Dermatomyositis-Related Encephalopathy: Clinical, Neuroimaging and Immunological Characterization
Abstract
1. Introduction
2. Materials and Methods
Operational Definitions
3. Results
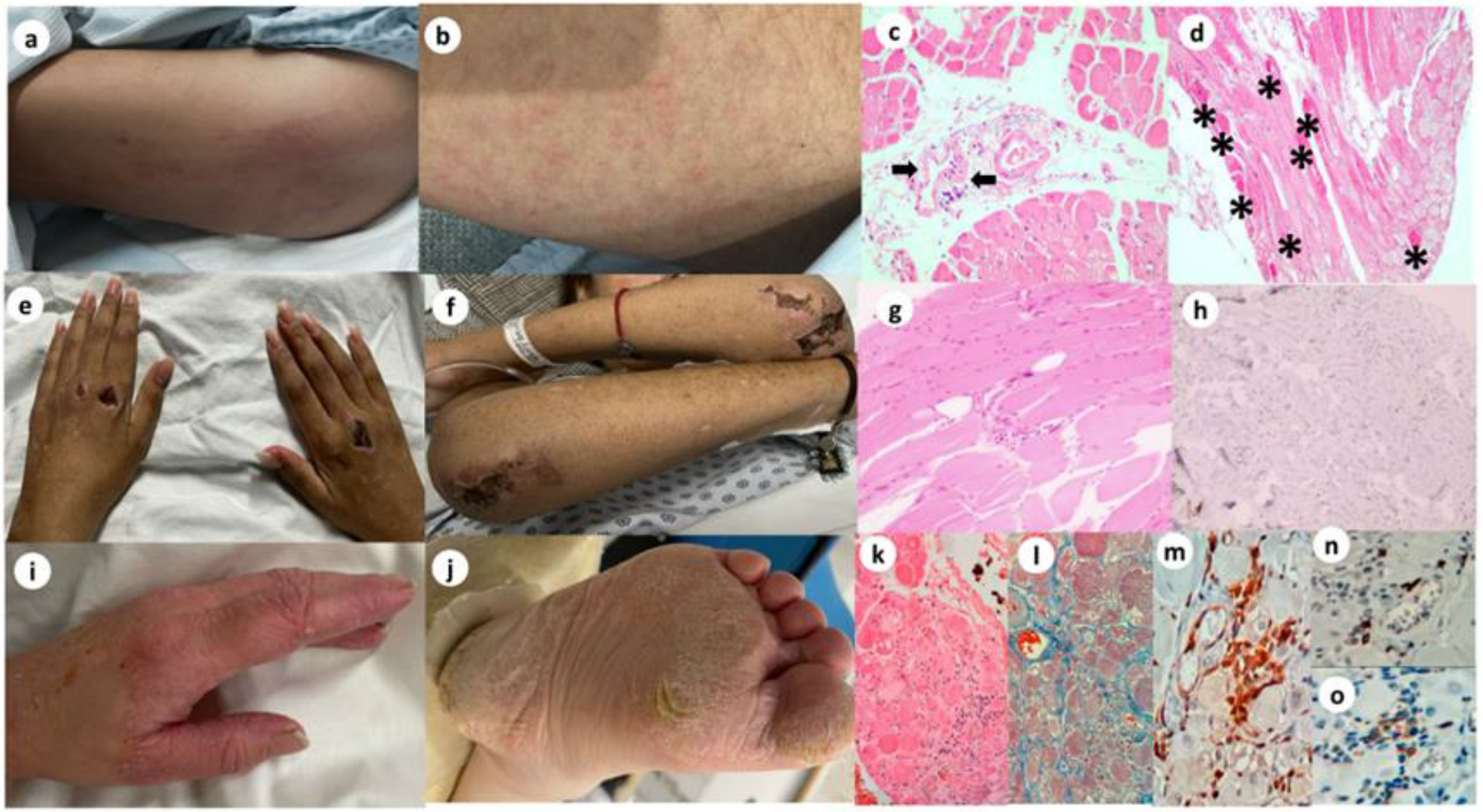
3.1. Main Clinical and Laboratory Features of Patients with Dermatomyositis with and Without Encephalopathy
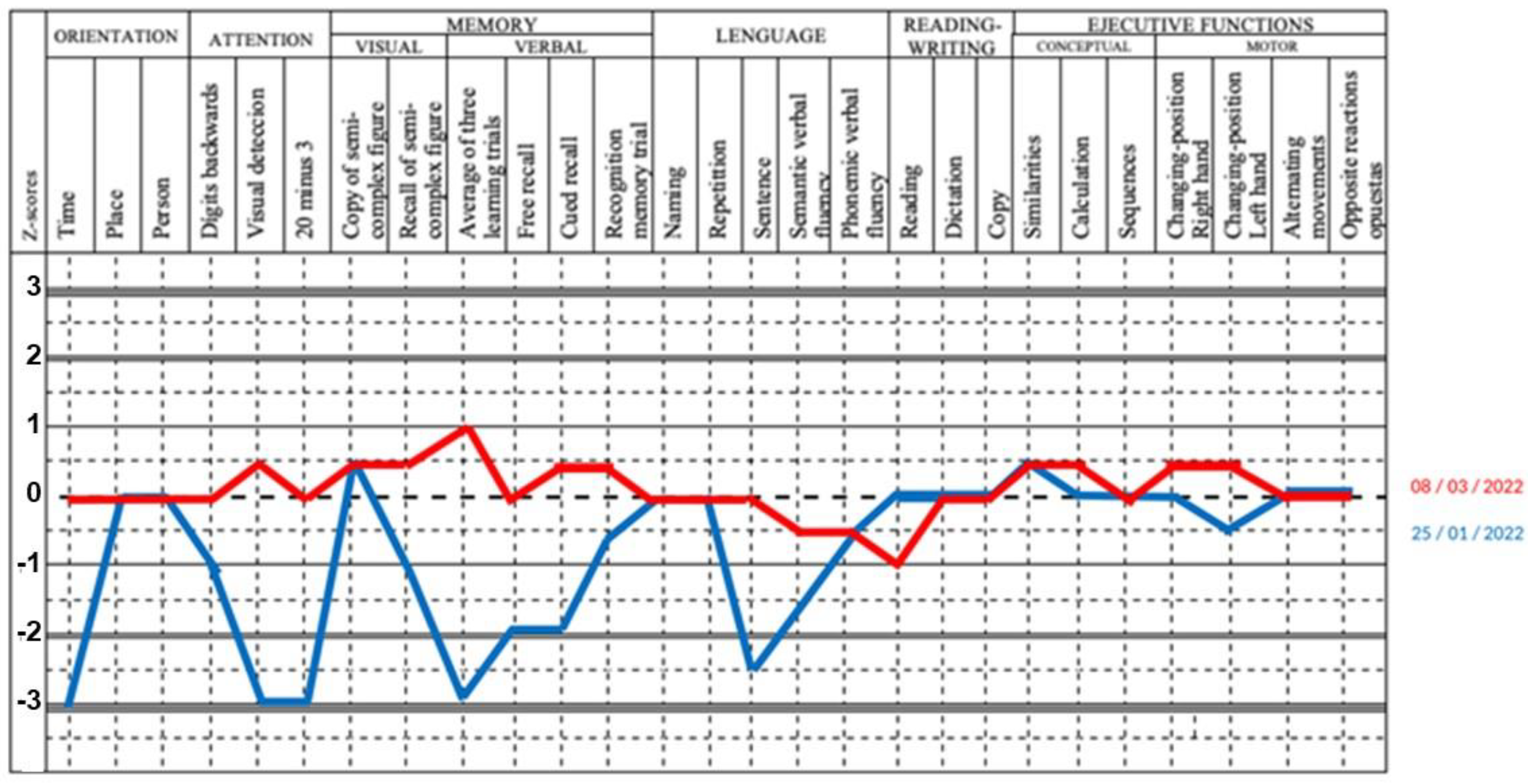
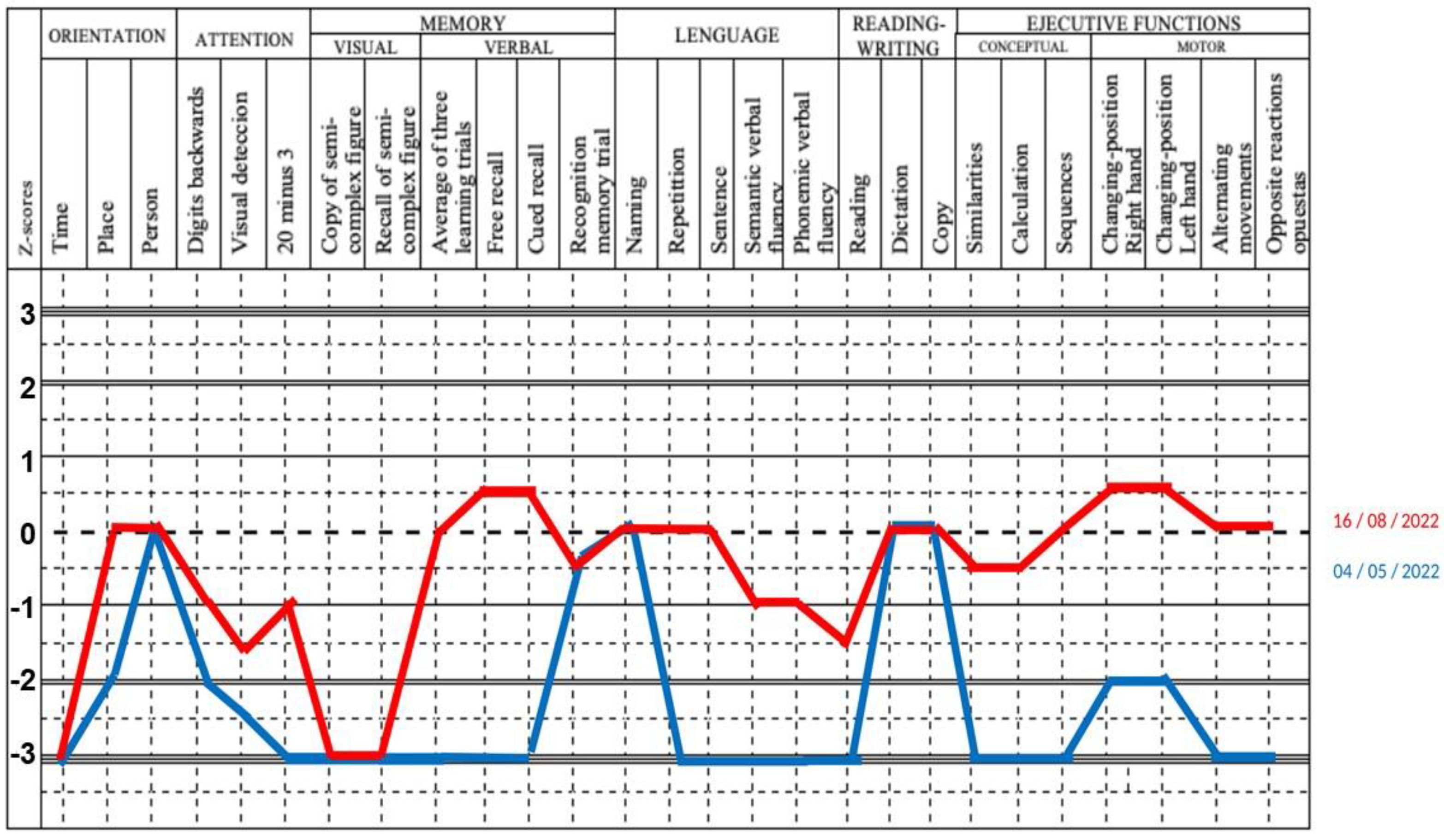
3.2. Main Clinical and Laboratory Features of Patients with Dermatomyositis with Encephalopathy
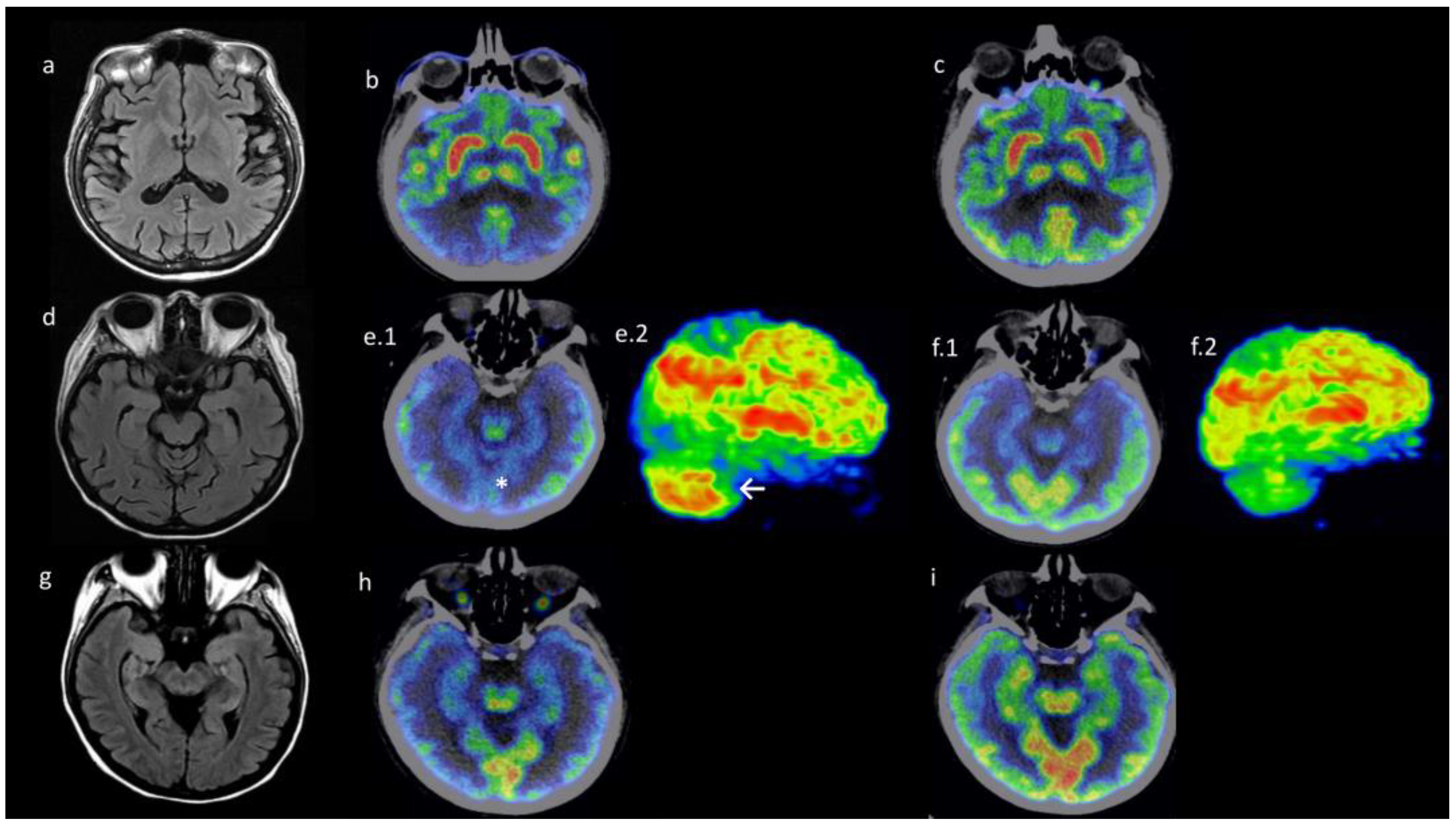
3.3. Neuroimaging Findings: Patients with Dermatomyositis-Related Encephalopathy Had Differential Metabolism Pattern in PET/CT Images
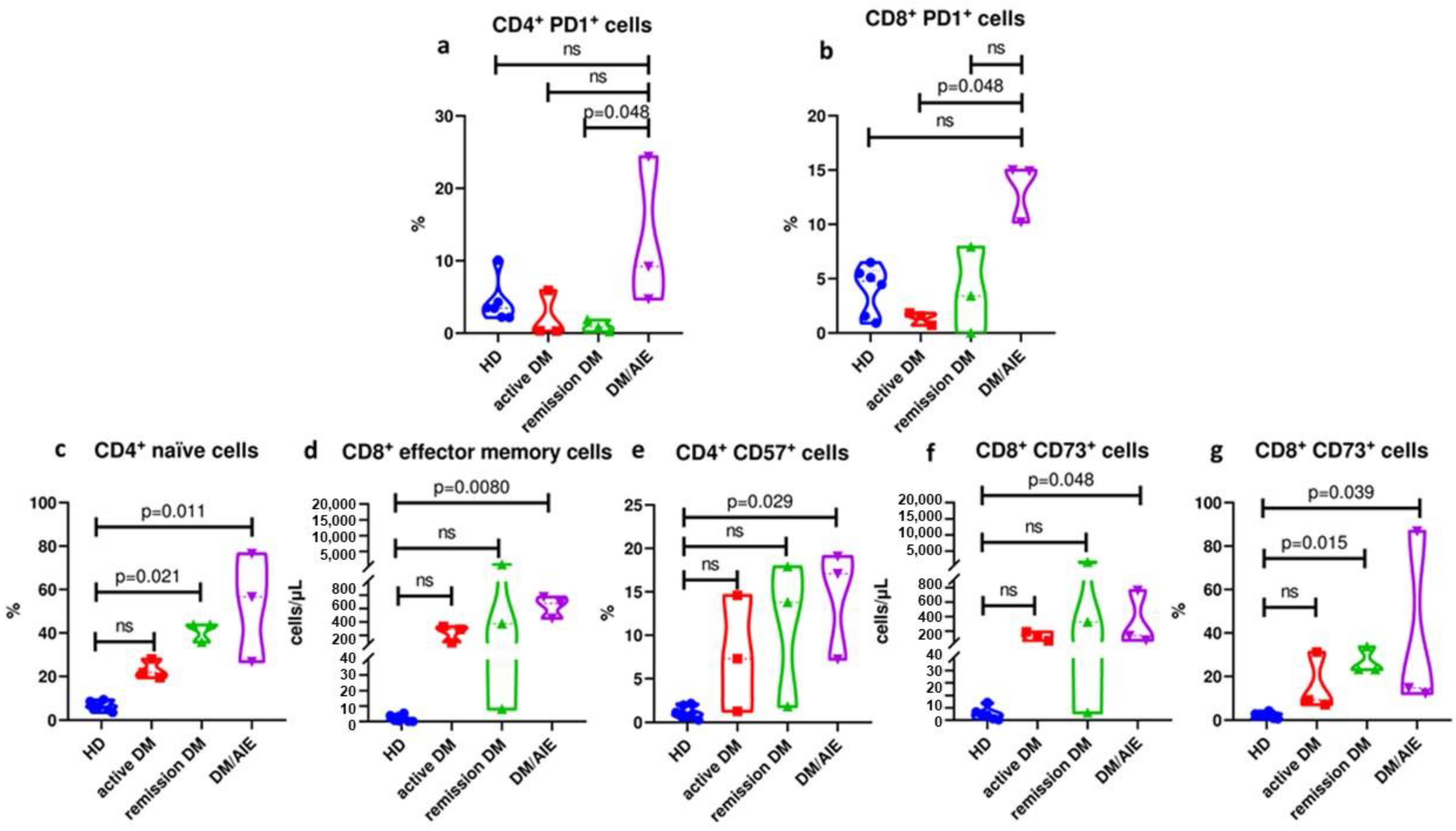
3.4. Patients with Dermatomyositis-Related Encephalopathy Are Characterized by a Differential T Cell Profile in Peripheral Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 2003, 362, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Seto, N.; Torres-Ruiz, J.J.; Carmona-Rivera, C.; Pinal-Fernandez, I.; Pak, K.; Purmalek, M.M.; Hosono, Y.; Fernandes-Cerqueira, C.; Gowda, P.; Arnett, N.; et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 2020, 5, e134189. [Google Scholar] [CrossRef] [PubMed]
- Elst, E.F.; Kamphuis, S.S.; Prakken, B.J.; Wulffraat, N.M.; van der Net, J.; Peters, A.C.; Kuis, W. Case report: Severe central nervous system involvement in juvenile dermatomyositis. J. Rheumatol. 2003, 30, 2059–2063. [Google Scholar]
- Kalim, H.; Wahono, C.S.; Permana, B.P.O.; Pratama, M.Z.; Handono, K. Association between senescence of T cells and disease activity in patients with systemic lupus erythematosus. Reumatologia 2021, 59, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Bocharnikov, A.V.; Keegan, J.; Wacleche, V.S.; Cao, Y.; Fonseka, C.Y.; Wang, G.; Muise, E.S.; Zhang, K.X.; Arazi, A.; Keras, G.; et al. PD-1hiCXCR5– T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight 2019, 4, e130062. [Google Scholar] [CrossRef]
- Wang, H.; Lan, L.; Chen, J.; Xiao, L.; Han, F. Peripheral blood T-cell subset and its clinical significance in lupus nephritis patients. Lupus Sci. Med. 2022, 9, e000717. [Google Scholar] [CrossRef]
- Mills, J.H.; Thompson, L.F.; Mueller, C.; Waickman, A.T.; Jalkanen, S.; Niemela, J.; Airas, L.; Bynoe, M.S. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9325–9330. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Nagata, N.; Kanazawa, N.; Mitsuhata, T.; Iizuka, M.; Nagashima, M.; Nakamura, M.; Kaneko, J.; Kitamura, E.; Nishiyama, K.; Iizuka, T. Neuronal surface antigen-specific immunostaining pattern on a rat brain immunohistochemistry in autoimmune encephalitis. Front. Immunol. 2023, 13, 1066830. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validez y confiabilidad del MoCA (Montreal Cognitive Assessment) para el tamizaje del deterioro cognoscitivo en méxico. Rev. Colomb. Psiquiatr. 2018, 47, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Solís, F.; Ardila, A.; Rosselli, M. NEUROPSI: A brief neuropsychological test battery in Spanish with norms by age and educational level. J. Int. Neuropsychol. Soc. 1999, 5, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Cistaro, A.; Caobelli, F.; Quartuccio, N.; Fania, P.; Pagani, M. Uncommon 18F-FDG-PET/CT findings in patients affected by limbic encephalitis: Hyper-hypometabolic pattern with double antibody positivity and migrating foci of hypermetabolism. Clin. Imaging 2015, 39, 329–333. [Google Scholar] [CrossRef]
- Moreno-Ajona, D.; Prieto, E.; Grisanti, F.; Esparragosa, I.; Sánchez Orduz, L.; Gállego Pérez-Larraya, J.; Arbizu, J.; Riverol, M. (18)F-FDG-PET Imaging Patterns in Autoimmune Encephalitis: Impact of Image Analysis on the Results. Diagnostics 2020, 10, 356. [Google Scholar] [CrossRef]
- Martin, C.; Maurer, T.; Mutizwa, M.M. Neuromyelitis Optica with Cutaneous Findings: Case Report and Review of the Literature. Dermatology 2015, 230, 289–292. [Google Scholar] [CrossRef]
- Yang, L.; Guan, W.; Liu, H.; Li, Y.; Gong, Y.; Lv, Q.; Zeng, Q.; Wei, Q.; Zhang, X.; Chen, W.; et al. Juvenile dermatomyositis with central nervous system involvement: Two case reports from a retrospective single-center cohort, with literature review. Front. Pediatr. 2024, 12, 1409950. [Google Scholar] [CrossRef]
- Lou, K.; Minhas, S.; Nayar, S. Dermatomyositis Related Paraneoplastic Encephalitis Presenting as Terminal Delirium in the Palliative Care Unit—A Case Report. J. Palliat. Care 2022, 37, 270–272. [Google Scholar] [CrossRef]
- Caselli, R.J.; Drazkowski, J.F.; Wingerchuk, D.M. Autoimmune Encephalopathy. Mayo Clin. Proc. 2010, 85, 878–880. [Google Scholar] [CrossRef]
- Flanagan, E.P.; McKeon, A.; Lennon, V.A.; Boeve, B.F.; Trenerry, M.R.; Tan, K.M.; Drubach, D.A.; Josephs, K.A.; Britton, J.W.; Mandrekar, J.N.; et al. Autoimmune Dementia: Clinical Course and Predictors of Immunotherapy Response. Mayo Clin. Proc. 2010, 85, 881–897. [Google Scholar] [CrossRef]
- Nguyen, M.; Groenendyk, J.; Steker, D.; Goyal, P.K.; Nguyen, C.V. Antimelanoma differentiation-associated gene 5 dermatomyositis associated with acute encephalopathy. JAAD Case Rep. 2021, 13, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Viengkhou, B.; Hofer, M.J. Breaking down the cellular responses to type I interferon neurotoxicity in the brain. Front. Immunol. 2023, 14, 1110593. [Google Scholar] [CrossRef]
- Regan, M.; Haque, U.; Pomper, M.; Pardo, C.; Stone, J. Central nervous system vasculitis as a complication of refractory dermatomyositis. J. Rheumatol. 2001, 28, 207–211. [Google Scholar] [PubMed]
- Delman, D.; Peng, X.; Zedek, D.C.; Jewells, V.; Chahin, N.; Markovic-Plese, S. Dermatomyositis as a presentation of neuromyelitis optica spectrum disorder. J. Neuroimmunol. 2015, 278, 108–111. [Google Scholar] [CrossRef]
- Abboud, H.; Probasco, J.C.; Irani, S.; Ances, B.; Benavides, D.R.; Bradshaw, M.; Christo, P.P.; Dale, R.C.; Fernandez-Fournier, M.; Flanagan, E.P.; et al. Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tao, L.; Li, H.; Deng, J. Dermatomyositis related to autoimmune thyroiditis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1085–1093. [Google Scholar] [CrossRef]
- Pîrşcoveanu, D.F.V.; Pirici, I.; Tudorică, V.; Bălşeanu, T.A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pîrşcoveanu, M. Tau protein in neurodegenerative diseases—A review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150. [Google Scholar]
- Do, L.D.; Chanson, E.; Desestret, V.; Joubert, B.; Ducray, F.; Brugière, S.; Couté, Y.; Formaglio, M.; Rogemond, V.; Thomas-Antérion, C.; et al. Characteristics in limbic encephalitis with anti-adenylate kinase 5 autoantibodies. Neurology 2017, 88, 514–524. [Google Scholar] [CrossRef]
- Constantinescu, R.; Krýsl, D.; Andrén, K.; Asztély, F.; Bergquist, F.; Zetterberg, H.; Andreasson, U.; Axelsson, M.; Menachem, E.B.; Jons, D.; et al. Cerebrospinal fluid markers of neuronal and glial cell damage in patients with autoimmune neurologic syndromes with and without underlying malignancies. J. Neuroimmunol. 2017, 306, 25–30. [Google Scholar] [CrossRef]
- Yamada, S.; Yamashita, H.; Nakano, M.; Hatano, H.; Sasaki, T.; Takahashi, Y.; Kaneko, H. Thrombotic Microangiopathy with Polymyositis/Dermatomyositis: Three Case Reports and a Literature Review. Intern. Med. 2018, 57, 2259–2265. [Google Scholar] [CrossRef]
- Ishizuka, M.; Watanabe, R.; Ishii, T.; Machiyama, T.; Akita, K.; Fujita, Y.; Shirota, Y.; Fujii, H.; Harigae, H. Long-term follow-up of 124 patients with polymyositis and dermatomyositis: Statistical analysis of prognostic factors. Mod. Rheumatol. 2016, 26, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Rider, L.G.; Aggarwal, R.; Machado, P.M.; Hogrel, J.Y.; Reed, A.M.; Christopher-Stine, L.; Ruperto, N. Update on outcome assessment in myositis. Nat. Rev. Rheumatol. 2018, 14, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, Q.; Fu, Q.; Li, B.; Huang, J.; Zeng, G. Tim-3, PD-1, CD244 and Foxp3 Positive T Cells’ Relation to the Prognosis of Dermatomyositis and Polymyositis Patients. J. Coll. Physicians Surg. Pak. 2023, 33, 421–426. [Google Scholar]
- Luo, Q.; Kong, Y.; Fu, B.; Li, X.; Huang, Q.; Huang, Z.; Li, J. Increased TIM-3+PD-1+ NK cells are associated with the disease activity and severity of systemic lupus erythematosus. Clin. Exp. Med. 2022, 22, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Koohini, Z.; Hossein-Nataj, H.; Mobini, M.; Hosseinian-Amiri, A.; Rafiei, A.; Asgarian-Omran, H. Analysis of PD-1 and Tim-3 expression on CD4+ T cells of patients with rheumatoid arthritis; negative association with DAS28. Clin. Rheumatol. 2018, 37, 2063–2071. [Google Scholar] [CrossRef]
- Wienke, J.; Veldkamp, S.R.; Struijf, E.M.; Yousef Yengej, F.A.; van der Wal, M.M.; van Royen-Kerkhof, A.; van Wijk, F. T cell interaction with activated endothelial cells primes for tissue-residency. Front. Immunol. 2022, 13, 827786. [Google Scholar] [CrossRef]
- Wang, E.C.; Lawson, T.M.; Vedhara, K.; Moss, P.A.; Lehner, P.J.; Borysiewicz, L.K. CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997, 40, 237–248. [Google Scholar] [CrossRef]
| Variable | Dermatomyositis with Encephalopathy Patients (n = 3) Median (IQR) | Active Dermatomyositis Patients Without Encephalopathy (n = 3) Median (IQR) | Remission Dermatomyositis Patients Without Encephalopathy (n = 3) Median (IQR) | Healthy Controls (n = 3) Median (IQR) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 49 (46–56) | 31 (31–58) | 45 (29–45) | 44 (36–58) | NS |
| Montreal Cognitive Assessment (MoCA) | 22 (18–25) | 26 (26–27) | 27 (26–29) | 29 (29–30) | 0.0167 |
| Manual muscle test 8 (MMT8) | 96 (80–120) | 92 (90–128) | 150 (150–150) | - | NS |
| Cutaneous dermatomyositis disease area and severity index (CDASI) acute | 19 (12–20) | 11 (2–29) | 0 (0–0) | - | NS |
| Cutaneous dermatomyositis disease area and severity index (CDASI) chronic | 3 (0–12) | 2 (1–7) | 1 (0–3) | - | NS |
| Creatine phosphokinase (U/L) | 43 (33–17,406) | 330 (26–7261) | 45 (39–74) | 29 (20–55) | NS |
| Aldolase (U/L) | 11.8 (3.9–266) | 17 (16–78) | 3.5 (3.5–4) | - | NS |
| Alanine aminotransferase (U/L) | 41.1 (27.8–626) | 163 (44–296) | 18 (10–19) | 28 (21.2–34) | 0.039 |
| Aspartate aminotransferase (U/L) | 52.1 (47.4–1130) | 115 (72–355) | 15 (13–15.9) | 34 (30–40) | 0.039 |
| Lactate dehydrogenase (U/L) | 347 (257–2718) | 436 (363–1124) | 141 (110–153) | 105 (79–210) | NS |
| C-reactive protein (mg/dL) | 15 (1.5–17) | 20 (1–21) | 0.15 (0.02–0.46) | 1 (0.6–1.12) | NS |
| Ferritin (ng/mL) | 1752 (1357–1994) | 340 (100–8237) | 21.8 (7.7–55.5) | 172 (127–193) | 0.039 |
| Gamma-globulins (g/dL) | 4 (3.1–4) | 4.3 (2–4.7) | 2.7 (2.5–3.1) | 3.34 (1.9–3.6) | NS |
| CSF cells (mm3) | 0 (0–3) | - | - | - | |
| CSF proteins (mg/dL) | 43 (27–73.4) | - | - | - | |
| Myositis Specific Antibodies (MSA) | TIF1γ, MDA5 and Mi2 | MDA5, Mi2 and seronegative | Mi2 (×2) and seronegative | ||
| Methylprednisolone pulses use (%) | 3 (100%) | 2 (66.6%) | 0 | ||
| 1 mg/kg/d of prednisone use (%) | 3 (100%) | 3 (100%) | 0 | ||
| IVIg use (%) | 2 (66.6%) | 2 (66.6%) | 1 (33.3%) | ||
| Plasmapheresis use (%) | 2 (66.6%) | 0 | 0 | ||
| Rituximab use (%) | 2 (66.6%) | 2 (66.6%) | 0 | ||
| Mycophenolate mofetil use (%) | 2 (66.6%) | 3 (100%) | 0 | ||
| Tacrolimus use (%) | 0 | 1 (33.3%) | 0 | ||
| Methotrexate use (%) | 0 | 2 (66.6%) | 2 (66.6%) | ||
| Azathioprine use (%) | 0 | 0 | 2 (66.6%) | ||
| Hydroxychloroquine use (%) | 1 (33.3%) | 3 (100%) | 2 (66.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Vázquez, D.A.; Davizon-López, C.A.; Gutiérrez-Castillo, A.; Torres-Ruiz, J.; Pérez-Fragoso, A.; Alcalá-Carmona, B.; Barrera-Godínez, A.; Juárez-Vega, G.; Gutiérrez-Gutiérrez, L.A.; Hernández-Ramírez, R.; et al. Dermatomyositis-Related Encephalopathy: Clinical, Neuroimaging and Immunological Characterization. Diagnostics 2025, 15, 700. https://doi.org/10.3390/diagnostics15060700
Carrillo-Vázquez DA, Davizon-López CA, Gutiérrez-Castillo A, Torres-Ruiz J, Pérez-Fragoso A, Alcalá-Carmona B, Barrera-Godínez A, Juárez-Vega G, Gutiérrez-Gutiérrez LA, Hernández-Ramírez R, et al. Dermatomyositis-Related Encephalopathy: Clinical, Neuroimaging and Immunological Characterization. Diagnostics. 2025; 15(6):700. https://doi.org/10.3390/diagnostics15060700
Chicago/Turabian StyleCarrillo-Vázquez, Daniel Alberto, Carlos Antonio Davizon-López, Alejandro Gutiérrez-Castillo, Jiram Torres-Ruiz, Alfredo Pérez-Fragoso, Beatriz Alcalá-Carmona, Alejandro Barrera-Godínez, Guillermo Juárez-Vega, Lidia Antonia Gutiérrez-Gutiérrez, Rodrigo Hernández-Ramírez, and et al. 2025. "Dermatomyositis-Related Encephalopathy: Clinical, Neuroimaging and Immunological Characterization" Diagnostics 15, no. 6: 700. https://doi.org/10.3390/diagnostics15060700
APA StyleCarrillo-Vázquez, D. A., Davizon-López, C. A., Gutiérrez-Castillo, A., Torres-Ruiz, J., Pérez-Fragoso, A., Alcalá-Carmona, B., Barrera-Godínez, A., Juárez-Vega, G., Gutiérrez-Gutiérrez, L. A., Hernández-Ramírez, R., & Gómez-Martín, D. (2025). Dermatomyositis-Related Encephalopathy: Clinical, Neuroimaging and Immunological Characterization. Diagnostics, 15(6), 700. https://doi.org/10.3390/diagnostics15060700






