Application of Liquid Chromatography/Tandem Mass Spectrometry for Quantitative Analysis of Plasmalogens in Preadolescent Children—The Hokkaido Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Sex-Specific Differences in Plasmalogen Levels in Children’s Plasma
3.2. Age-Dependent Changes in Plasmalogen Levels in Children’s Plasma
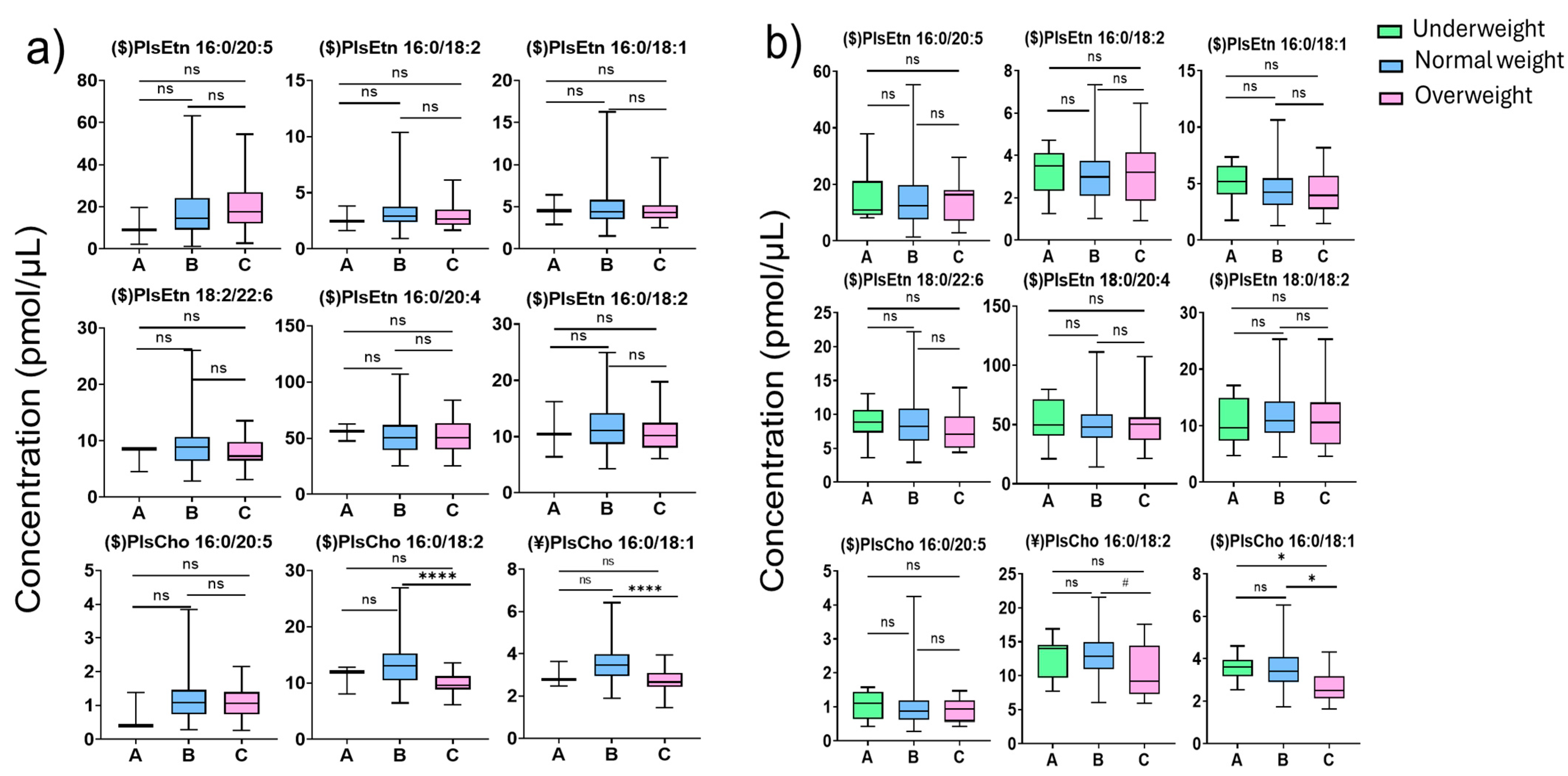
3.3. Change in Plasma Plasmalogens in Overweight Children’s Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pls | plasmalogens |
| LC-MS/MS | liquid chromatography/tandem mass spectrometry |
| PlsEtn | ethanolamine plasmalogen |
| PlsCho | choline plasmalogen |
| GPs | glycerophospholipids |
| PUFAs | polyunsaturated fatty acids |
| AD | Alzheimer’s disease |
| POW | percentage of weight |
| HPLC | high-performance liquid chromatography |
| BHT | Butylated hydroxytoluene |
| ART | aligned rank transformation |
| EPA | eicosapentaenoic acid |
| AA | arachidonic acid |
| DHA | docosahexaenoic acid |
| LCFAs | long-chain fatty acids |
| FAs | fatty acids |
References
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Mawatari, S.; Katafuchi, T.; Miake, K.; Fujino, T. Dietary plasmalogen increases erythrocyte membrane plasmalogen in rats. Lipids Health Dis. 2012, 11, 161. [Google Scholar] [CrossRef]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Heymans, H.S.A.; Schutgens, R.B.H.; Tan, R.; Bosch, H.v.D.; Borst, P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature 1983, 306, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.L.; Rapport, M.M. The biochemistry of plasmalogens. I. Isolation and characterization of phosphatidal choline, a pure native plasmalogen. J. Biol. Chem. 1962, 237, 329–333. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Maeba, R.; Araki, A.; Fujiwara, Y. Serum Ethanolamine Plasmalogen and Urine Myo-Inositol as Cognitive Decline Markers. Adv. Clin. Chem. 2018, 87, 69–111. [Google Scholar]
- Dorninger, F.; Moser, A.B.; Kou, J.; Wiesinger, C.; Forss-Petter, S.; Gleiss, A.; Hinterberger, M.; Jungwirth, S.; Fischer, P.; Berger, J. Alterations in the Plasma Levels of Specific Choline Phospholipids in Alzheimer’s Disease Mimic Accelerated Aging. J. Alzheimer’s Dis. 2018, 62, 841–854. [Google Scholar] [CrossRef]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; de Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 41. [Google Scholar] [CrossRef]
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Wiest, M.; German, J.; Harvey, D.; Watkins, S.; Hertz-Picciotto, I. Plasma fatty acid profiles in autism: A case-control study. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 221–227. [Google Scholar] [CrossRef]
- Gonzalez, P.A.; Simcox, J.; Raff, H.; Wade, G.; Von Bank, H.; Weisman, S.; Hainsworth, K. Lipid signatures of chronic pain in female adolescents with and without obesity. Lipids Health Dis. 2022, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Labadaridis, I.; Moraitou, M.; Theodoraki, M.; Triantafyllidis, G.; Sarafidou, J.; Michelakakis, H. Plasmalogen levels in full-term neonates. Acta Paediatr. 2009, 98, 640–642. [Google Scholar] [CrossRef]
- Hui, S.-P.; Chiba, H.; Kurosawa, T. Liquid chromatography–mass spectrometric determination of plasmalogens in human plasma. Anal. Bioanal. Chem. 2011, 400, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Vasku, G.; Acar, N.; Berdeaux, O. Rapid Analysis of Plasmalogen Individual Species by High-Resolution Mass Spectrometry. Methods Mol. Biol. 2023, 2625, 259–267. [Google Scholar]
- Otoki, Y.; Kato, S.; Kimura, F.; Furukawa, K.; Yamashita, S.; Arai, H.; Miyazawa, T.; Nakagawa, K. Accurate quantitation of choline and ethanolamine plasmalogen molecular species in human plasma by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 134, 77–85. [Google Scholar] [CrossRef]
- Kishi, R.; Sasaki, S.; Yoshioka, E.; Yuasa, M.; Sata, F.; Saijo, Y.; Kurahashi, N.; Tamaki, J.; Endo, T.; Sengoku, K.; et al. Cohort profile: The Hokkaido study on environment and Children’s Health in Japan. Leuk. Res. 2011, 40, 611–618. [Google Scholar] [CrossRef]
- Kishi, R.; Araki, A.; Minatoya, M.; Hanaoka, T.; Miyashita, C.; Itoh, S.; Kobayashi, S.; Ait Bamai, Y.; Yamazaki, K.; Miura, R.; et al. The Hokkaido Birth Cohort Study on Environment and Children’s Health: Cohort profile updated. Environ. Health Prev. Med. 2017, 22, 46–62. [Google Scholar] [CrossRef]
- Kishi, R.; Araki, A.; Minatoya, M.; Hanaoka, T.; Miyashita, C.; Itoh, S.; Kobayashi, S.; Ait Bamai, Y.; Yamazaki, K.; Miura, R.; et al. Hokkaido birth cohort study on environment and children’s health: Cohort profile 2021. Environ. Health Prev. Med. 2021, 26, 59. [Google Scholar] [CrossRef]
- Li, Y.; Gowda, S.G.; Gowda, D.; Ikeda, A.; Bamai, Y.A.; Ketema, R.M.; Kishi, R.; Chiba, H.; Hui, S.P. Alterations in plasma short-chain fatty acids in preadolescence children: The Hokkaido study. J. Chromatogr. B 2024, 1242, 124191. [Google Scholar] [CrossRef]
- Ketema, R.M.; Bamai, Y.A.; Miyashita, C.; Saito, T.; Kishi, R.; Ikeda-Araki, A. Phthalates mixture on allergies and oxidative stress biomarkers among children: The Hokkaido study. Environ. Int. 2022, 160, 107083. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, H.; Ikeda-Araki, A.; Bamai, Y.A.; Ito, S.; Inao, T.; Yokota, I.; Miyashita, C.; Kishi, R.; Konno, S. Potential determinants of T helper 2 markers and their distribution in school-aged children. Allergol. Int. 2023, 72, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Gao, Z.-J.; Chen, Z.; Abe, T.; Hori, S.; Fukiya, S.; Ishizuka, S.; Yokota, A.; Chiba, H.; Hui, S.-P. Untargeted lipidomic analysis of plasma from high fat diet-induced obese rats using UHPLC-linear trap quadrupole-Orbitrap MS. Anal. Sci. 2020, 36, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Tajima, Y.; Murayama, M.; Senoo, Y.; Maekawa, K.; Saito, Y. Plasma and serum from nonfasting men and women differ in their lipidomic profiles. Biol. Pharm. Bull. 2013, 36, 682–685. [Google Scholar] [CrossRef]
- Shirasawa, T.; Ochiai, H.; Nanri, H.; Nishimura, R.; Ohtsu, T.; Hoshino, H.; Tajima, N.; Kokaze, A. Trends of Underweight and Overweight/Obesity Among Japanese Schoolchildren From 2003 to 2012, Defined by Body Mass Index and Percentage Overweight Cutoffs. J. Epidemiol. 2015, 25, 482–488. [Google Scholar] [CrossRef]
- Rosalia, C.; Salvatore, C. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef]
- Serefko, A.; Jach, M.E.; Pietraszuk, M.; Świąder, M.; Świąder, K.; Szopa, A. Omega-3 Polyunsaturated Fatty Acids in Depression. Int. J. Mol. Sci. 2024, 25, 8675. [Google Scholar] [CrossRef]
- Pottala, J.; Talley, J.; Churchill, S.; Lynch, D.; von Schacky, C.; Harris, W. Red Blood Cell Fatty Acids are Associated with Depression in a Case-Control Study of Adolescents. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 161–165. [Google Scholar] [CrossRef]
- Emery, S.; Häberling, I.; Berger, G.; Baumgartner, N.; Strumberger, M.; Albermann, M.; Nalani, K.; Schmeck, K.; Erb, S.; Bachmann, S.; et al. Verbal Memory Performance in Depressed Children and Adolescents: Associations with EPA but Not DHA and Depression Severity. Nutrients 2020, 12, 3630. [Google Scholar] [CrossRef]
- Riva, D. Sex and gender difference in cognitive and behavioral studies in developmental age: An introduction. J. Neurosci. Res. 2023, 101, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.-M.; Swaab, D.F. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocrinol. 2011, 32, 214–226. [Google Scholar] [CrossRef]
- Lim, W.L.; Huynh, K.; Chatterjee, P.; Martins, I.; Jayawardana, K.S.; Giles, C.; Mellett, N.A.; Laws, S.M.; Bush, A.I.; Rowe, C.C.; et al. Relationships Between Plasma Lipids Species, Gender, Risk Factors, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 303–315. [Google Scholar] [CrossRef] [PubMed]
- van der Wurff, I.; von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.; Kirschner, P.; de Groot, R. Effect of one year krill oil supplementation on depressive symptoms and self-esteem of Dutch adolescents: A randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102208. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Ω-3 fatty-acids modulate symptoms of depressive disorder, serum levels of ω-3 fatty acids and omega-6/ω-3 ratio in children: A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef]
- Maher, A.C.; Akhtar, M.; Vockley, J.; Tarnopolsky, M.A. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. PLoS ONE 2010, 5, e12025. [Google Scholar] [CrossRef]
- Maeba, R.; Maeda, T.; Kinoshita, M.; Takao, K.; Takenaka, H.; Kusano, J.; Yoshimura, N.; Takeoka, Y.; Yasuda, D.; Okazaki, T.; et al. Plasmalogens in human serum positively correlate with high-density lipoprotein and decrease with aging. J. Atheroscler. Thromb. 2007, 14, 12–18. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Jr. ; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoon, Y.C.; Im, H.; Son, Y.; Kim, M.; Saha, A.; Choi, C.; Lee, J.; Lee, S.; Kim, J.H.; et al. Adipocyte lysoplasmalogenase TMEM86A regulates plasmalogen homeostasis and protein kinase A-dependent energy metabolism. Nat. Commun. 2022, 13, 4084. [Google Scholar] [CrossRef]
- Kimura, T.; Kimura, A.K.; Ren, M.; Monteiro, V.; Xu, Y.; Berno, B.; Schlame, M.; Epand, R.M. Plasmalogen loss caused by remodeling deficiency in mitochondria. Life Sci. Alliance 2019, 2, e201900348. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Ozeki, T.; Sugihara, S.; Ito, K.; Okada, T.; Tamai, H.; Takaya, R.; Hanaki, K.; Murata, M. Criteria for medical intervention in obese children: A new definition of ‘Obesity disease’ in Japanese children. Pediatr. Int. 2003, 45, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Jenkins, N.; Luckasen, G.; Rao, S.; Ryan, E.P. Plasma metabolomics of children with aberrant serum lipids and inadequate micronutrient intake. PLoS ONE 2018, 13, e0205899. [Google Scholar] [CrossRef]
- Ikuta, A.; Sakurai, T.; Nishimukai, M.; Takahashi, Y.; Nagasaka, A.; Hui, S.-P.; Hara, H.; Chiba, H. Composition of plasmalogens in serum lipoproteins from patients with non-alcoholic steatohepatitis and their susceptibility to oxidation. Clin. Chim. Acta 2019, 493, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nishimukai, M.; Maeba, R.; Ikuta, A.; Asakawa, N.; Kamiya, K.; Yamada, S.; Yokota, T.; Sakakibara, M.; Tsutsui, H.; Sakurai, T.; et al. Serum choline plasmalogens-those with oleic acid in sn-2-are biomarkers for coronary artery disease. Clin. Chim. Acta 2014, 437, 147–154. [Google Scholar] [CrossRef]
- Nishimukai, M.; Maeba, R.; Yamazaki, Y.; Nezu, T.; Sakurai, T.; Takahashi, Y.; Hui, S.-P.; Chiba, H.; Okazaki, T.; Hara, H. Serum choline plasmalogens, particularly those with oleic acid in sn-2, are associated with proatherogenic state. J. Lipid Res. 2014, 55, 956–965. [Google Scholar] [CrossRef]
- Meletis, C.D. Alkyl-acylglycerols and the important clinical ramifications of raising plasmalogens in dementia and Alzheimer’s disease. Integr. Med. (Encinitas) 2020, 19, 12–16. [Google Scholar]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef]
- Chen, Y.; Miura, Y.; Sakurai, T.; Chen, Z.; Shrestha, R.; Kato, S.; Okada, E.; Ukawa, S.; Nakagawa, T.; Nakamura, K.; et al. Comparison of dimension reduction methods on fatty acids food source study. Sci. Rep. 2021, 11, 18748. [Google Scholar] [CrossRef]



| PlsEtn (pmol/uL) | PlsCho (pmol/uL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Boys | 16:0/20:5 | 16:0/18:2 | 16:0/18:1 | 18:0/22:6 | 18:0/20:4 | 18:0/18:2 | 16:0/20:5 | 16:0/18:2 | 16:0/18:1 |
| MAX | 63.06 | 10.37 | 16.27 | 26 | 106.68 | 24.89 | 3.84 | 26.91 | 6.42 |
| MIN | 1.17 | 0.94 | 1.53 | 2.87 | 25.4 | 4.26 | 0.26 | 6.12 | 1.44 |
| M ± SD | 18.57 ± 13.14 | 3.11 ± 1.28 | 4.77 ± 1.95 | 8.78 ± 3.25 | 51.85 ± 15.36 | 10.84 ± 3.99 | 1.20 ± 0.67 | 12.58 ± 3.33 | 3.34 ± 0.79 |
| Girls | 16:0/20:5 | 16:0/18:2 | 16:0/18:1 | 18:0/22:6 | 18:0/20:4 | 18:0/18:2 | 16:0/20:5 | 16:0/18:2 | 16:0/18:1 |
| MAX | 55.31 | 7.33 | 10.63 | 22.22 | 111.13 | 25.33 | 4.24 | 21.59 | 6.52 |
| MIN | 1.43 | 0.92 | 1.29 | 2.94 | 14.24 | 4.48 | 0.29 | 5.93 | 1.62 |
| M ± SD | 15.14 ± 10.16 | 3.12 ± 1.29 | 4.56 ± 1.89 | 8.65 ± 3.24 | 0.73 ± 17.04 | 11.64 ± 4.31 | 0.97 ± 0.52 | 12.941 ± 3.41 | 3.48 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gowda, S.G.B.; Gowda, D.; Jayaprakash, J.; Nath, L.R.; Ikeda, A.; Bamai, Y.A.; Ketema, R.M.; Kishi, R.; Chiba, H.; et al. Application of Liquid Chromatography/Tandem Mass Spectrometry for Quantitative Analysis of Plasmalogens in Preadolescent Children—The Hokkaido Study. Diagnostics 2025, 15, 743. https://doi.org/10.3390/diagnostics15060743
Chen Y, Gowda SGB, Gowda D, Jayaprakash J, Nath LR, Ikeda A, Bamai YA, Ketema RM, Kishi R, Chiba H, et al. Application of Liquid Chromatography/Tandem Mass Spectrometry for Quantitative Analysis of Plasmalogens in Preadolescent Children—The Hokkaido Study. Diagnostics. 2025; 15(6):743. https://doi.org/10.3390/diagnostics15060743
Chicago/Turabian StyleChen, Yifan, Siddabasave Gowda B. Gowda, Divyavani Gowda, Jayashankar Jayaprakash, Lipsa Rani Nath, Atusko Ikeda, Yu Ait Bamai, Rahel Mesfin Ketema, Reiko Kishi, Hitoshi Chiba, and et al. 2025. "Application of Liquid Chromatography/Tandem Mass Spectrometry for Quantitative Analysis of Plasmalogens in Preadolescent Children—The Hokkaido Study" Diagnostics 15, no. 6: 743. https://doi.org/10.3390/diagnostics15060743
APA StyleChen, Y., Gowda, S. G. B., Gowda, D., Jayaprakash, J., Nath, L. R., Ikeda, A., Bamai, Y. A., Ketema, R. M., Kishi, R., Chiba, H., & Hui, S.-P. (2025). Application of Liquid Chromatography/Tandem Mass Spectrometry for Quantitative Analysis of Plasmalogens in Preadolescent Children—The Hokkaido Study. Diagnostics, 15(6), 743. https://doi.org/10.3390/diagnostics15060743









