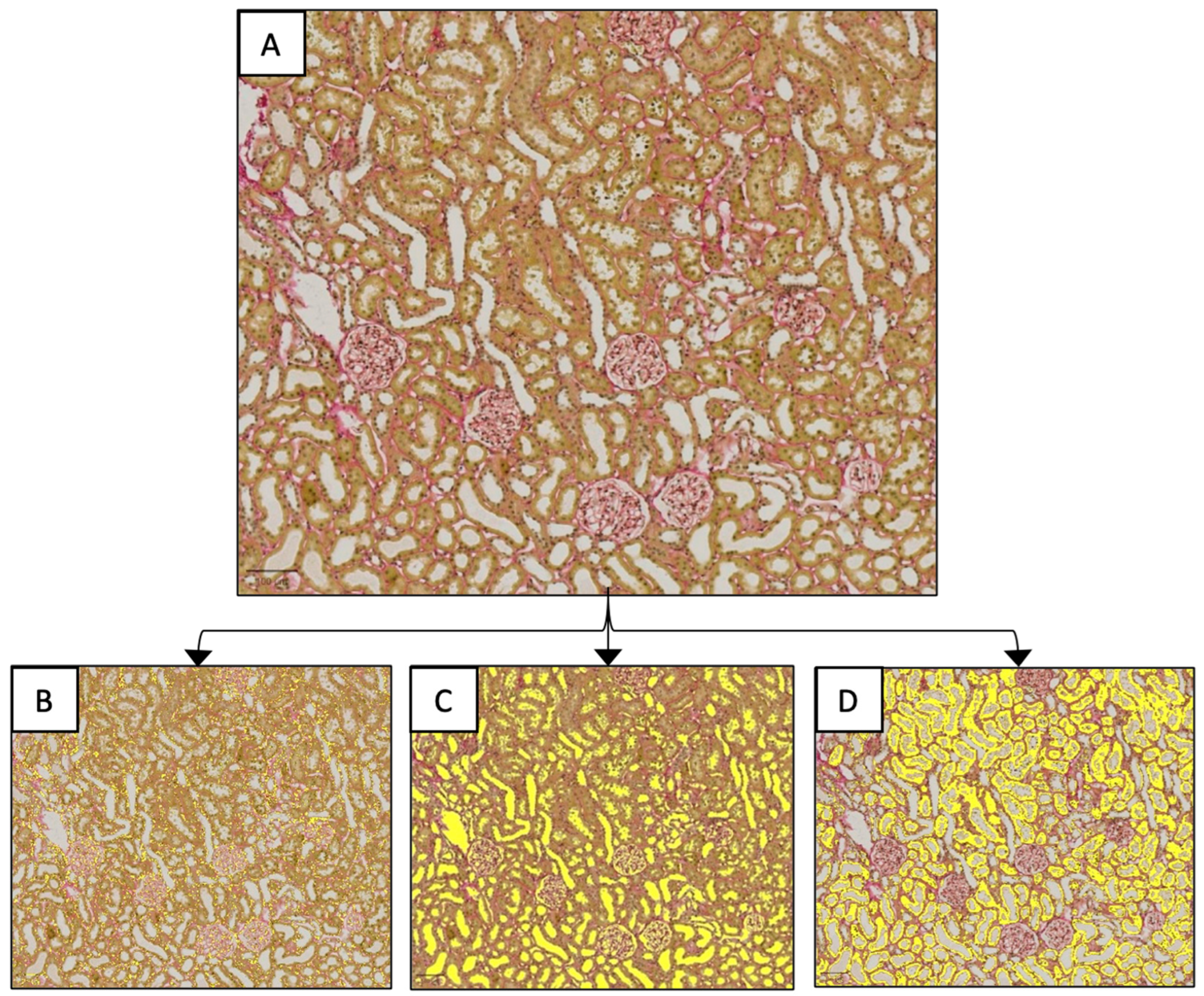
2.3. Renal Histology
2.4. Statistical Analysis
3. Results
3.1. Study Population
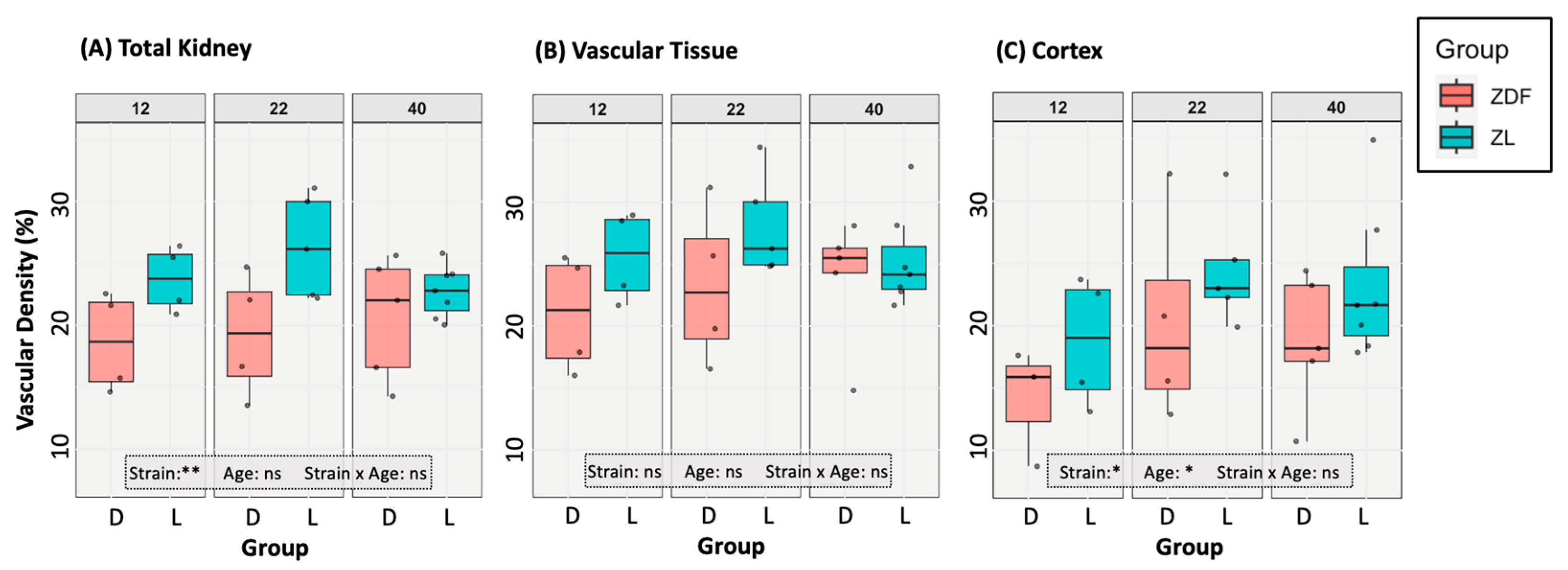
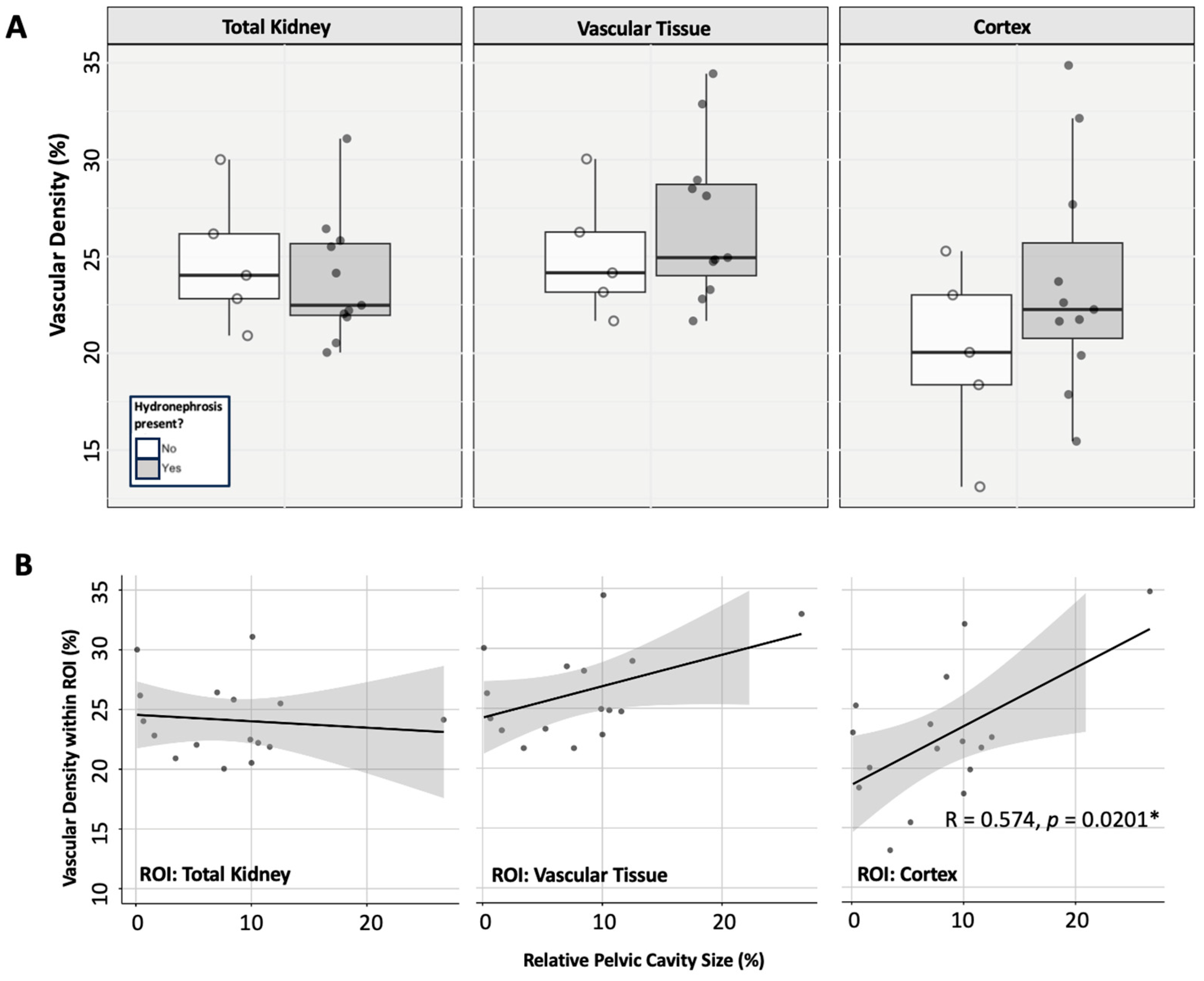
3.2. Vascular Modelling of Diabetic Kidney Disease
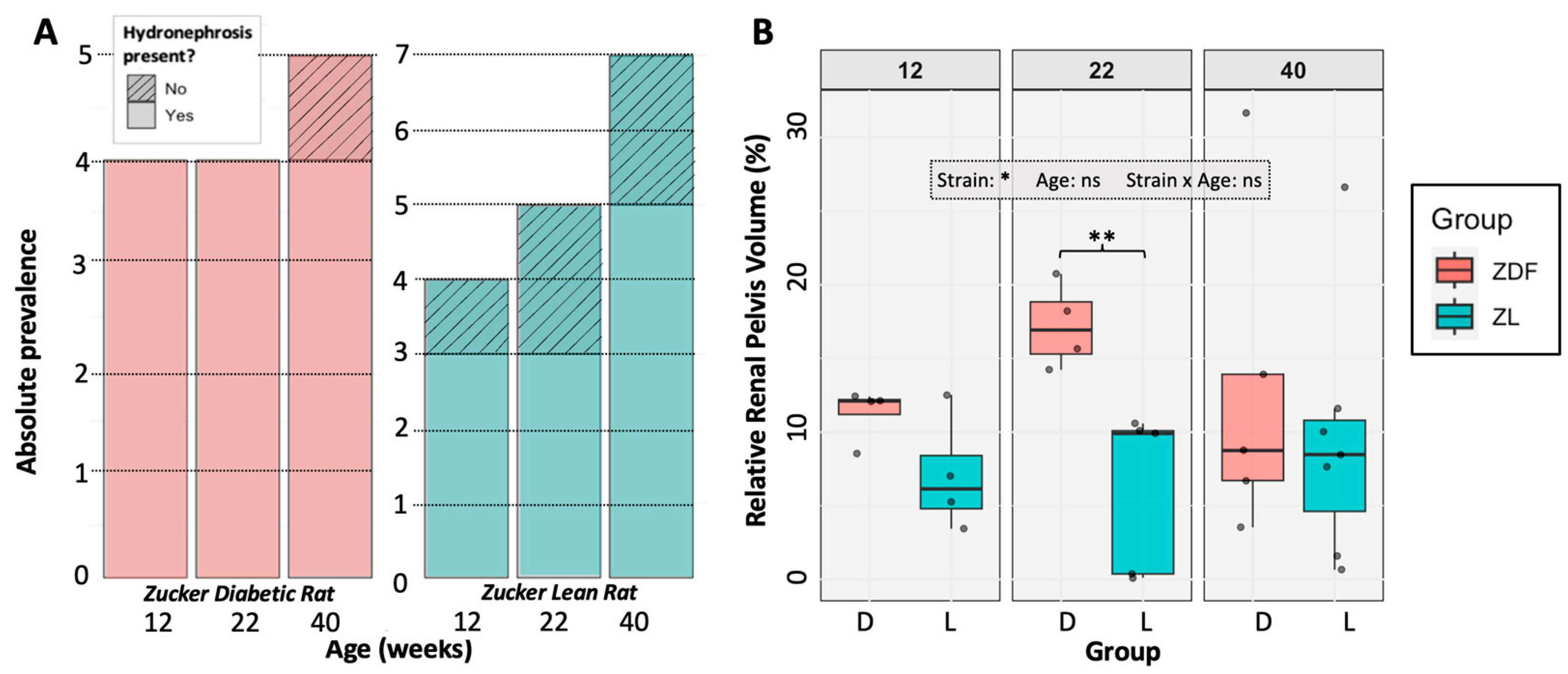
3.3. Hydronephrosis Prevalence and Severity
3.4. The Impact of Hydronephrosis on Renal Function and Anatomy
3.4.1. Renal Vascularity
3.4.2. Histological Tissue Architecture
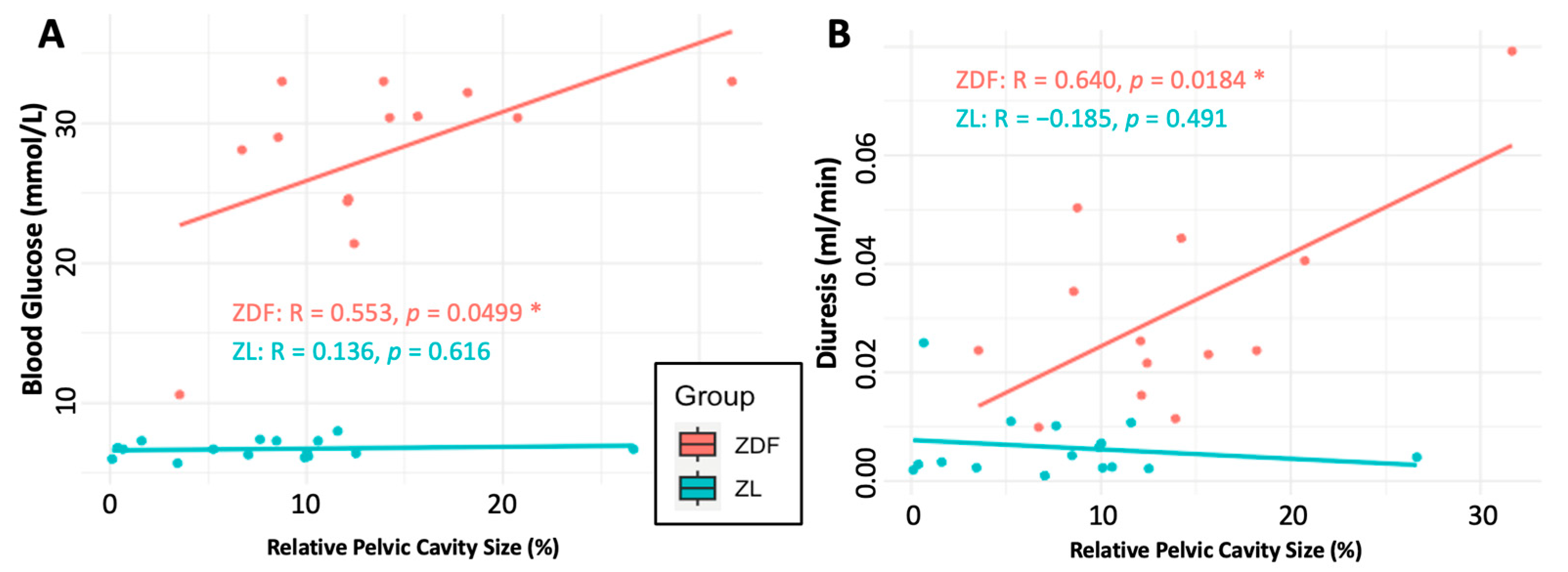
3.4.3. Renal Functional Parameters
4. Discussion
4.1. The Effects of Hydronephrosis on Healthy (ZL) Rats
4.2. Implications for Diabetes and DKD Research
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DKD | Diabetic kidney disease |
| ZDF | Zucker Diabetic Fatty |
| ZL | Zucker Lean |
| ROI | Region of Interest |
| ANOVA | Analysis of Variance |
| MANOVA | Multivariate Analysis of Variance |
| IQR | Interquartile Range |
References
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic kidney disease: World wide difference of prevalence and risk factors. J. Nephropharmacol. 2016, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Liu, Y.; Li, H.J.; Zhang, P.R. Pathophysiology of diabetic kidney disease and autophagy: A review. Medicine 2023, 102, e33965. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, D.; Jandeleit-Dahm, K.; Cooper, M. Diabetic nephropathy: Diagnosis and treatment. Nat. Rev. Endocrinol. 2013, 9, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, I.; van Koppen, A.; Joles, J.A. Animal Models of Diabetic Kidney Disease. In Diabetic Nephropathy: Pathophysiology and Clinical Aspects; Roelofs, J.J., Vogt, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 375–413. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Peterson, R.G.; Shaw, W.N.; Neel, M.-A.; Little, L.A.; Eichberg, J. Zucker Diabetic Fatty Rat as a Model for Non-insulin-dependent Diabetes Mellitus. ILAR J. 1990, 32, 16–19. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Kumar, V.P.; Sankaran, U. Experimental animal models for diabetes and its related complications-a review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Marsh, S.A.; Powell, P.C.; Agarwal, A.; Dell’Italia, L.J.; Chatham, J.C. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: Role of hydronephrosis. Am. J. Physiol.-Heart C 2007, 293, H292–H298. [Google Scholar] [CrossRef]
- Vora, J.P.; Zimsen, S.M.; Houghton, D.C.; Anderson, S. Evolution of metabolic and renal changes in the ZDF/Drt-fa rat model of type II diabetes. J. Am. Soc. Nephrol. 1996, 7, 113–117. [Google Scholar] [CrossRef]
- Kopp, U.C.; Cicha, M.Z.; Yorek, M.A. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: Role of angiotensin. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R858–R866. [Google Scholar] [CrossRef]
- Baynes, J.; Murray, D.B. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid. Med. Cell Longev. 2009, 2, 328–334. [Google Scholar] [CrossRef]
- Søgaard, S.B.; Andersen, S.B.; Taghavi, I.; Schou, M.; Christoffersen, C.; Jacobsen, J.C.B.; Kjer, H.M.; Gundlach, C.; McDermott, A.; Jensen, J.A.; et al. Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease. Diagnostics 2023, 13, 3197. [Google Scholar] [CrossRef] [PubMed]
- Komers, R.; Zdychova, J.; Cahova, M.; Kazdova, L.; Lindsley, J.N.; Anderson, S. Renal cyclooxygenase-2 in obese Zucker (fatty) rats. Kidney Int. 2005, 67, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Corcos, J.; Przydacz, M. Hydronephrosis. In Consultation in Neurourology: A Practical Evidence-Based Guide; Corcos, J., Przydacz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 213–228. [Google Scholar] [CrossRef]
- Ahmadzadeh, A.; Tahmasebi, M.; Gharibvand, M.M. Causes and Outcome of Prenatally Diagnosed Hydronephrosis. Saudi J. Kidney Dis. Transplant. 2009, 20, 246–250. [Google Scholar]
- Yousefichaijan, P.; Safi, F.; Saleh Jafari, A.; Movahedkia, R.; Rezagholizamnjany, M.; Rafiei, M.; Arjmand, A. Etiology of Hydronephrosis in Neonates. Nephro-Urol. Mon. 2018, 10, e65307. [Google Scholar] [CrossRef]
- Thom, R.P.; Rosenblum, N.D. A translational approach to congenital non-obstructive hydronephrosis. Pediatr. Nephrol. 2013, 28, 1757–1761. [Google Scholar] [CrossRef]
- Floege, J.; Johnson, R.J.; Feehally, J. Comprehensive Clinical Nephrology, 4th ed.; Floege, J., Johnson, R.J., Feehally, J., Eds.; Mosby: Philadelphia, PA, USA, 2010. [Google Scholar] [CrossRef]
- Siegelman, S.S.; Bosniak, M.A. Renal arteriography in hydronephrosis. Its value in diagnosis and management. Radiology 1965, 85, 609–616. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Lacy, E.R. The mammalian renal pelvis: Physiological implications from morphometric analyses. Anat. Embryol. 1980, 160, 131–144. [Google Scholar] [CrossRef]
- Klahr, S.; Morrissey, J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Ren. Physiol. 2002, 283, F861–F875. [Google Scholar] [CrossRef]
- Hester, A.G.; Krill, A.; Shalaby-Rana, E.; Rushton, H.G. Initial observational management of hydronephrosis in infants with reduced differential renal function and non-obstructive drainage parameters. J. Pediatr. Urol. 2022, 18, 661.e1–661.e6. [Google Scholar] [CrossRef]
- Fan, G.; Jiang, C.; Huang, Z.; Tian, M.; Pan, H.; Cao, Y.; Lei, T.; Luo, Q.; Yuan, J. 3D autofluorescence imaging of hydronephrosis and renal anatomical structure using cryo-micro-optical sectioning tomography. Theranostics 2023, 13, 4885–4904. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDermott, A.; Panduro, N.S.; Taghavi, I.; Kjer, H.M.; Søgaard, S.B.; Nielsen, M.B.; Jensen, J.A.; Sørensen, C.M. The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis. Diagnostics 2025, 15, 782. https://doi.org/10.3390/diagnostics15060782
McDermott A, Panduro NS, Taghavi I, Kjer HM, Søgaard SB, Nielsen MB, Jensen JA, Sørensen CM. The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis. Diagnostics. 2025; 15(6):782. https://doi.org/10.3390/diagnostics15060782
Chicago/Turabian StyleMcDermott, Amy, Nathalie Sarup Panduro, Iman Taghavi, Hans Martin Kjer, Stinne Byrholdt Søgaard, Michael Bachmann Nielsen, Jørgen Arendt Jensen, and Charlotte Mehlin Sørensen. 2025. "The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis" Diagnostics 15, no. 6: 782. https://doi.org/10.3390/diagnostics15060782
APA StyleMcDermott, A., Panduro, N. S., Taghavi, I., Kjer, H. M., Søgaard, S. B., Nielsen, M. B., Jensen, J. A., & Sørensen, C. M. (2025). The Zucker Diabetic Fatty Rat as a Model for Vascular Changes in Diabetic Kidney Disease: Characterising Hydronephrosis. Diagnostics, 15(6), 782. https://doi.org/10.3390/diagnostics15060782






